Abstract
Formation of bone and dentin are classical examples of matrix-mediated mineralization. The mineral phase is essentially the same in these two tissues and primarily consists of a carbonated hydroxyapatite, but the difference lies in the crystal size and shape. There are three components that are necessary for proper mineralization, namely the proper synthesis and secretion of the non-collagenous proteins (NCPs), self-assembly of the collagenous matrix, and delivery of calcium and phosphate ions to the extracellular matrix. Three major NCPs present in the dentin matrix are dentin matrix protein 1 (DMP1), dentin phosphophorin (DPP), and dentin sialoprotein (DSP). In this study, we show the temporal and spatial localization of these NCPs and correlate their expression with the presence of collagenous matrix and calcified deposits in developing mouse incisors and molars. DMP1, an acidic protein, is present predominantly at the mineralization front and in the nucleus of undifferentiated preodontoblast cells. DPP, the major NCP, is present in large amounts at the mineralization front and might function to regulate the size of the growing hydroxyapatite crystals. For the first time, we report the localization of DPP in the nucleus of preodontoblast cells, suggesting a signaling function during the odontoblast differentiation process. DSP is localized predominantly in the dentinal tubules at the site of peritubular dentin, which is highly mineralized in nature. Thus, the precise localization of DMP1, DPP, and DSP in the dentin tissue suggests that a concerted effort between several NCPs is necessary for dentin formation. (J Histochem Cytochem 57:227–237, 2009)
Keywords: dentin matrix protein 1, dentin phosphophorin, dentin sialoprotein, mineralization
In vertebrates, the mineralization process involves a sequential and localized series of events that leads to the controlled growth and formation of carbonated apatite mineral within an extracellular matrix (Boskey 1996). Each mineralizing tissue provides both a structural and chemical framework, which acts as a scaffold for mineral deposition at specific sites (Hao et al. 2004; He and George 2004). In bone and dentin, type I collagen is intimately associated in a well-defined manner with calcium phosphate crystals. A common feature prevalent in mineralized tissues is the presence of acidic macromolecules (Linde 1989; Gorski 1992). Many of these macromolecules bind calcium ions and apatite and some inhibit mineral formation from spontaneously precipitating solutions (Schinke et al. 1996; MacDougall et al. 1998; Gorski et al. 2004). Specific roles in the mineralization process have been proposed for many of these macromolecules. These include nucleation of the mineral, control of postnucleation growth, and transformation of calcium phosphate deposits to hydroxyapatite (Denhardt and Guo 1993; Wazen et al. 2007).
Odontoblasts are terminally differentiated ectomesenchymal cells that synthesize several collagenous and non-collagenous proteins. The major phosphoproteins of the non-collagenous group are now known as the SIBLING (small integrin binding ligand, N-linked glycoprotein) family (Fisher and Fedarko 2003). The SIBLING family consists of dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP), osteopontin (OPN), matrix extracellular phosphoglycoprotein (MEPE), and bone sialoprotein (BSP). Each of these proteins plays an important role in either promoting tissue mineralization or inhibiting the process. Our interest lies in the characterization of the function of three proteins: DMP1, dentin sialoprotein (DSP), and dentin phosphophorin (DPP). These proteins are synthesized by the odontoblasts during the start of the mineralization process and play a regulatory role during the formation of the dentin matrix.
DMP1 is a non-collagenous phosphoprotein identified and isolated from the mineralized matrix of bone and dentin (George et al. 1993; D'Souza et al. 1997; Qin et al. 2007). Using confocal microscopy and live bone cells, we have shown that DMP1 resides in the nucleus of undifferentiated osteoblasts, and during the maturation process, DMP1 is exported out from the nucleus into the extracellular matrix (Narayanan et al. 2003). The release of calcium ions from the endoplasmic reticulum stores and its influx into the nucleus, during the differentiation of osteoblasts, facilitates the export of DMP1 into the extracellular matrix (Narayanan et al. 2003). The full-length native DMP1 isolated from the bone extracellular matrix is processed into two distinct forms: the N-terminal (37 kDa) and C-terminal (57 kDa) fragments. The C-terminal portion of DMP1 self-assembles to form a fibrillar template that facilitates deposition of hydroxyapatite crystals elongated in the c-axis direction (Steiglitz et al. 2004; Tartaix et al. 2004; Gajjeraman et al. 2007). Recent studies have shown that mutation of DMP1 in humans causes autosomal recessive hypophosphatemic rickets (Lorenz-Depiereux et al. 2006). This finding has also been confirmed in the DMP1-null mouse (Feng et al. 2006).
DPP is thought to have a primary role in the nucleation of calcium phosphate mineral (Boskey et al. 1990; He et al. 2005). In solution, it inhibits mineral formation from spontaneously precipitating solutions (Fujisawa et al. 1986). A significant fraction of phosphophorin is cross-linked to dentin collagen in vivo (Huq et al. 2005). Results show that a single DPP molecule can interact with and effectively cross-link two or more collagen molecules and that such cross-linking does lead to the formation of molecularly staggered collagen-PP aggregates even at acid pH (Dahl et al. 1998; Dahl and Veis 2003). When native DPP was immobilized on agarose beads or on collagen fibrils by in vivo or in vitro cross-linking, it induced apatite formation from metastable calcium phosphate solutions that do not spontaneously precipitate (Saito et al. 1997).
DSP is a cleaved product of DSPP (MacDougall et al. 1997). DSP exists as proteolytically processed fragments that result from scission of X-Asp bonds (Yamakoshi et al. 2003; Qin et al. 2004). It is hypothesized that the processing of DSPP is catalyzed by the PHEX enzyme, an endopeptidase that is predominantly expressed in bone and teeth and has a strong preference for cleavage at the NH2 terminus of the aspartyl residue (Qin et al. 2004). DSP has a large number of sialic acid residues, and experiments on the in vitro formation of hydroxyapatite showed that DSP has little or no effect on mineralization (Boskey et al. 2000). Genetic studies of human defects in biomineralization and analysis of transgenic mice have identified DMP1 and DSPP as important mediators of mineralization (Sreenath et al. 2003; Ye et al. 2004).
Several published studies showed the gene expression profiles for DMP1, DPP, and DSP in the tooth (D'Souza et al. 1997; Begue-Kirn et al. 1998a,b; Bleicher et al. 1999; Qin et al. 2002,2003); however, a systematic study correlating protein localization along with mineral deposition has not yet been studied. Therefore, in this study, we used immunohistochemical techniques to identify the expression of these matrix proteins during the mineralization process.
Materials and Methods
Preparation of Tissues and General Histology
Developing heads or mandibles were collected from mouse embryonic (E) day 18 and postnatal (P) days 1, 3, 5, 7, and 20. The specimens were fixed in 4% paraformaldehyde and embedded in paraffin, and serial sections, 5 μm thick, were cut and mounted on charged slides. Only day 20 mandibles were demineralized in buffered 10% EDTA for 7 days at 4C and processed as above.
Masson's Trichrome Staining
The sections were deparaffinized and hydrated in distilled water, mordant in Bouin's solution for 1 hr at 56C, and stained with phosphomolybdic–phosphotungstic acid for 10 min and aniline blue solution for 5 min. The sections were counterstained with hematoxylin solution and examined under a light microscope.
von Kossa Staining
The non-decalcified (days 1, 3, 5, and 7) 5-μm sections were washed with distilled water, treated with 1% AgNO3 for 1 hr, washed again with distilled water, and treated with 2.5% sodium thiosulfate for 5 min. The specimens were counterstained and examined under a light microscope.
Antibody Production
Rat DMP1 and mouse DSP proteins spanning the entire coding region were expressed respectively in bacteria using pGEX-4T3 (GE Healthcare; Piscataway, NJ). The constructs created fusion proteins with glutathione S-transferase tag at the C terminus. Native DPP was extracted from fetal calves' teeth using established protocols with 0.5 M EDTA in the presence of enzyme inhibitors. Crude phosphophorin extract was purified using a diethylaminoethyl ion exchange column. The purified DMP1, DPP, and DSP antigens were independently mixed with complete Freund's adjuvant and injected into rabbits according to standard protocols for generating anti-DMP1, anti-DPP, and anti-DSP antibodies. Serum was collected from each rabbit before immunization and 7 days after each booster injection.
Affinity Purification of Anti-DMP1, Anti-DSP, and Anti-DPP Antibodies
The resulting immune serum was purified using HiTrap N-hydroxysuccinimide-activated high performance affinity columns according to standard protocols (GE Healthcare). Briefly, 1 mg of recombinant (r) DMP1, rDSP, or rDPP protein was coupled to 1-ml affinity columns. Three ml of immune serum was added to the column and allowed to bind for 4 hr at 4C. The column was washed with 0.2 M NaHCO3 and 0.5 M NaCl at pH 8.3. The antibody was eluted with 2 M glycine-HCl, pH 2.0. The eluate was collected in 25 μl of 1 M Tris, pH 8.0. Monospecificity of the antibodies was established using Western blot analysis.
Western Blot Analysis
Total proteins were extracted from 3-day-old mouse first molars and calvaria as follows. Briefly, 1 g of the powdered teeth or calvarial bone was demineralized in 50 ml of 0.5 M EDTA, pH 7.4 for 3–4 days. Protease inhibitors were also used and included 50 mM 6-amino-n-caproic acid, 25 mM benzamidine HCl, 0.5 mM N-ethylmaleimide, and 0.3 mM PMSF. The solution was centrifuged at 12,000 × g for 45 min, and the supernatant was dialyzed with water overnight. The precipitate was dissolved in 1 M calcium chloride (1 liter) for 2 days and centrifuged at 14,000 × g for 60 min. The precipitate was demineralized again with EDTA and dialyzed with water. To reduce the volume, the supernatant was passed through a concentrator (molecular mass cut-off 20,000). The concentration of the total proteins was estimated by Bradford microassay reagent (Bio-Rad; Hercules, CA). Subsequently, 20 μg of the complex was fractionated on a 10% SDS-PAGE gel. The proteins were transferred to nitrocellulose according to the protocol by Towbin et al. (1979). The blots were incubated with 1:200 diluted affinity-purified anti-DMP1 and anti-DPP and 1:2000 diluted anti-DSP antibodies and then with alkaline phosphatase–conjugated anti-rabbit secondary antibody. The blots were washed with distilled water and developed in 20 mM Tris-Cl, pH 7.5, and 200 mM NaCl using an alkaline phosphatase–conjugated substrate kit (Bio-Rad). The total protein was also resolved on SDS-PAGE gels and stained with Coomassie blue to visualize the total proteins from teeth and bone.
IHC Staining
Tissue sections from mouse heads or mandibles at different developmental stages were used for IHC staining. Briefly, the paraffin sections were deparaffinized in xylene, rehydrated, and rinsed three times (5 min each) in PBS (pH 7.4) before quenching of endogenous peroxidase activity (3% H2O2 in PBS at room temperature for 10 min). The sections were rinsed three times (5 min each) in PBS and blocked with 10% normal goat serum for 45 min at room temperature. The sections were again rinsed in PBS solution and incubated with primary antibodies against DMP1, DPP, and DSP at 1:200, 1:2000, and 1:200 dilutions, respectively (overnight at 4C). A negative control was included by using an equal concentration of rabbit preimmune serum as primary antibody. After incubation with the primary antibody, the tissue sections were rinsed three times (15 min each) in PBS and incubated with a biotinylated rabbit anti-goat immunoglobulin (1:500) for 60 min at room temperature. Amplification of the antigen–antibody complex was achieved using avidin–biotin–peroxidase (ABC kit; Vector Laboratories, Burlingame, CA) for 60 min at room temperature. The color reaction was precipitated using DAB (Vector Laboratories, Burlingame, CA) for 1–5 min at room temperature. The tissue sections were counterstained with hematoxylin.
Results
Characterization of Anti-DMP1, Anti-DPP, and Anti-DSP Antibodies
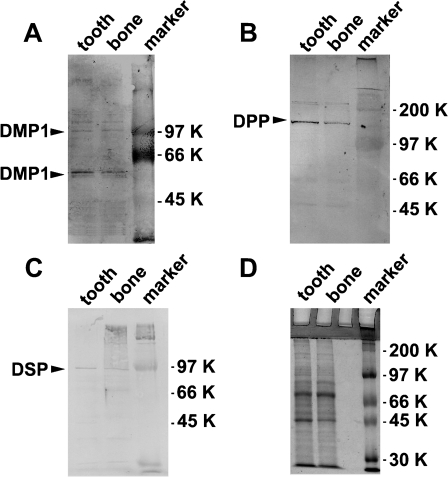
Monospecificity of the affinity-purified anti-DMP1, anti-DPP, and anti-DSP antibody was determined by Western blot analysis on total protein extracted from mouse teeth and calvaria bone. Results in Figure 1A show that anti-DMP1 antibody identified a major band at ∼60 kDa in teeth and bone. However, bands around 105 kDa could correspond to the full-length DMP1 as reported by Huang et al (2008). As expected, anti-DPP antibody identified a single band at ∼100–120 kDa (Figure 1B) with more DPP protein being present in the tooth than in the bone. The fainter band above ∼200 kDa could correspond to the intact DSPP protein. The anti-DSP antibody identified a single band at ∼95 kDa (Figure 1C). Thus, the polyclonal antibodies were specific and could identify native proteins from the tissue extract.
Figure 1.
Expression of dentin matrix protein 1 (DMP1), dentin phosphophorin (DPP), and dentin sialoprotein (DSP) in bone and teeth as determined by Western blotting. Total protein extracts were prepared as described in Materials and Methods from mouse molars and calvaria bone in postnatal 3-day-old mice. (A) Anti-DMP1 polyclonal antibody. (B) Anti-DPP polyclonal antibody. (C) Anti-DSP polyclonal antibody. (D) Coomassie blue staining of total proteins isolated from 3-day-old mouse molar and calvaria bone.
Developmental Expression of DMPs at E18
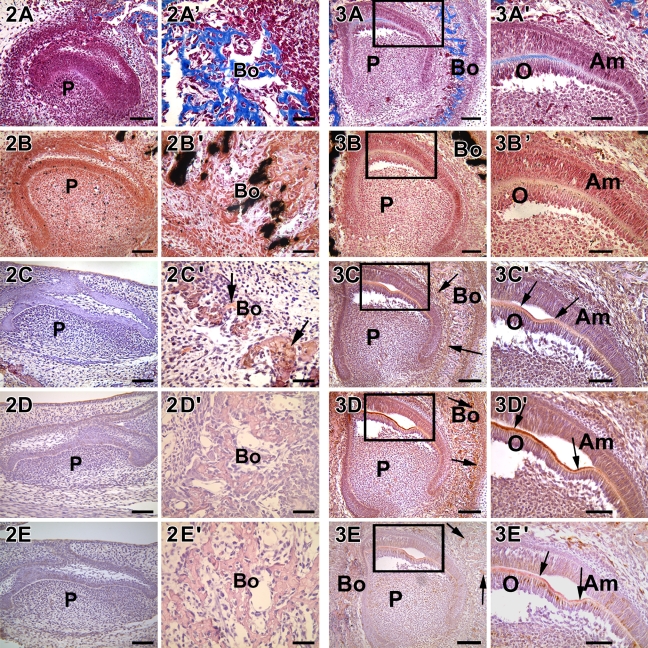
Histological evaluation of the E18 developing tooth germ showed that development was at the late bell stage (Figure 2A). Both the preameloblasts and the preodontoblasts were undergoing polarization and had not yet secreted an organic dentin matrix. This was indicated by the lack of mineral deposits in the dentin matrix (Figure 2B). However, the osteoblasts in the surrounding alveolar bone were actively synthesizing a mineralized collagenous matrix (Figures 2A′ and 2B′). Of the matrix proteins, DMP1 was identified by immunostaining in the alveolar bone matrix only (Figure 2C′, arrow). Both DPP and DSP were not expressed in either the dentin or the bone matrix (Figures 2D, 2D′, 2E, and 2E′). Thus, at E18, the preodontoblasts were undergoing polarization and did not synthesize and secrete the components of the organic dentin matrix.
Figure 2.
Localization of collagen, mineral deposits, DMP1, DSP, and DPP in 18-day-old mouse embryos. Developing heads were collected from E18.5 mouse embryo, fixed, embedded, and cut into 5-μm-thick sections. DMP1 was expressed only in the osteoblasts of alveolar bone (C′, arrows). DPP and DSP were not expressed at this stage of development. Presence of collagenous matrix and calcium deposits were determined by Masson's trichrome stain (A,A′) and von Kossa stain (B,B′), respectively. Both were seen in trace amounts only in the mineralized matrix of bone. (C,C′) Anti-DMP1 antibody. (D,D′) Anti-DPP antibody. (E,E′) Anti-DSP antibody. Bo, bone; P, dental pulp. Bars: A–E = 20 μm; A′–E′ = 10 μm.
Localization of DMPs at P1
Histological analysis at P1 showed the polarized nature of the odontoblasts at the cusp region. Mason's trichrome staining of the sections showed the deposition of the collagenous matrix only in regions where the odontoblasts were secretory in nature (Figures 3A and 3A′). Along with collagen synthesis, the secretory odontoblasts were actively synthesizing DMP1, DPP, and DSP macromolecules (Figures 3C′, 3D′, and 3E′, arrow). However, staining with von Kossa showed the absence of calcified deposits in the dentin matrix (Figures 3B and 3B′), whereas its presence was clearly seen in the surrounding alveolar bone. This suggested that matrix proteins including collagen, DMP1, DPP, and DSP were secreted to the extracellular matrix before the calcification process.
Figure 3.
Localization of collagen, mineral deposits, DMP1, DPP, and DSP in a postnatal 1-day-old mouse (P1). (A,A′) The incisor is at the late bell stage and trichrome Masson staining showed the deposition of the collagen matrix in the dentin and in the surrounding bone. (B,B′) Sparse deposits of calcified deposits were observed in dentin, whereas abundant calcified matrix was observed in the surrounding bone. (C,C′) DMP1, (D,D′) DPP, and (E,E′) DSP were localized to secretory odontoblasts near the cuspal region, which were actively synthesizing a mineralized matrix. Arrows indicate mineralized dentin and bone matrix. A′–E′ represent the boxed areas in A–E. Am, ameloblast; O, odontoblast; P, pulp. Bars: A–E = 20 μm; A′–E′ = 10 μm.
Localization of DMPs at P3
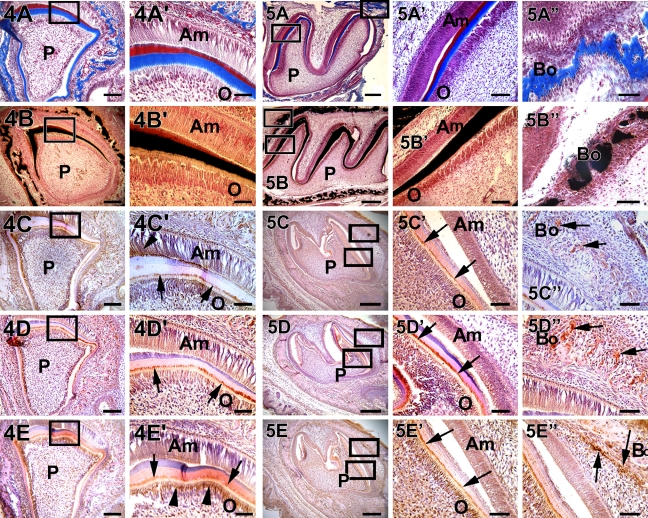
At day 3, intense staining was observed for collagen in the dentin matrix and the presence of a well-calcified dentin matrix as shown by von Kossa staining in both the incisor (Figures 4A and 4B) and the molars (Figures 5A and 5B). Expression of DMP1 was particularly interesting because it was observed in the nucleus of some of the undifferentiated pulp cells (Figure 4C′). Most of the expression was seen at the mineralization front of the dentin matrix (Figure 4C′, arrow). Expression of DMP1 was also observed in the Tomes' process region of actively matrix synthesizing ameloblasts (Figure 4C′, arrowhead). DPP, the major non-collagenous protein of the dentin matrix, was localized predominantly in the odontoblasts and in great amounts at the mineralization front (Figures 4D′ and 5D′, arrow). DSP, on the other hand, was found mostly at the junction between the odontoblast cell body and the process (Figure 4E′, arrow and arrowhead). In the molars, the same pattern of expression was observed (Figure 5E′). In the surrounding alveolar bone, expression of DMP1, DSP, and DPP was clearly evident in the osteoblasts (Figures 5C″, 5D″, and 5E″, arrow). Thus, DMP1 was constitutively expressed, whereas DPP and DSP were expressed at later stages during development in the alveolar bone.
Figure 4.
Localization of collagen, mineral deposits, DMP1, DPP, and DSP in a postnatal 3-day-old mouse incisor. (A,A′ and B,B′) Progressive increase in collagen deposition and calcified matrix formation, respectively. DMP1 was localized at the mineralization front, which was between predentin and dentin, and in the odontoblasts (C,C′, arrow). (C) The surrounding alveolar bone also showed DMP1 localization. However, DMP1 was transiently expressed in the ameloblasts (C′, arrowhead). DPP and DSP were both localized at the mineralization front (D′,E′, arrow). DSP was highly expressed in the cytoplasm of the odontoblasts (E′, arrowhead). Both DPP and DSP were expressed in the ameloblasts. Bars: A–E = 20 μm; A′–E′ = 10 μm.
Figure 5.
Localization of collagen, mineral deposits, DMP1, DPP, and DSP in postnatal 3-day-old mouse molars. Deposition of collagen and mineralized matrix are seen in dentin (A,A′ and B,B′) and in the surrounding bone (A″,B″). Localization of DMP1, DPP, and DSP were predominantly expressed at the mineralization front as shown in C–E, respectively. Arrows in C′–E′ indicate the mineralization front. Localization of these proteins is clearly seen in the alveolar bone as indicated by arrows in C″–E″. Bars: A–E = 20 μm; A′–E′= 10 μm; A″–E″ = 2 μm.
Localization of DMPs at P5
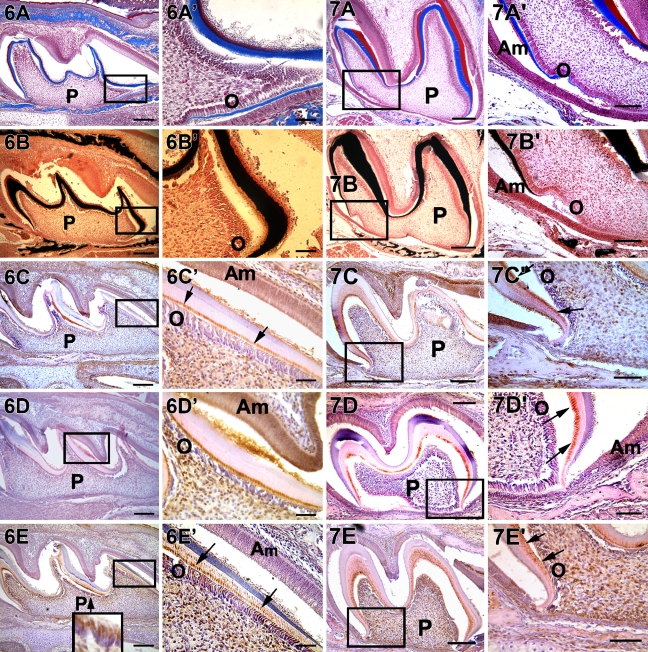
Extensive mineralization of the dentin matrix was observed on P5. DMP1 was localized specifically at the mineralization front (Figure 6C′, arrow). High levels of DMP1, DPP, and DSP expression were observed in both the odontoblasts and the mineralized matrix (Figures 6C′, 6D′, and 6E′). DSP was localized at the mineralization front (Figure 6E′, arrow) and was clearly observed in the dentinal tubules. DSP was found consistently in the odontoblast cell body (Figure 6E, inset).
Figure 6.
Localization of collagen, mineral deposits, DMP1, DPP, and DSP in a postnatal 5 day mouse first molar. Deposition of collagen and mineralized matrix are observed (A,A′,B,B′). Localization of DMP1, DPP, and DSP were predominantly observed at the mineralization front (C′–E′, arrow). All three matrix proteins were also localized in the nucleus of pulp cells. The insert in E depicts the localization of DSP throughout the cytoplasm in the odontoblasts. A′–E′ are higher magnification from the corresponding boxed portion from A–E. P, pulp; O, odontoblasts; Am, ameloblasts. Bars: A–E = 20 μm; A′–E′ = 10 μm.
Localization of DMPs at P7
At P7 the thick collagenous matrix as indicated by Mason's trichrome (Figures 7A and 7A′) was calcified as determined by von Kossa staining (Figures 7B and 7B′). Expression patterns of DMP1, DPP, and DSP remained similar to P5 (Figures 7C, 7C′, 7D, 7D′, 7E, and 7E′). Results from the expression pattern suggested that these non-collagenous proteins were essential throughout the dentin assembly process.
Figure 7.
Localization of collagen, mineral deposits, DMP1, DSP, and DPP in a postnatal 7 day mouse first molar. Deposition of collagen and mineralized matrix seen in A, B, A′, and B′. A′–E′ are higher magnification from the corresponding boxed portion from A–E. Arrows indicate localization of proteins at the mineralization front. Localization of DMP1, DPP, and DSP observed at the mineralization front and at the cervical loop region. DSP is localized in the bone at this stage of development. Bars: A–E = 20 μm; A′–E′ = 10 μm.
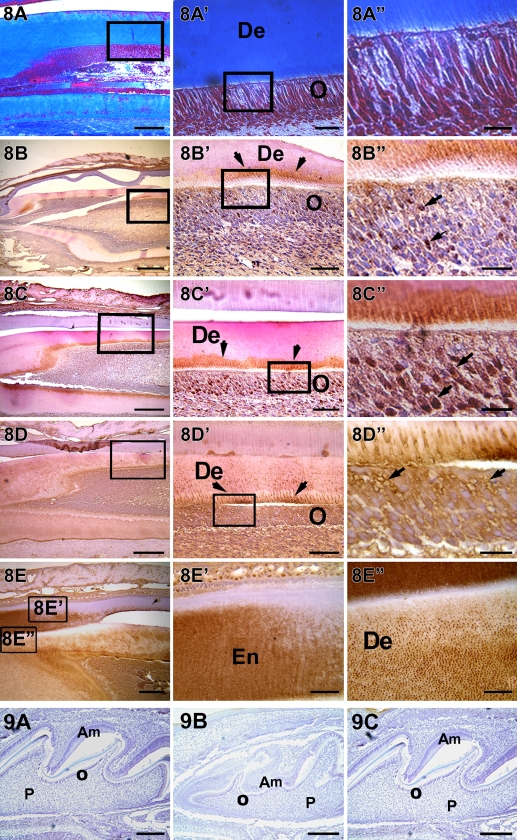
Localization of DMPs at P20
In the maturing teeth at P20, collagenous matrix was abundantly present (Figures 8A, 8A′, and 8A″). DMP1 expression was clearly observed at the mineralization front (Figure 8C′, arrow) and in the nucleus of differentiated odontoblasts (Figure 8C″, arrow). Strong expression of DPP was observed in the odontoblastic layer and in the nucleus (Figure 8D″, arrow). Interestingly, DSP expression was observed throughout the dentinal tubules (Figures 8E′ and 8F″) and in the newly formed enamel matrix (Figures 8F and 8F′). These results indicated that the odontoblasts were actively engaged in synthesizing and secreting DMPs. In all experiments, sections were processed with preimmune serum to determine the specificity of the DMP1, DPP, and DSP antibodies. A representative image from day 3 is shown in Figure 9.
Figure 8.
Localization of collagen, DMP1, DSP, and DPP in a postnatal 20-day-old mouse incisor. Abundant collagenous matrix are localized in A,A′, and A″. DMP1 is localized at the mineralization front and also observed in the nucleus of differentiating odontoblasts (B,B′,B″). DPP is localized at the mineralization front and in the nucleus of the odontoblasts (C,C′,C″). DSP is observed in the dentinal tubules and around the plasma membrane of the odontoblasts (D,D′,D″). DSP is also localized in the early formed enamel matrix (E′) and in the dentin matrix (E″). Am, ameloblast; De, dentin; En, enamel; O, odontoblast; P, pulp. Bars: A–E = 20 μm; A′–E′ = 10 μm; A″–E″ = 2 μm.
Figure 9.
IHC analysis of a 3-day-old mouse first molar with preimmune serum of DMPs. (A) DMP1. (B) DPP. (C) DSP. Am, ameloblast; O, odontoblast; P, pulp. Bar = 20 μm.
Discussion
During development of the craniofacial apparatus, neural crest cells from midbrain and rostral hindbrain migrate depending on the environmental cues and occupy the first visceral arch. These cells give rise to the dental papilla cells, cells of the periodontium, and the alveolar bone cells. The odontoblasts, which are derived from the dental papillae, are highly specialized cells. The terminal differentiation of odontoblasts is characterized by a sequence of cytological and functional changes (Ruch et al. 1995). Onset of the expression of DMPs is the hallmark of secretory odontoblasts. Components of the predentin matrix are synthesized first and then the specialized macromolecules necessary for dentin mineralization are synthesized (Lisi et al. 2003).
Some of the macromolecules secreted by the odontoblasts are proteoglycans such as decorin, biglycan, and fibromodulin; Gla proteins such as osteocalcin; members of the SIBLING family such as DMP1, DSPP, OPN, MEPE, and BSP; and other non-collagenous proteins such as amelogenin and other serum-derived proteins (Begue-Kirn et al. 1998b; Ruch 1998). Among these dentin constituents, we focused on the temporal and spatial localization of three major secretory products of the odontoblasts, DMP1, DSP, and DPP, during dentinogenesis.
Our observations indicate that, at E20, expression of DMP1 is first observed in the mineralized matrix of the alveolar bone. The surrounding osteoblasts of the alveolar bone synthesize a calcified matrix even before dentin mineralization. Histological evaluation of the developing tooth germ showed that the preodontoblasts are in the process of terminal differentiation; hence, the collagenous matrix is absent in the predentin. Furthermore, the presence of a basement membrane suggests that the reciprocal epithelial–mesenchymal interactions necessary for progressive terminal differentiation of the odontoblasts are in progress. Hence, the onset of the expression of the DMPs, associated with the terminally differentiation state, is clearly absent.
At P1, the odontoblasts are highly polarized and are actively involved in the synthesis of the collagenous and the non-collagenous matrix. Lack of mineral deposition suggests that the assembly of the organic template is first required before matrix mineralization. To promote epitactic nucleation, a non-collagenous protein facilitating nucleation must be immobilized on the organic matrix before mineral formation.
DPP is the principle non-collagenous protein in the dentin matrix and is secreted by fully polarized odontoblasts. The results from this study confirmed previous studies that DPP is secreted directly to the mineralization front through the odontoblastic processes (Weinstock and Leblond 1973). In the presence of calcium, DPP can form an insoluble complex on the collagen template at the predentin–dentin border. Interestingly, in this study, we observed DPP localized in the nuclei of a defined population of odontoblasts. This is the first report that showed nuclear localization in vertebrates. Similar nuclear localization of DPP was seen in the cells of the unmineralized syncytia of the sea urchin (Veis et al. 2002). On day 3, the dentin matrix is highly mineralized with high expression levels of DPP at the mineralization front. Another interesting feature is that the osteoblasts of the surrounding alveolar bone of P20 mice expressed DPP, suggesting that this protein might also be involved in regulating later stages of osteogenesis.
Even though DSP and DPP are derived from the same precursor (DSPP), it is apparent from this study that the localization of DSP is quite unique. The DSP antibody stained extensively on the plasma membrane at the junction between the odontoblast body and the process, implicating a distinct function that is different from DPP. Thus, even though DSP and DPP are derived from DSPP, their spatial localization suggest independent functions during dentinogenesis. Furthermore, strong DSP expression was observed in the enamel matrix, particularly in the interprismatic region. White et al. (2007) have shown that overexpression of DSP in mouse ameloblasts significantly increased enamel hardness. Thus, expression of DSP during early enamel formation suggests that DSP might contribute to the mechanical properties of the dentin–enamel junction (DEJ), confirming published data (White et al. 2007). However, Baba et al. (2004), in decalcified rat molars, showed the presence of DSP only in the dentinal tubules of all stages of rat molar development. Relative expression levels of DMP1, DPP, and DSP during development in alveolar bone and tooth are summarized in Table 1.
Table 1.
Summary of expression levels of DMP1, DPP, and DSP during development in alveolar bone and tooth
| DMP1
|
DPP
|
DSP
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Am | Od | Os | Am | Od | Os | Am | Od | Os | |
| E18 | − | − | + | − | − | − | − | − | − |
| P1 | − | + | + | + | ++ | + | + | ++ | + |
| P3 | + | ++ | ++ | + | +++ | ++ | + | +++ | ++ |
| P5 | + | ++ | + | + | +++ | + | + | +++ | + |
| P7 | + | ++ | + | + | +++ | + | + | +++ | + |
| P20 | + | ++ | + | + | ++ | + | ++ | +++ | + |
+, Low expression level; ++, medium expression level; +++, high expression level; DMP1, dentin matrix protein 1; DPP, dentin phosphophorin; DSP, dentin sialoprotein; Am, ameloblast; Od, odontoblast; Os, osteoblast; E, embryonic; P, postnatal.
It is evident that the DMPs synthesized by differentiated odontoblasts are localized in well-defined regions of the dentin matrix. This suggests that the biomineralization of dentin is a highly regulated process requiring a precise molecular role for the organic matrix. Specific macromolecules are necessary to function as crystal nucleators, direct crystal orientation, stabilize the mineral phase, and regulate crystal size. Presence of DMP1, DSP, and DPP throughout development suggests that these proteins are needed for the homeostasis of the dentin matrix. Another emerging paradigm is that several of these extracellular matrix proteins, besides their structural role, might play an important role in cell signaling and communication. Further studies are warranted to establish and identify the precise signaling mechanisms and the peptide fragments responsible for such a function.
Acknowledgments
This work was supported by National Institutes of Health Grants DE 11657 and DE 13836.
References
- Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT (2004) Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biology 23:371–379 [DOI] [PubMed] [Google Scholar]
- Begue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT (1998a) Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci 106:963–970 [DOI] [PubMed] [Google Scholar]
- Begue-Kirn C, Ruch JV, Ridall AL, Butler WT (1998b) Comparative analysis of mouse DSP and DPP expression in odontoblasts, preameloblasts, and experimentally induced odontoblast-like cells. Eur J Oral Sci 106(suppl 1):254–259 [DOI] [PubMed] [Google Scholar]
- Bleicher F, Couble ML, Farges JC, Couble P, Magloire H (1999) Sequential expression of matrix protein genes in developing rat teeth. Matrix Biol 18:133–143 [DOI] [PubMed] [Google Scholar]
- Boskey A, Spevak L, Tan M, Doty SB, Butler WT (2000) Dentin sialoprotein (DSP) has limited effects on in vitro apatite formation and growth. Calcif Tissue Int 67:472–478 [DOI] [PubMed] [Google Scholar]
- Boskey AL (1996) Matrix proteins and mineralization: an overview. Connect Tissue Res 35:357–363 [DOI] [PubMed] [Google Scholar]
- Boskey AL, Maresca M, Doty S, Sabsay B, Veis A (1990) Concentration-dependent effects of dentin phosphophorin in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner 11:55–65 [DOI] [PubMed] [Google Scholar]
- Dahl T, Sabsay B, Veis A (1998) Type I collagen-phosphophorin interactions: specificity of the monomer-monomer binding. J Struct Biol 123:162–168 [DOI] [PubMed] [Google Scholar]
- Dahl T, Veis A (2003) Electrostatic interactions lead to the formation of asymmetric collagen-phosphophorin aggregates. Connect Tissue Res 44(suppl 1):206–213 [PubMed] [Google Scholar]
- Denhardt DT, Guo X (1993) Osteopontin: a protein with diverse functions. FASEB J 7:1475–1482 [PubMed] [Google Scholar]
- D'Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res 12:2040–2049 [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, et al. (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS (2003) Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res 44(suppl 1):33–40 [PubMed] [Google Scholar]
- Fujisawa R, Kuboki Y, Sasaki S (1986) Changes in interaction of bovine dentin phosphophorin with calcium and hydroxyapatite by chemical modifications. Calcif Tissue Int 39:248–251 [DOI] [PubMed] [Google Scholar]
- Gajjeraman S, Narayanan K, Hao J, Qin C, George A (2007) Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem 282:1193–1204 [DOI] [PubMed] [Google Scholar]
- George A, Sabsay B, Simonian PA, Veis A (1993) Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem 268:12624–12630 [PubMed] [Google Scholar]
- Gorski JP (1992) Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralization. Calcif Tissue Int 50:391–396 [DOI] [PubMed] [Google Scholar]
- Gorski JP, Wang A, Lovitch D, Law D, Powell K, Midura RJ (2004) Extracellular bone acidic glycoprotein-75 defines condensed mesenchyme regions to be mineralized and localizes with bone sialoprotein during intramembranous bone formation. J Biol Chem 279:25455–25463 [DOI] [PubMed] [Google Scholar]
- Hao J, Zou B, Narayanan K, George A (2004) Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone 34:921–932 [DOI] [PubMed] [Google Scholar]
- He G, George A (2004) Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem 279:11649–11656 [DOI] [PubMed] [Google Scholar]
- He G, Ramachandran A, Dahl T, George S, Schultz D, Cookson D, Veis A, et al. (2005) Phosphorylation of phosphophorin is crucial for its function as a mediator of biomineralization. J Biol Chem 280:33109–33114 [DOI] [PubMed] [Google Scholar]
- Huang B, Maciejewska I, Sun Y, Peng T, Qin D, Lu Y, Bonewald L, et al. (2008) Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int 82:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq NL, Loganathan A, Cross KJ, Chen YY, Johnson NI, Willetts M, Veith PD, et al. (2005) Association of bovine dentine phosphophorin with collagen fragments. Arch Oral Biol 50:807–819 [DOI] [PubMed] [Google Scholar]
- Linde A (1989) Dentin matrix proteins: composition and possible functions in calcification. Anat Rec 224:154–166 [DOI] [PubMed] [Google Scholar]
- Lisi S, Peterkova R, Peterka M, Vonesch JL, Ruch JV, Lesot H (2003) Tooth morphogenesis and pattern of odontoblast differentiation. Connect Tissue Res 44(suppl 1):167–170 [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, et al. (2006) DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38:1248–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Gu TT, Luan X, Simmons D, Chen J (1998) Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res 13:422–431 [DOI] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem 272:835–842 [DOI] [PubMed] [Google Scholar]
- Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A (2003) Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem 278:17500–17508 [DOI] [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT (2004) Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126–136 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Butler WT (2003) Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res 44(suppl 1):179–183 [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, et al. (2002) The expression of dentin sialophosphoprotein gene in bone. J Dent Res 81:392–394 [DOI] [PubMed] [Google Scholar]
- Qin C, D'Souza R, Feng JQ (2007) Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res 86:1134–1141 [DOI] [PubMed] [Google Scholar]
- Ruch JV (1998) Odontoblast commitment and differentiation. Biochem Cell Biol 76:923–938 [PubMed] [Google Scholar]
- Ruch JV, Lesot H, Begue-Kirn C (1995) Odontoblast differentiation. Int J Dev Biol 39:51–68 [PubMed] [Google Scholar]
- Saito T, Arsenault AL, Yamauchi M, Kuboki Y, Crenshaw MA (1997) Mineral induction by immobilized phosphoproteins. Bone 21:305–311 [DOI] [PubMed] [Google Scholar]
- Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W (1996) The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem 271:20789–20796 [DOI] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, et al. (2003) Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem 278:24874–24880 [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS (2004) Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem 279:980–986 [DOI] [PubMed] [Google Scholar]
- Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, et al. (2004) In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem 279:18115–18120 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis A, Barss J, Dahl T, Rahima M, Stock S (2002) Mineral-related proteins of sea urchin teeth: Lytechinus variegatus. Microsc Res Tech 59:342–351 [DOI] [PubMed] [Google Scholar]
- Wazen RM, Tye CE, Goldberg HA, Hunter GK, Smith CE, Nanci A (2007) In vivo functional analysis of polyglutamic acid domains in recombinant bone sialoprotein. J Histochem Cytochem 55:35–42 [DOI] [PubMed] [Google Scholar]
- Weinstock M, Leblond CP (1973) Radioautographic visualization of the deposition of a phosphoprotein at the mineralization front in the dentin of the rat incisor. J Cell Biol 56:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SN, Paine ML, Ngan AY, Miklus VG, Luo W, Wang H, Snead ML (2007) Ectopic expression of dentin sialoprotein during amelogenesis hardens bulk enamel. J Biol Chem 282:5340–5345 [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Liu S, Zhang C, Oida S, Fukae M, Simmer JP (2003) Characterization of porcine dentin sialoprotein (DSP) and dentin sialophosphoprotein (DSPP) cDNA clones. Eur J Oral Sci 111:60–67 [DOI] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, et al. (2004) Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem 279:19141–19148 [DOI] [PubMed] [Google Scholar]