Abstract
The present study shows that increased Abeta production in hippocampal neurons, due to a failure of NGF signal, induces an unexpected phosphorylation of tyrosine kinase receptor A (TrkA), followed by activation of the phospholipase C γ (PLCγ) pathway and neuronal death. Such phosphorylation seems causally connected with 2 kinases known be involved in amyloidogenesis, Src and CDK5, and associated with α and γ secretase–mediated p75 processing. Pharmacologic inhibition of TrkA phosphorylation and partial silencing of TrkA and/or p75 receptors prevent PLCγ activation and protect neurons from death. Concomitantly with these events, TrkA, p75, Abeta peptides, and PS1 protein coimmunoprecipitate, suggesting their direct interplay in the subsequent onset of apoptotic death. Together, these findings depict a cellular mechanism whereby the same cellular transducing system may invert its intracellular message from trophic and antiapoptotic to a death signaling, which could also have relevance in the onset of Alzheimer's disease.
Keywords: Alzheimer's disease, neurodegeneration, neurotrophin, NGF, NGF receptors
NGF mediates survival, differentiation, growth, and apoptosis of neurons by binding to 2 types of cell-surface receptors. Whereas tyrosine kinase receptor A (TrkA) has been shown to transduce specific prosurvival signals via an intricate network of intracellular pathways (1), the pan-p75 receptor (p75) modulates NGF affinity for TrkA and has been shown to be involved in transducing or directly carrying out death signals (2).
Recently several articles have summarized evidence for a causal link between deficit in NGF signaling, transport, and processing and the onset of Alzheimer's disease (AD) (3, 4).
We have recently demonstrated that the interruption of NGF signaling in neurons activates the amyloidogenic pathway and induces death through a still-unclear mechanism (5, 6).
Here we show that 24 h after NGF deprivation, TrkA undergoes an unexpected NGF-independent phosphorylation. This posttranslational modification is induced either by the overproduced pool of Abeta or by exogenously added Abeta and is strictly connected to neuronal death. Such TrkA phosphorylation is largely prevented by inhibitors of CDK5 and Src intracellular pathways, both involved in amyloid processing (7–9), and by TrkA and p75 silencing.
Concomitantly with TrkA phosphorylation, the amounts of p75 C-terminal fragments, which are the products of α and γ secretase cleavage on p75 (10, 11), increase and associate with TrkA to form a molecular complex also including a full length of p75, Abeta peptides, and PS1 protein.
Results
An Anomalous Phosphorylation of TrkA Receptor Occurs in Hippocampal Neurons After NGF Deprivation.
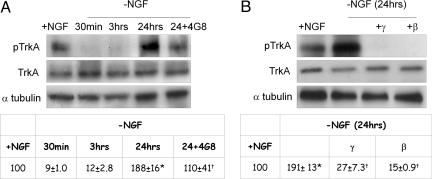
It is largely known that TrkA autophosphorylates after NGF binding and undergoes dephosphorylation, indicative of a resting state, after NGF detachment (1). Surprisingly, we found that at a much longer time after NGF removal (24 h), a sort of paradoxical, marked TrkA(pY490) phosphorylation (which is much higher than that measurable in the presence of NGF) becomes detectable, although the full-length form is substantially unchanged (Fig. 1 A and B). Such anomalous phosphorylation is strictly related to amyloid peptide(s) production, as shown by the finding that the anti-Abeta antibody 4G8, and, even more markedly, γ and β secretase inhibitors (Fig. 1 A and B), largely or totally prevent TrkA phosphorylation, concomitantly with an inhibition of neuronal death, previously reported in the same experimental model (5). Because the antibody used to detect TrkA(pY490) may also cross-react with TrkB and TrkC, to enrich the lysate to be analyzed, we have immunoprecipitated TrkA protein (IP TrkA) with a TrkA-selective antibody and subsequently blotted the immunocomplex with anti-TrkA(pY490). Supporting information (SI) Fig. S1 (Top) shows that TrkA is phosphorylated after 24 h of NGF removal and that the event is blocked by anti-Abeta antibody and γ and β secretase inhibitors. The lower panel shows the amount of TrkA brought down with the IP procedure.
Fig. 1.
Time course of phospho-TrkA(pY490) (pTrkA) and full-length TrkA (TrkA) protein levels in hippocampal neurons exposed to NGF (+NGF) or deprived of NGF in a time ranging from 30 min to 24 h (-NGF). The amount of pTrkA due to 24 h of NGF deprivation is compared with that detectable after exposure to anti-Abeta antibody (4G8) (A) or to secretase inhibitors (γ and β) (B). Densitometric analyses of the corresponding experiments are reported in the Tables a and b. Values from phosphorylated TrkA were normalized with respect to full-length TrkA levels and were expressed as percentage of +NGF samples. *, P < 0.05 vs. +NGF; †, P < 0.05 vs. -NGF values (24 h). The identification of the 140-kDa Trk (pY490) species was obtained by immunoprecipitation of TrkA with a specific anti-TrkA antibody (see Methods) followed by immunoblotting with TrkA(pY490) antibodies before NGF exposure.
Inhibition or Silencing of TrkA and p75 Paradoxically Favors Neuronal Survival Under NGF Deprivation.
To confirm TrkA involvement in the neuronal death mechanism, hippocampal neurons were exposed to 2 different TrkA phosphorylation inhibitors, K-252a and CEP-2563 (CEP), and then deprived of NGF for 24 h, when intact and condensed nuclei were assessed. K-252a is a powerful cell-permeable protein kinase inhibitor largely used to inhibit TrkA phosphorylation (12), whereas CEP is a highly selective inhibitor of TrkA receptor, acting at its ATP binding pocket (13). As shown in Fig. S2 a and b, K-252a and CEP largely inhibit TrkA phosphorylation under NGF removal and almost totally protect neurons from death (Table 1). Note also that Abeta 1-42 peptides also induce TrkA phosphorylation (Fig. S2c), and Abeta-mediated neuronal death is counteracted by TrkA phosphorylation inhibitors (Table in Fig. S2c).
Table 1.
Extent of intact and condensed nuclei in NGF-deprived neurons (24 h) after exposure to TrkA inhibitors K-252a and CEP-2563
| Variable | Intact nuclei | Condensed nuclei |
|---|---|---|
| +NGF | 100 | 2.0 ± 0.7 |
| -NGF | 50 ± 3* | 4.5 ± 0.9* |
| K-252a | 80 ± 2 | 3 ± 0.4 |
| CEP | 78 ± 5 | 3 ± 0.8 |
| K-252a/-NGF | 83 ± 7† | 2.5 ± 0.5† |
| CEP/-NGF | 81 ± 4† | 2.0 ± 0.8† |
Two hours after incubation with K-252a (100 nM) and CEP-2563 (CEP) (200 nM), cells were washed 3 times and re-exposed for 24 h to a fresh medium with (K-252a; CEP) or without NGF (K-252a/-NGF; CEP/-NGF). The corresponding Western blot analysis showing the effect of TrkA inhibitors on TrkA(pY490) levels is reported in Fig. S2 a and b. Parallel experiments were performed with 10 μM Abeta 1-42 peptides in the presence or absence of K-252a and CEP-2563 (K-252a/Ab; CEP/Ab) (Fig. S2c and Table). Condensed nuclei are expressed as the mean ± SE of 10 fields of duplicate determinations of 5 independent experiments. Intact nuclei are reported as percentage of controls (+NGF).
*, P < 0.05 vs. cell survival of +NGF samples.
†, P < 0.05 vs. cell survival of -NGF or Abeta samples.
Moreover, partial silencing of TrkA (Fig. S2d) induces 30% of neuronal death in the presence of NGF (Table 2), confirming the prosurvival role normally exerted by TrkA receptor also in hippocampal neurons. Surprisingly, however, after NGF removal, when 47% of scramble neurons die, TrkA-silenced neurons seem to be protected (81%), further depicting a molecular mechanism in which TrkA is essential for neuronal survival in the presence of NGF but exerts a noxious action and induces neuronal death after its withdrawal. It is worth noting that in TrkA partially silenced neurons, TrkA remains phosphorylated after NGF removal. This event is probably due to the fact that only 20%–30% of TrkA is silenced. However, it may also suggest that other Trk receptors, such as TrkB or TrkC, may be involved in this anomalous Abeta-mediated phosphorylation.
Table 2.
Cell survival (evaluated as number of intact nuclei) of TrkA, p75, and TrkA + p75 siRNA neurons (TrkAi; p75i; TrkAi + p75i) and of the corresponding scramble samples (Sble) deprived of NGF for 24 h (-NGF)
| Variable | Intact nuclei |
|
|---|---|---|
| +NGF | -NGF | |
| Sble | 100 | 47 ± 7.2* |
| TrkAi | 70 ± 6.0 | 81 ± 4.2† |
| p75i | 81 ± 6.8 | 97 ± 3.1† |
| TrkAi + p75 | 116 ± 6.4 | 95 ± 2.9† |
See SI Methods. Western blot analysis of p75 and TrkA receptor extent after neuronal silencing (TrkAi; p75i) and of the corresponding scramble samples are reported in Figs. S2d and S3b. Data are representative of 4 different experiments.
*, P < 0.05 vs. +NGF.
†, P < 0.05 vs. -NGF of scramble samples.
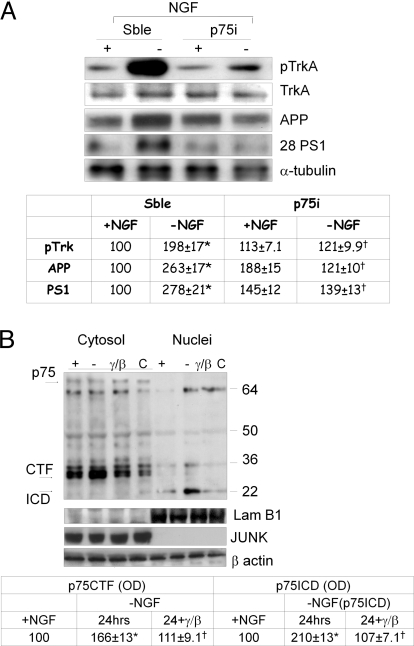
Several studies suggest that p75 neurotrophin receptor may mediate TrkA phosphorylation by a direct or indirect binding to its transmembrane domain, via a still-unclear mechanism (14–16), modulating NGF affinity for TrkA receptor. To assess whether p75 affects TrkA activity, we have resorted to p75 RNA silencing (p75i). As can be seen in Table 2, partial p75 silencing (see also Fig. S3a) largely protected neurons from death due to NGF deprivation, concomitantly with a marked prevention of TrkA phosphorylation (Fig. 2A), indicating an active participation of this receptor in TrkA-mediated death mechanism(s). Note that cosilencing of p75 and TrkA receptors further potentiates neuronal survival after NGF removal, demonstrating a cooperation of both receptors in these events (Table 2). Furthermore, the levels of amyloid precursor protein (APP) and of the 28-kDa PS1 N-terminus subunit are significantly reduced in the same p75-silenced NGF-deprived neurons (Fig. 2A), pointing to an involvement of p75 receptor in the mechanism leading to the activation of amyloidogenesis previously reported in the same experimental model (5).
Fig. 2.
(A) Western blot analysis of pTrkA, TrkA, APP, and 28-kDa PS1 N-terminus fragment (28 PS1) in p75-silenced neurons (p75i) after 24 h of NGF removal and in the corresponding scramble samples. pTrkA densitometric values were normalized using full-length TrkA (TrkA) values as internal control (see Table at bottom). All data represent means ± SEM from 4 independent experiments. *, P < 0.05 vs. +NGF values; †, P < 0.05 vs. -NGF values of the corresponding scramble samples. (B) Cytosolic and nuclear fractions Western blot analysis performed with p75 C-terminus antibody (see Methods). C, neurons before NGF exposure; +, neurons after NGF exposure; -, 24-h NGF- deprived neurons; γ/β, NGF-deprived neurons incubated with γ/β secretase inhibitors. Optical density analysis of CTF and ICD fragments is reported in the Table at bottom. Data were normalized on the basis of the correspondent β-actin values to assess that equal amounts of protein were loaded on gel. *, P < 0.05 vs. +NGF; †, P < 0.05 vs. -NGF values. The same samples loaded in equal amount and run on different gel were probed with JUNK and Lamin B1 antibodies to determine the purity of nuclear and cytoplasmic fractions. Arrows mark CTF (30–32 kDa) and ICD (22 kDa) bands. Western blot showing the effect of Abeta 1-42 exposure on p75 processing is reported in Fig. S3a.
It was also recently demonstrated that p75 may mediate the Abeta 1-42 neuronal death mechanism via its processing (17) to produce p75 C-terminal fragment (CTF). To evaluate whether p75 processing also occurs in NGF-deprived neurons, we have performed Western blot analysis of these fragments in cytosolic and nuclear compartments. As shown in Fig. 2B, concomitantly with TrkA phosphorylation, p75 CTF increases in the cytoplasmic compartment, whereas p75 intracellular domain (ICD) accumulates into nuclei. The same events are also induced by Abeta peptides (Fig. S3b) and are prevented by γ and β secretase inhibitors (Fig. 2B). Relevantly, as previously reported and as shown in Fig. S3b, Abeta exposure induces an overload of p75 CTF, but not of p75 ICD fragments, probably because of an inhibition of γ secretase activity (17). In contrast, in NGF-deprived neurons the accumulation of Abeta (5) and of p75 ICD fragments (Fig. 2B and Fig. S3b) indicates an overactivation of γ secretase and points to the possibility that Abeta, when externally added, may activate different unknown mechanism(s) of death.
TrkA Phosphorylation Induces Phospholipase C γ Activation Concomitantly with AKT Signal Interruption.
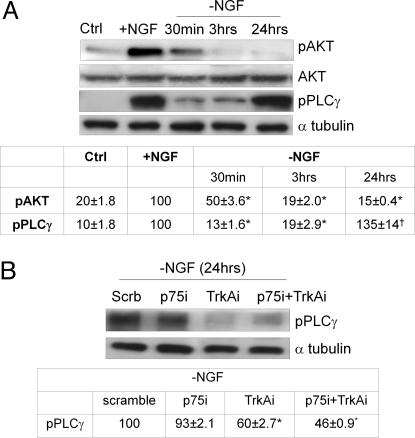
As shown in Fig. 3A, 3 h after NGF removal, the NGF prosurvival pAKT-dependent pathway (18) is totally interrupted, as expected. A somewhat more complex pattern concerns the phospholipase C γ (PLCγ) phosphorylation pathway (pPLCγ), which falls down immediately after NGF removal, but 24 h later significantly increases to the former level (Fig. 3A), concomitantly with anomalous TrkA phosphorylation (Fig. 1 A and B), Abeta accumulation, and neuronal death (5). Because PLCγ is phosphorylated by TrkA (1) under physiologic conditions and because an imbalance of PLCγ expression in AD brains has recently been described (19), it was of interest to investigate whether TrkA anomalous phosphorylation is connected with PLCγ activation at long incubation times. To this aim, pPLCγ levels have been assessed in TrkAi, p75i, and cosilenced neurons 24 h after NGF removal. As shown in Fig. 3B, TrkA silencing and p75+TrkA cosilencing markedly prevented the increase of pPLCγ levels, whereas no significant effect was detected in p75 silenced neurons, indicating that the PLCγ pathway was TrkA dependent.
Fig. 3.
(A) Western blot analysis of AKT (pAKT) and phospholipase C γ (pPLCγ) phosphorylation levels from cultures before (Ctrl) and after 48 h of NGF (+NGF) exposure or deprivation of NGF (-NGF) in a time ranging from 30 min to 24 h. The Table at bottom reports the corresponding densitometric analysis. Values from pAKT were normalized using full-length AKT (AKT), whereas pPLCγ values were normalized using α-tubulin as internal control. All data were expressed as percentage of +NGF samples and are representative of 4 different experiments. *, P < 0.05 vs. +NGF values; †, P < 0.05 vs. 30 min of NGF removal values. (B) Western blot of PLCγ of neurons preincubated with scramble RNAi or silenced for p75, TrkA, or with both together after 24 h of NGF removal. All data were expressed as percentage of -NGF scramble samples and are representative of 4 different experiments. *, P < 0.05 vs. -NGF scramble values.
CDK5 and Src Family Are Involved in pTrkA-Mediated Neuronal Death Mechanism.
It is widely recognized that several kinases, such as CDK5 and Src proteins, trigger amyloidogenesis by affecting APP phosphorylation and processing (7–9, 20). It is also known that the Src family is involved in NGF-independent mechanism leading to TrkA phosphorylation (21). To evaluate whether such anomalous TrkA and PLCγ phosphorylation is connected with these kinases, neurons were exposed to CDK5 and Src inhibitors (roscovitine and PP1, respectively), and the number of intact and condensed nuclei were assessed 24 h after NGF removal. As shown in Fig. S4 a and b, both PP1 and roscovitine compounds reduce TrkA and PLCγ phosphorylation and prevent apoptotic death (Table in Fig. S4), supporting the hypothesis of an involvement of these enzymes in TrkA-mediated neuronal death signal via Abeta-increased production.
pTrkA, p75 CTF, Abeta, and PS1 N-Terminus Association Occurs After the Interruption of NGF Signal.
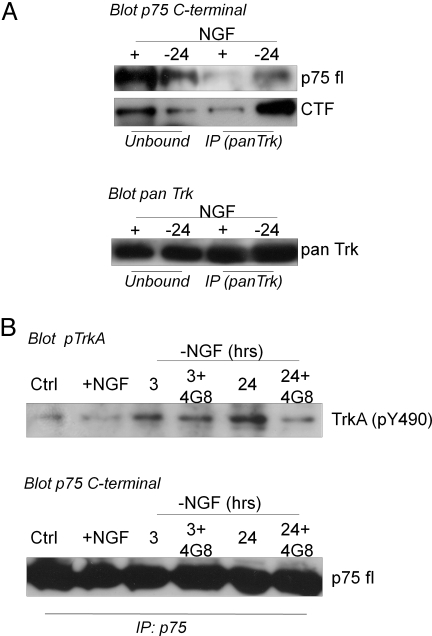
To assess a possible direct interplay between the NGF receptor system and some players of the amyloidogenic pathway within the context of cellular compartments, we performed coimmunoprecipitation assay and immunofluorescence analysis. Immunoprecipitation with anti-pan-Trk, followed by blot with p75 C-terminus antibody, shows that p75 (full length and CTF) is bound to TrkA receptor after 24 h of NGF deprivation (Fig. 4A). Similar results are obtained when p75 is immunoprecipitated with anti-p75 C-terminus antibody and analyzed with TrkA(pY490) antibody. This complex is already formed 3 h after NGF removal and prevented by incubation with an Abeta antibody (Fig. 4B).
Fig. 4.
(A) Immunoprecipitation analysis performed with anti-pan-Trk (A) and p75 C-terminal antibodies (B) on lysates 24 h after NGF removal. Immunocomplex amounts were analyzed by immunoblotting with anti-p75 C-terminal (A). Note that to detect full-length p75 species (fl p75), longer time of exposure was needed than those necessary for CTF band. To demonstrate that equal amounts of protein were brought down after the immunoprecipitation procedure, membranes were stripped and reprobed with pan-Trk antibody. (B) Immunoprecipitation analysis performed with p75 C-terminal antibody (see Methods). Immunocomplexes were analyzed by immunoblotting with TrkA(pY490) antibody. The amount of p75 brought down by immunoprecipitation was analyzed by blotting with p75 C-terminal antibody (fl p75). Ctrl, samples before NGF exposure; +NGF, samples exposed to NGF; 3, 24, 24-h NGF-deprived samples; 3 + 4G8, 24 + 4G8, 24-h NGF-deprived samples incubated with Abeta antibody (MAb4G8).
To evaluate whether p75 also coimmunoprecipitates with Abeta peptides, 3- and 24-h NGF-deprived samples were immunoprecipitated with anti-Abeta (MAb 6E10) and blotted with p75 C-terminus and p75 N-terminus antibodies. As shown in Fig. S5a, p75 CTF but not full-length p75 species immunoprecipitate with Abeta peptides as soon as 3 h after NGF removal; the immunocomplexes decrease at 24 h and totally disappear at 48 h, when, however, both p75 and APP (full-length and Abeta peptides) seem to be localized in the same neuritic compartment (Fig. S5b). Moreover, 28-kDa PS1 N-terminus subunit increases 3 h after NGF removal (5) and preferentially localizes in the nuclear compartment (Fig. S6 a and b) where p75 ICD fragment is also present (Fig. 2B). Furthermore, immunoprecipitation assay performed with anti-p75 C-terminal and blotted with anti-PS1 N-terminus antibodies shows that PS1 (holoprotein and 28-kDa N-terminal fragment) also binds p75 (Fig. S6c).
Therefore, immunoprecipitation assays allow us to conclude that after NGF removal an interaction between the APP processing system and the NGF receptors occurs. This interaction is accompanied by the formation of a molecular complex in which p75 binds TrkA, Abeta peptides, and PS1 (holoprotein and 28-kDa fragment), eventually leading to hippocampal neuronal death. It remains to be established whether such a multimolecular complex actually occurs and operates in a living, intact neuron and whether it also involves other neurotrophin receptors.
Discussion
Growing evidence suggests that imbalance in the NGF signal, transport, and processing are crucial factors in AD (3, 4, 22). Gene therapy trials using NGF-grafted autologous fibroblasts injected into the basal nucleus of Meynert (23) or drugs that maintain a homeostatic balance between TrkA and p75 (24) further validate the hypothesis of an involvement of neurotrophins in AD (25) and are promising in terms of AD therapy. The NGF receptor system is also affected in AD. TrkA decreases in the basal forebrain and in the cortex (26) of AD patients. A switch from TrkA to p75 has been described during neuronal aging, resulting in increased amyloidogenic processing of APP (27). An Abeta pathway, inducing TrkA phosphorylation directly by itself and indirectly by promoting NGF secretion (28), has recently been reported.
p75 expression is also linked to changes occurring in AD (17, 29), probably through its direct binding to Abeta 1-42 peptides (29, 30). In addition, p75 mediates APP promoter activity, leading to an increase of secreted APP (31, 32), and undergoes intramembrane α and γ secretase–mediated processing to generate p75 CTF and p75 ICD fragments, respectively (11). Our recent findings have demonstrated a direct link among NGF withdrawal, activation of amyloidogenic pathway, tau processing, and neuronal death (5, 6, 33), providing evidence for a mechanism in which a discontinued or limited supply of NGF can activate the amyloidogenic pathway, triggering apoptotic death. The rationale of the present study was to investigate the possible connection between the NGF receptor system and the amyloidogenic pathway in an experimental model in which both events are directly linked.
Here we show that (i) TrkA undergoes an anomalous phosphorylation when NGF is removed from its “physiologic” binding site; (ii) such phosphorylation may be directly or indirectly mediated by CDK5 and/or Src enzymes, as indicated by the finding that their respective antagonists contextually inhibit this posttranslational event and the ensuing death; (iii) both TrkA and p75 receptors play a role in apoptotic death after NGF removal, as shown by the finding that when they are partially silenced the extent of death is proportionally reduced; (iv) the products of α and γ secretase–mediated cleavage of p75, CTF and ICD, respectively distribute into different cellular compartments, where they probably contribute to apoptotic death also affecting gene expression (2, 3); and (v) the trigger of all these events appears to be Abeta, although whether it acts upstream or contemporarily to them remains to be elucidated. The finding that these events can also be induced by Abeta extracellular exposure confirms the primary role attributed to Abeta in this hypothesis. However, the fact that in its intracellular produced form due to NGF withdrawal Abeta is capable of inducing changes different from those reported when externally added (17), and at concentrations that are an order of magnitude lower than those necessary to cause analogous cellular events when exogenously added, suggests that its toxic action may involve some intracellular players such as, for instance, NGF receptors, via their anomalous activation or processing.
An NGF-independent TrkA autophosphorylation has been described previously (34, 35), and concomitantly to our study an Abeta-mediated, NGF-independent TrkA phosphorylation mechanism was described in hippocampal neurons (28). We are unable to establish the exact sequence of events leading to this NGF-independent TrkA phosphorylation, but the findings that TrkA inhibitors fail to completely prevent its phosphorylation state suggest that other kinases, besides Src and CDK5, take part in this anomalous and unexpected TrkA phosphorylation. Recent findings carried out in animal models of AD and aging demonstrate that BDNF administration, initiated after disease onset, prevented cortical and hippocampal neuronal death (36). Because similar effects were also described after NGF administration in AD11 mouse (37) and AD patients (23), we are prompted to hypothesize that TrkB could also undergo the same fate described here for TrkA.
On the basis of the findings reported here, we hypothesize a scenario summarized in Fig. S7. When NGF is present and bound to TrkA, the crucial players, APP, the β and γ secretases, and p75 receptors are in some way sequestered away from each other and/or kept inactive. Under this “physiologic state,” TrkA can transduce its numerous intracellular signals and, on the other side, α secretase can catalyze the physiologic, nonamyloidogenic pathway. But if TrkA is deprived of its natural ligand (i.e., NGF), the CDK5 and Src enzymes may catalyze, in a still unknown fashion, the anomalous phosphorylation of TrkA. This event is probably induced by an imbalance of secretase activities with a consequent increase of Abeta peptides and p75 fragments, which lead to apoptotic death.
Altogether, these findings unravel a new neuronal death mechanism in which TrkA switches from a prosurvival to proapoptotic receptor via Abeta-mediated p75 processing and depict a link between a deficit of NGF—and probably of other neurotrophins—and all instances in which neurotrophins play their biologic role.
Methods
Cell Cultures.
Hippocampal neurons were prepared from embryonic day 17 to 18 (E17/E18) embryos from timed pregnant Wistar rats (Charles River), as previously reported (4).
Half of the medium was changed every 3 to 4 days. All experimental treatments were performed on 6- to 7-day-old cultures in Neurobasal B27 medium. Culmsee et al. (38) have previously reported that neuronal TrkA levels increase from day 1 through day 4 in culture and that a remarkable decline occurs at days 7 through 14 as compared with the initial expression levels. Therefore, to obtain the highest response to NGF exposure, hippocampal neurons plated for 3 to 4 days in Neurobasal plus B27 medium (control) were incubated with NGF (50 ng/mL) for 48 h (+NGF, +) and then washed twice and exposed to NGF-free medium (-NGF). Duplicate experiments were carried out in the presence of an anti-NGF rabbit polyclonal antibody to confirm data obtained by simple NGF withdrawal and to exclude any NGF interference (Fig. S8).
Anti-Aβ antibody (MAb 4G8, 1 μg/mL; Signet), γ secretase (50 nM, L-685,458; Calbiochem) or β secretase inhibitors (240 nM; MBL) were used at the highest nontoxic concentration (4).
CDK(s) inhibitors (roscovitine; Sigma), Src inhibitor (PP1; Calbiochem), and TrkA phosphorylation inhibitors (K-252a and CEP-2563; Calbiochem) were preliminarily tested in control neurons in the presence or absence of NGF and used at the highest nontoxic concentration.
Cell Viability.
Viable hippocampal neurons were quantified by counting the number of intact nuclei (39) or by the DNA-binding fluorochrome Hoechst 33258 (Molecular Probes) (condensed nuclei). The stained cells were examined immediately with a standard fluorescence microscope.
Western Blot.
Whole-cell lysates were obtained from neurons washed twice with ice-cold PBS and lysed in PhosphoProtein Lysis Buffer (Qiagen).
Equivalent amounts of cell extracts were mixed with sample buffer according to Invitrogen Nu PAGE. After heating at 70 °C for 10 min, proteins were subjected to SDS-PAGE (Nu PAGE) and transferred electrophoretically to PVDF membrane (Amersham). Incubation with primary antibodies was performed overnight at 4 °C, and subsequently membranes were incubated with donkey antirabbit peroxidase-coupled secondary antibodies (1:5,000) for 1 h at room temperature. Immunoreactivity was developed by an enhanced chemiluminescence system (Amersham). To normalize loaded sample, the blots were stripped (Restore Western Blot Stripping; Pierce) and reprobed with an antibody against α-tubulin (Sigma; 1/10,000, overnight at 4 °C). For analysis of the Western blotting data, densitometric analysis was performed using Scan Analysis software for Macintosh (Biosoft).
The antibodies used were as follows: MAb anti-APP (22C11) from Chemicon; MAb anti-Abeta (clones 4G8 and 6E10), p75 C-terminal (#PRB-608P), and JUNK1 (#PRB-536) from Covance; MAb anti-PS1 N-terminus (clone APS11) and TrkA antibody, which detects basal levels of TrkA receptor, from Novus Biol (#R152–100); MAb anti-α-tubulin, p35/25 CDK5, and MAb p75 N-terminal from Sigma; and TrkA(pY490), phospho-AKT(Ser-473), AKT (full length), and phospho PLCγ-1 (pY783) from Cell Signaling Technology. To better detect the full-length p75 band, which p75 C-terminus antibody from Sigma fails to show at short exposure times, the Western blot shown in Fig. S3b was performed with anti-p75-C-terminus from Covance.
siRNA Treatment.
The pools of siRNA duplexes designed against rat p75 and/or Trk were obtained from Invitrogen (catalog no. 10620312; sequence RSS302377 for p75 and sequence RSS329366 for TrkA). An RNA fluorescent oligo was used as the scramble control of siRNA (BLOCK-iT; Invitrogen). siRNAs were transfected into cells by using the Lipofectamine 2000 (Invitrogen) as suggested by the manufacturer. Three-day-cultured neurons were incubated in OPTIMEM medium with siRNA against p75 and/or Trk (p75i; Trki; p75i+Trki) or with a scramble RNA control oligos (scramble). Six hours later the oligos were removed, and neurons were exposed to NGF for 24 h. Subsequently the medium was rinsed, and the cultures were washed 3 times and incubated in a fresh NGF-deprived medium for 24 h.
Immunofluorescence.
Hippocampal neurons were fixed for 20 min in PBS containing 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 (20 min, 20 °C), and processed for labeling with anti-p75 C-terminal (N3908) from Sigma, anti-Abeta MAb 6E10 from Covance, and anti-PS1 N-terminal (clone APS11) from Novus Biological. Nuclei were visualized by staining with DAPI (1 μg/mL; Sigma). Secondary antibodies coupled to Alexa dyes (488 and 594) were from Molecular Probes (Invitrogen). Digital images were obtained with an Olympus BX51 microscope (×60 oil objectives) equipped with a Diagnostic Instruments SPOT camera and collected with SPOT image analysis software. Controls were performed either by omitting the primary antibody or by preincubating the primary antibody with the corresponding peptide.
Subcellular Fractionation.
Subcellular fractionation was carried out using the Nuclei Ez Prep Isolation kit from Sigma. Intact nuclei were lysed according to the procedure previously described (40). Anti-JUNK (#PRB-536P; Covance) and anti-Lamin B1 (#M20; Santa Cruz Biotechnology) were used as loading control to determine the purity of cytoplasmic and nuclear extracts, respectively. Beta-actin Western blots were routinely carried out as loading control. For corresponding samples, equal protein amounts were loaded in different gels.
Immunoprecipitation.
Immunoprecipitation was performed by cross-linking the Ig's antibodies to Protein G on bead surface. Samples containing the target protein antigen were added to Dynabeads Protein G complex, according to the procedure described by the manufacturer (Invitrogen), and eluted with 0.1 M citrate buffer (pH 2.3). pH was adjusted by adding Tris/HCl 2 M. Immunoprecipitation of TrkA reported in Fig. S1 and in Fig. S5 was performed with anti-TrkA antibody (30 μg/100 μL of Dynabeads Protein G) from Novus Biological (#R152–100). For the immunoprecipitation reported in Figs. S5 and S6, p75 C-terminus antibody (Covance), pan-Trk antibody (#G1581; Promega), and anti-Abeta antibody (6E10; Covance) were used at concentrations of 10 μg/100 μL of Dynabeads Protein G. Note that, because anti-p75 C-terminal from Sigma needs a long exposure time to detect full-length species, all immunoprecipitation procedures were carried out with anti-p75 C-terminus from Covance to be sure to recover also full length p75 species. Western blot analysis of Fig. 5 A and B and Fig. 6 (Top) was performed with anti-p75 C-terminus from Sigma.
Statistical Analysis.
Values are expressed as mean ± SE. Statistical analysis was performed with ANOVA followed by the Newman-Keuls test. Statistical significance was accepted at the 95% confidence level (P < 0.05).
Supplementary Material
Acknowledgments.
We thank Professor Moses Chao for kindly providing the phosphoTrk antibodies. Financial support was received from the Regione Lazio, Fondo Investimento Ricerca di Base 2003, Progetti di Rilevante Interesse Nazionale 2006, and Istituto Nazionale Riposo e Cura per Anziani 2008 (to P.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904998106/DCSupplemental.
References
- 1.Huang EJ, Reichardt F. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 2.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo A, Capsoni S, Paoletti F. Towards non invasive nerve growth factor therapies for Alzheimer disease. J Alzheimers Dis. 2009;15:255–283. doi: 10.3233/jad-2008-15210. [DOI] [PubMed] [Google Scholar]
- 4.Calissano P, Matrone C, Amadoro G. Apoptosis and in vitro Alzheimer disease neuronal models. Communicative Integrative Biol. 2009;2:1–7. doi: 10.4161/cib.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matrone C, et al. Activation of the amyloidogenic route by NGF deprivation induces apoptotic death in PC12 cells. J Alzheimers Dis. 2008;13:81–96. doi: 10.3233/jad-2008-13109. [DOI] [PubMed] [Google Scholar]
- 6.Matrone C, Ciotti MT, Mercanti D, Marolda R, Calissano P. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:13139–13144. doi: 10.1073/pnas.0806133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer's disease. Trends Mol Med. 2004;10:452–458. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds CH, et al. Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2, and Src family kinases. J Biol Chem. 2008;283:18177–18186. doi: 10.1074/jbc.M709715200. [DOI] [PubMed] [Google Scholar]
- 9.Williamson R, et al. Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: Involvement of Src family protein kinases. J Neurosci. 2002;22:10–20. doi: 10.1523/JNEUROSCI.22-01-00010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronfman FC. Metalloproteases and gamma-secretase: New membrane partners regulating p75 neurotrophin receptor signaling? J Neurochem. 2007;103(Suppl 1):91–100. doi: 10.1111/j.1471-4159.2007.04781.x. [DOI] [PubMed] [Google Scholar]
- 11.Urra S, et al. TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal gamma-secretase-mediated release of the p75 intracellular domain. J Biol Chem. 2007;282:7606–7615. doi: 10.1074/jbc.M610458200. [DOI] [PubMed] [Google Scholar]
- 12.Kase H, et al. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 13.Wood ER, et al. Discovery and in vitro evaluation of potent TrkA kinase inhibitors: Oxindole and aza-oxindoles. Bioorg Med Chem Lett. 2004;14:953–957. doi: 10.1016/j.bmcl.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Zaccaro MC, Ivanisevic L, Perez P, Meakin SO, Saragovi HU. p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J Biol Chem. 2001;276:31023–31029. doi: 10.1074/jbc.M104630200. [DOI] [PubMed] [Google Scholar]
- 15.Diolaiti D, et al. Functional cooperation between TrkA and p75(NTR) accelerates neuronal differentiation by increased transcription of GAP-43 and p21(CIP/WAF) genes via ERK1/2 and AP-1 activities. Exp Cell Res. 2007;313:2980–2992. doi: 10.1016/j.yexcr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sotthibundhu A, et al. Beta-amyloid(1-42) induces neuronal death through the p75 neurotrophin receptor. J Neurosc. 2008;28:3941–3946. doi: 10.1523/JNEUROSCI.0350-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke TF, Kaplan DR, Cantley LC. PI3K: Downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Dhillon H, Prasad MR, Markesbery WR. Regional levels of brain phospholipase Cgamma in Alzheimer's disease. Brain Res. 1998;811:161–165. doi: 10.1016/s0006-8993(98)00935-4. [DOI] [PubMed] [Google Scholar]
- 20.Russo C, et al. Signal transduction through tyrosine-phosphorylated C-terminal fragments of amyloid precursor protein via an enhanced interaction with Shc/Grb2 adaptor proteins in reactive astrocytes of Alzheimer's disease brain. J Biol Chem. 2002;277:35282–35288. doi: 10.1074/jbc.M110785200. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopal R, Chao MV. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol Cell Neurosci. 2006;33:36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Salehi A, et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:1–3. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Tuszynski MH, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 24.Longo FM, et al. Small molecule neurotrophin receptor ligands: Novel strategies for targeting Alzheimer's disease mechanisms/ Curr Alzheimer Res. 2007;4:503–506. doi: 10.2174/156720507783018316. [DOI] [PubMed] [Google Scholar]
- 25.Capsoni S, Giannotta S, Cattaneo A. Beta-amyloid plaques in a model for sporadic Alzheimer's disease based on transgenic anti-nerve growth factor antibodies. Mol Cell Neurosci. 2002;21:15–28. doi: 10.1006/mcne.2002.1163. [DOI] [PubMed] [Google Scholar]
- 26.Mufson EJ, et al. Reduction in p140-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer's disease. Exp Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- 27.Costantini C, Scrable H, Puglielli L. An aging pathway controls the TrkA to p75NTR receptor switch and amyloid beta-peptide generation. EMBO J. 2006;25:1997–2006. doi: 10.1038/sj.emboj.7601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulbarelli A, et al. TrkA pathway activation induced by amyloid-beta (Abeta) Mol Cell Neurosci. 2009;40:365–373. doi: 10.1016/j.mcn.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Yaar M, et al. Amyloid beta binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J Biol Chem. 2002;277:7720–7725. doi: 10.1074/jbc.M110929200. [DOI] [PubMed] [Google Scholar]
- 30.Coulson EJ. Does the p75 neurotrophin receptor mediate Abeta-induced toxicity in Alzheimer's disease? J Neurochem. 2006;98:654–660. doi: 10.1111/j.1471-4159.2006.03905.x. [DOI] [PubMed] [Google Scholar]
- 31.Ge YW, Lahiri DK. Regulation of promoter activity of the APP gene by cytokines and growth factors: Implications in Alzheimer's disease. Ann N Y Acad Sci. 2002;973:463–467. doi: 10.1111/j.1749-6632.2002.tb04684.x. [DOI] [PubMed] [Google Scholar]
- 32.Rossner S, Ueberham U, Schliebs R, Perez-Polo JR, Bigl V. p75 and TrkA receptor signaling independently regulate amyloid precursor protein mRNA expression, isoform composition and protein secretion in PC12 cells. J Neurochem. 1998;71:757–766. doi: 10.1046/j.1471-4159.1998.71020757.x. [DOI] [PubMed] [Google Scholar]
- 33.Corsetti V, et al. Identification of a caspase-derived N-terminal tau fragment in cellular and animal Alzheimer's disease models. Mol Cell Neurosci. 2008;38:381–392. doi: 10.1016/j.mcn.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagahara AH, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capsoni S, et al. Delivery of NGF to the brain: Intranasal versus ocular administration in anti-NGF transgenic mice. J Alzheimers Dis. 2009;16:371–388. doi: 10.3233/JAD-2009-0953. [DOI] [PubMed] [Google Scholar]
- 38.Culmsee C, et al. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor p75. Neuroscience. 2002;115:1089–1108. doi: 10.1016/s0306-4522(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 39.Volontè C, Ciotti MT, Battistini L. Development of a method for measuring cell number: Application to CNS primary neuronal cultures. Cytometry. 1994;17:274–276. doi: 10.1002/cyto.990170311. [DOI] [PubMed] [Google Scholar]
- 40.Matrone C, et al. HIF-1alpha reveals a binding activity to the promoter of iNOS gene after permanent middle cerebral artery occlusion. J Neurochem. 2004;90:368–378. doi: 10.1111/j.1471-4159.2004.02483.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.