Abstract
1. The apparent 3-oxoacyl-CoA thiolase activity of rat brain extracts is due to two different acetoacetyl-CoA thiolases, one cytoplasmic and the other mitochondrial. By the methods developed in the preceding paper (Middleton, 1973), the changes in activities of these two enzymes were determined during postnatal development. 2. Although the total brain acetoacetyl-CoA thiolase activity changes not more than 2-fold from birth to adulthood this masks large changes in the relative proportions of the two types of thiolase present. 3. Cytoplasmic acetoacetyl-CoA thiolase activity declines slowly from 4 units/g fresh wt. at birth to an adult value of 1.3 units/g fresh wt. 4. The mitochondrial acetoacetyl-CoA thiolase (activated by K+) rises rapidly in activity from 1 unit/g fresh wt. at birth to a peak value of 5 units/g fresh wt. at 20 days. After weaning the activity declines to 2.3 units/g fresh wt. in the adult. 5. These different developmental patterns are discussed in terms of the probable metabolic roles of the two brain acetoacetyl-CoA thiolases.
Full text
PDF

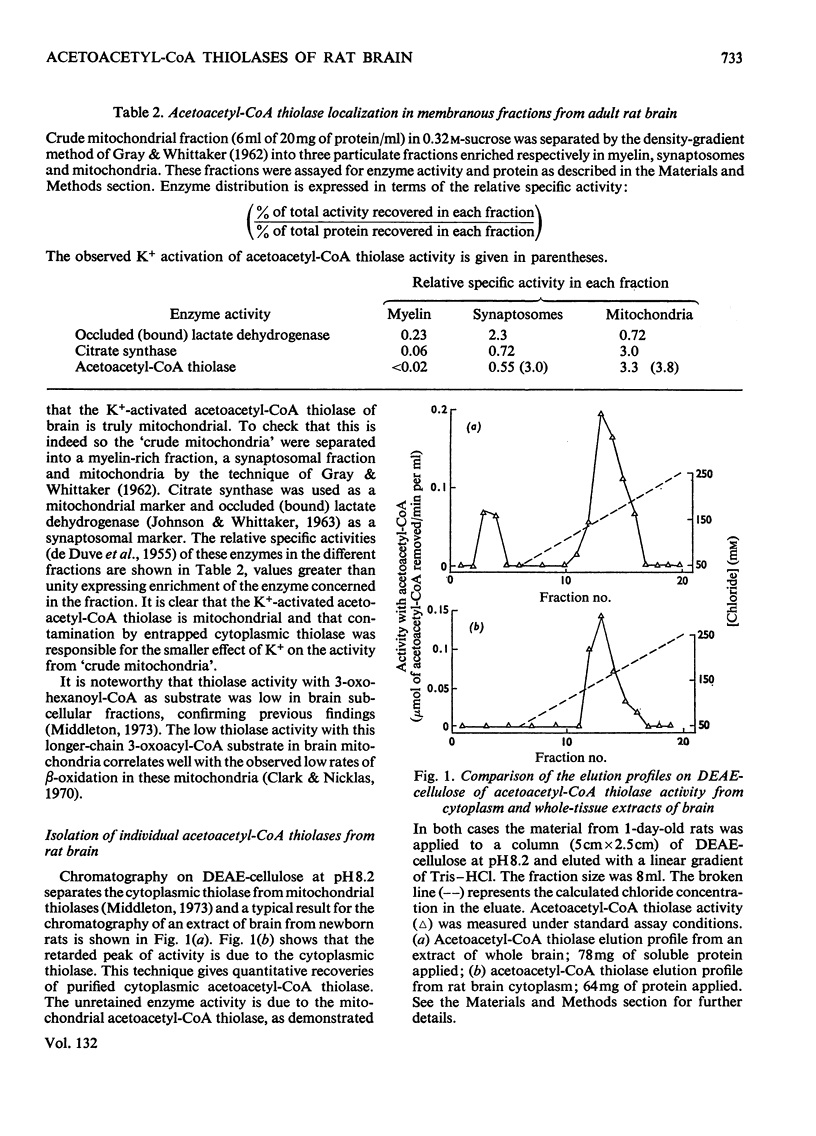
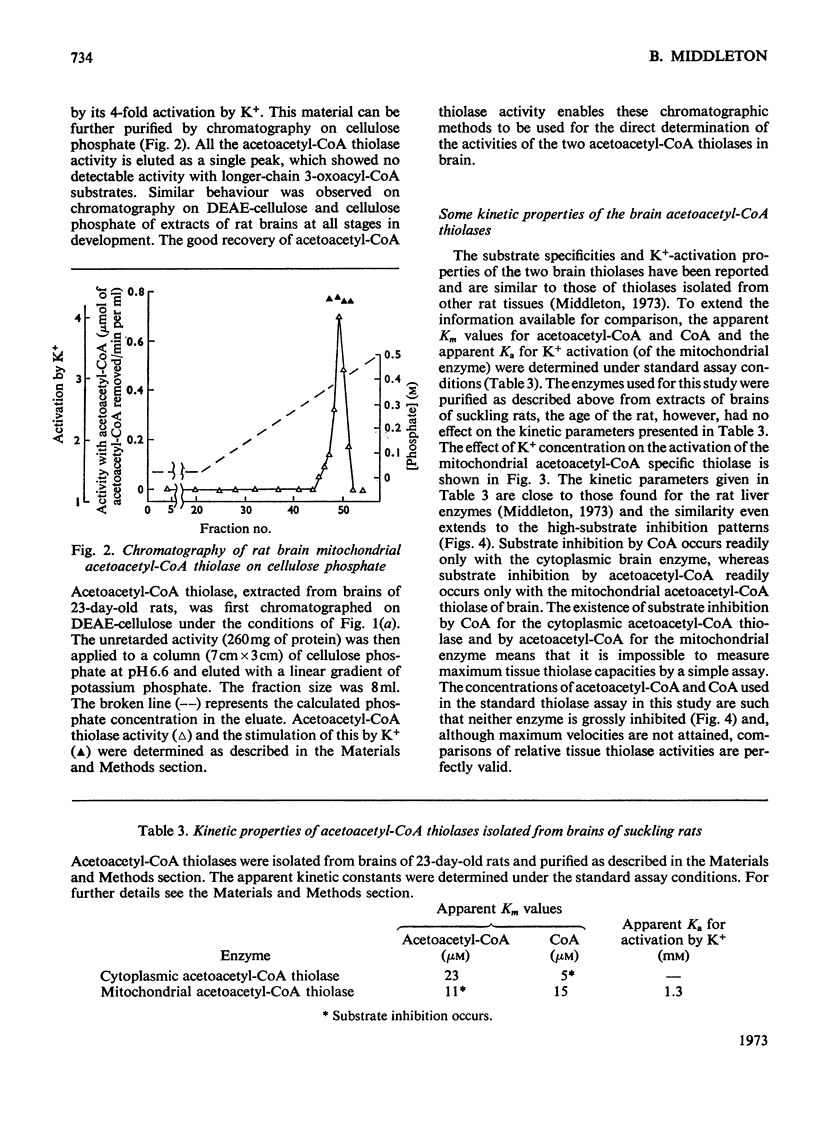
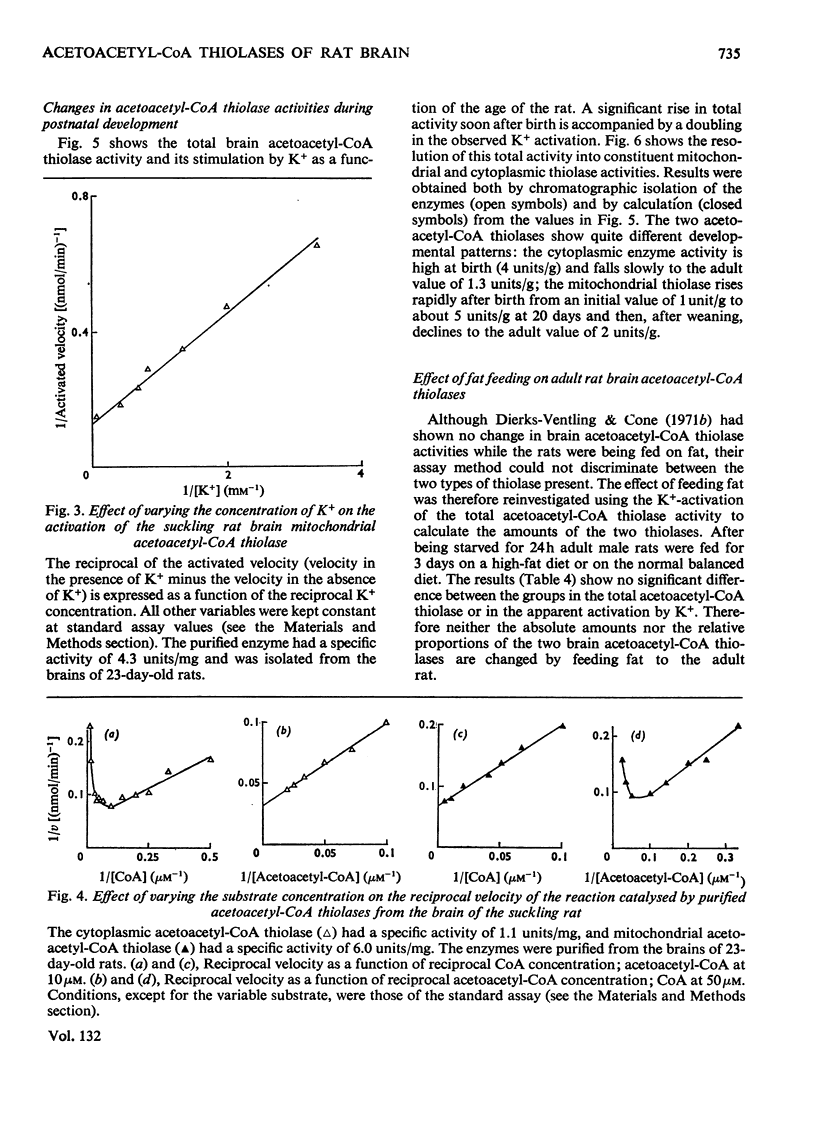
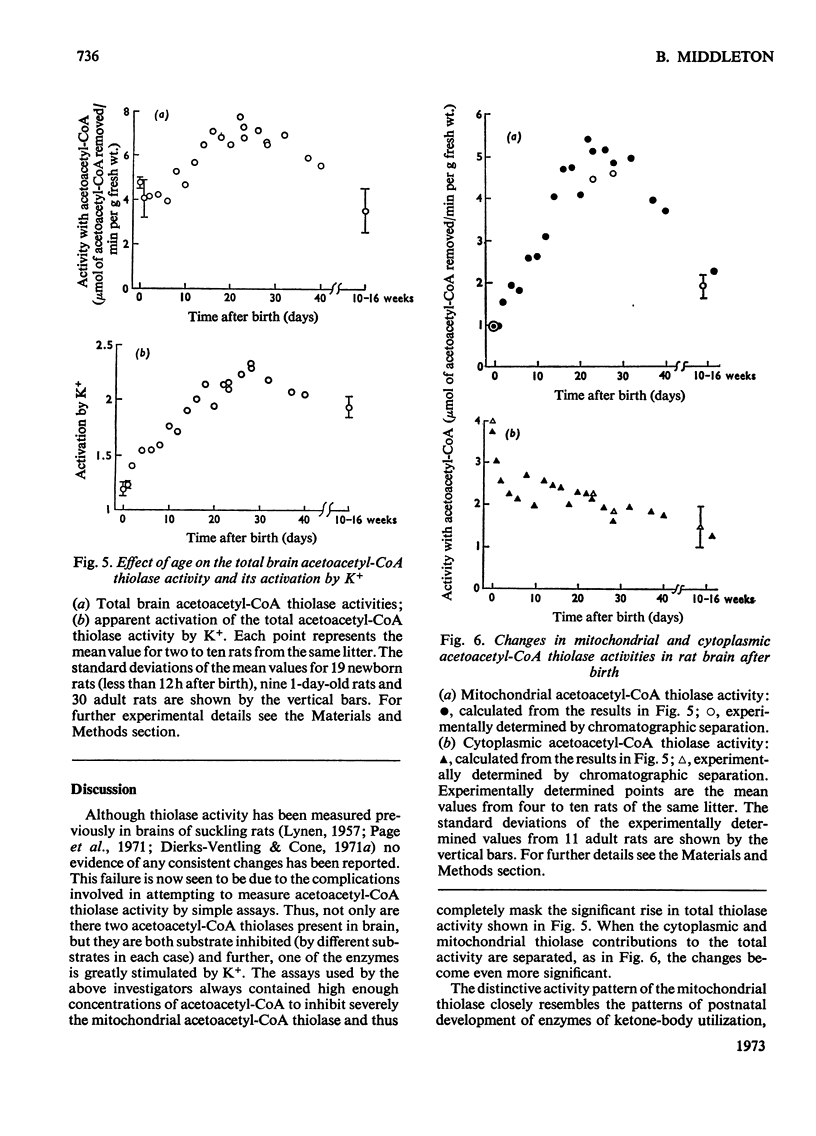

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks-Ventling C., Cone A. L. Acetoacetyl--coenzyme A thiolase in brain, liver, and kidney during maturation of the rat. Science. 1971 Apr 23;172(3981):380–382. doi: 10.1126/science.172.3981.380. [DOI] [PubMed] [Google Scholar]
- Dierks-Ventling C., Cone A. L. Ketone body enzymes in mammalian tissues. Effect of high fat diet. J Biol Chem. 1971 Sep 10;246(17):5533–5534. [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. K., WHITTAKER V. P. LACTATE DEHYDROGENASE AS A CYTOPLASMIC MARKER IN BRAIN. Biochem J. 1963 Sep;88:404–409. doi: 10.1042/bj0880404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Sokoloff L. Changes in D(--)-beta-hydroxybutyric dehydrogenase activity during brain maturation in the rat. J Biol Chem. 1967 Sep 10;242(17):3880–3883. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Middleton B. The oxoacyl-coenzyme A thiolases of animal tissues. Biochem J. 1973 Apr;132(4):717–730. doi: 10.1042/bj1320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tildon J. T., Cone A. L., Cornblath M. Coenzyme A transferase activity in rat brain. Biochem Biophys Res Commun. 1971 Apr 2;43(1):225–231. doi: 10.1016/s0006-291x(71)80111-0. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]


