Abstract
Cystic fibrosis is caused by impaired ion transport due to mutated cystic fibrosis transmembrane conductance regulator, accompanied by elevated activity of the amiloride-sensitive epithelial Na+ channel (ENaC). Here we show that knockout of the ubiquitin ligase Nedd4L (Nedd4-2) specifically in lung epithelia (surfactant protein C-expressing type II and Clara cells) causes cystic fibrosis-like lung disease, with airway mucus obstruction, goblet cell hyperplasia, massive inflammation, fibrosis, and death by three weeks of age. These effects of Nedd4L loss are likely caused by enhanced ENaC function, as reflected by increased ENaC protein levels, increased lung dryness at birth, amiloride-sensitive dehydration of lung explants, and elevated ENaC currents in primary alveolar type II cells analyzed by patch clamp recordings. Moreover, the lung defects were rescued with administration of amiloride into the lungs of young knockout pups via nasal instillation. Our results therefore suggest that the ubiquitin ligase Nedd4L can suppress the onset of cystic fibrosis symptoms by inhibiting ENaC in lung epithelia.
Cystic fibrosis (CF) is caused by mutations in cystic fibrosis transmembrane conductance regulator (CFTR). It leads to severe morbidity and mortality from lung disease that is characterized by small airway obstruction due to mucus accumulation, inflammation, repeated infections, and bronchiectasis (1). Among the hypotheses that have been proposed to explain the defects in CF lungs (e.g., refs. 2 and 3), the low volume hypothesis (4, 5) suggests that impaired Cl- secretion coupled with enhanced Na+ absorption caused by hyperactivity of the amiloride-sensitive epithelial Na+ channel (ENaC) (6, 7) leads to a reduction in the volume of airway surface liquid (ASL) (4). This airway dehydration, in turn, impairs cilia movement and mucociliary clearance, thereby leading to mucus buildup in the airways seen in lungs of CF patients. CFTR normally inhibits ENaC function in the airways, but in CF, where CFTR is mutated and nonfunctional, ENaC activity is enhanced (7).
ENaC is comprised of three subunits, α, β, and γ (8). It regulates salt and fluid absorption in the distal nephron, distal colon, lung, and other salt-sensitive tissues. In the kidney, it regulates Na+ and fluid reabsorption, which controls blood pressure (9). Each ENaC subunit contains a cytoplasmic PY motif (PPxY), which binds to the Nedd4 family of ubiquitin ligases (10), particularly Nedd4-2 (Nedd4L) (11). Nedd4L is composed of a C2 domain, 4 WW domains, and a ubiquitin ligase HECT domain (12, 13). Its WW domains mediate binding to the PY motifs of ENaC (10). Mutation of these PY motifs leads to impaired binding to Nedd4L, impaired channel ubiquitination, and endocytosis, and thus to increased cell-surface stability and function of ENaC (11, 14–16), which results in increased fluid absorption in the kidney (17). Although ENaC is expressed in both airway and alveolar type II epithelia (18, 19) (much like Nedd4L, as our preliminary results suggest) and plays an important role in fluid absorption in the lung, especially at birth (20), the function of Nedd4L in that tissue is unclear and no genetic models have been described. Here we show that specific ablation of Nedd4L in lung epithelia in mice leads to the development of cystic fibrosis-like symptoms, most likely caused by increased ENaC function. These results provide evidence for a protective regulatory role of a ubiquitin ligase, Nedd4L, against the development of cystic fibrosis.
Results
Generation of Nedd4L Conditional Knockout in Lung Epithelia.
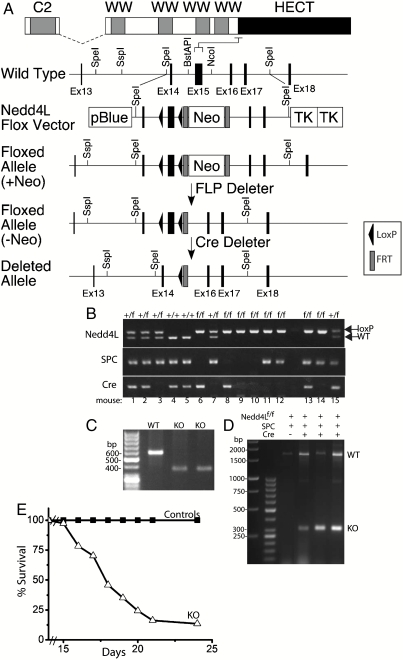
To investigate Nedd4L function in the lung, we generated Nedd4L conditional knockout mice, in which loxP sites were introduced into a sequence flanking exon15 (at the N terminus of the HECT domain—see Fig. 1A and Materials and Methods). This approach leads to ablation of expression from Exon 15 and downstream residues (i.e., the HECT domain) due to frameshift. It was chosen, rather than interference with the N-terminal start site, to ensure inactivation of Nedd4L and to avoid possible splicing around the start site, which is known to occur at the N terminus of Nedd4L (21, 22). The presence of the LoxP sites in the lungs of the Nedd4L floxed (Nedd4Lf/f) mice was verified by PCR and Southern blotting (Fig. 1B and Fig. S1A). Infection of mouse embryonic fibroblast cells generated from the Nedd4Lf/f mice with Cre-expressing retroviruses revealed loss of the whole Nedd4L protein, not just the HECT domain (Fig. S1B).
Fig. 1.
Generation of Nedd4L conditional knockout in lung epithelia that express SPC. (A) General strategy for generation of floxed Nedd4L allele in mice, targeting the N-terminal region of the HECT domain (exon 15), to inactivate catalytic activity. After confirmation of proper homologous recombination by Southern blotting, the heterozygous mouse with the exon 15 flanked by two loxP sites (black triangles) and the neomycin resistance cassette (Neo) flanked by two FRT sites (gray boxes) [Floxed allele (+Neo)] were crossed with the FLP deleter mouse to generate heterozygous Nedd4Lf/+ mouse without the neomycin resistance cassette [Floxed allele (−Neo)]. This mouse was crossed with the SPCrtTA;teton-Cre mouse to conditionally inactivate the Nedd4L allele (deleted allele). (B) PCR analysis of genomic DNA to test for the presence of Nedd4L floxed (f) allele, SPC, or Cre. In this example, only mouse 13 was a knockout, expressing Nedd4Lf/f, SPC, and Cre. (C) RT-PCR of isolated alveolar-type II cells of P14 mice proving loss of the N-terminal region of the HECT domain, as also verified by sequencing. Removal of exon15 causes a frameshift that deletes the whole HECT domain. (D) Loss of exon 15 in lungs from E18.5 knockout embryos analyzed by PCR on genomic DNA and verified by sequencing. (E) Survival rate of Nedd4Lf/f;SPC-rtTA;teto-Cre KO mice and controls (all other phenotypes), revealing death of most of the KO mice at 2–3 wk of age (n = 37 for Nedd4Lf/f;SPC-rtTA;teto-Cre and 109 for the controls). Some of the KO mice died immediately after birth from an unknown cause (not included in the figure).
To inactivate Nedd4L specifically in lung epithelia (alveolar type II cells and Clara cells), we crossed our Nedd4Lf/f mice to surfactant protein C (SPC)-rtTA;teto-Cre mice [(23) and Materials and Methods]. The presence of Cre and SPC-rtTA expression in lungs of these mice was verified by PCR (Fig. 1B). As reported earlier (24, 25), we also observed that Cre expression was confined to the lung, with no expression detected in the kidney, liver, or heart (Fig. S1C), but was “leaky” (Fig. S1D), i.e., detectable even in the absence of tetracycline (doxycycline, Dox) treatment. Because the leaky Cre expression was sufficient to cause severe phenotypic changes in the lungs of the Nedd4-2f/f;SPC-rtTA;teto-Cre mice but had no effect in WT or heterozygous mice (see below), all experiments (unless otherwise indicated) were carried out without Dox treatment. Deletion of Nedd4-2 (loss of exon 15) was verified by RT-PCR of mRNA isolated from alveolar type II cells of Nedd4-2f/f;SPC-rtTA;teto-Cre mice (Fig. 1C), or by PCR of genomic DNA isolated from lungs if E18.5 knockout embryos (Fig. 1D).
Mice Lacking Nedd4L in Lung Epithelia (SPC-Expressing Cells) Develop Cystic Fibrosis-Like Lung Disease.
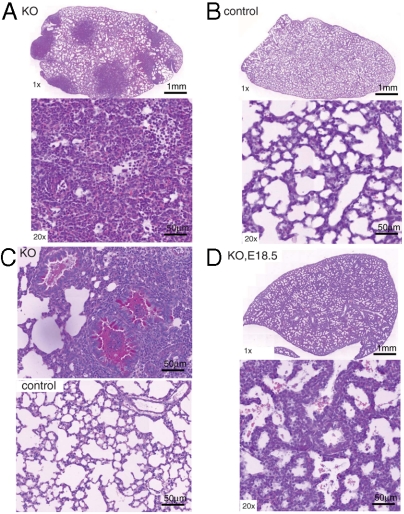
Following crossing of Nedd4f/f mice to SPC-rtTA;teto-Cre mice, we analyzed the lung phenotype of the Nedd4-2f/f;SPC-rtTA;teto-Cre animals. Most of the Nedd4Lf/f;SPC-rtTA;teto-Cre mice died between two and three weeks of age (Fig. 1E), whereas Cre-expressing Nedd4L+/+ or Nedd4L+/f animals, or Nedd4Lf/f mice lacking SPC-rtTA or teto-Cre, were unaffected. To investigate the lungs of the Nedd4Lf/f;SPC-rtTA;teto-Cre mice and the cause of their death, we sacrificed 10-d-old (P10) animals and analyzed their lungs. H&E staining of lungs from the Nedd4Lf/f;SPC-rtTA;teto-Cre mice revealed large dense patches covering extensive regions, which were infiltrated with neutrophils (Fig. 2A). This effect was not seen in control animals lacking Cre expression (Nedd4Lf/f;SPC-rtTA mice) (Fig. 2B), or in Cre-expressing Nedd4L+/+ or Nedd4L+/f mice (Fig. S2 A and B). Analysis of bacterial growth in bronchioalveolar lavage fluid and minced lungs revealed no bacterial infection in the knockout lungs at P10 or P14, suggesting that lung inflammation was not secondary to infection, in agreement with observations in CF infants in which inflammation often precedes fulminant infection (26). Periodic acid/Schiff (PAS) staining of the lungs demonstrated severe mucus accumulation (mucus plugs) in airways of the Nedd4Lf/f;SPC-rtTA;teto-Cre animals (Fig. 2C), but not in the controls (Nedd4Lf/f;SPC-rtTA). In addition, collagen (Trichrome) staining of the knockout lungs, to mark connective tissue and interstitial fibrosis (27), revealed expansion of fibrotic areas (Fig. S3). It is thus likely that Nedd4Lf/f;SPC-rtTA;teto-Cre mice die from airway obstruction and asphyxiation. This severe lung phenotype is developed postnatally over time, because lungs of Nedd4Lf/f;SPC-rtTA;teto-Cre E18.5 embryos just before birth (of mothers that had been treated with Dox to maximize Cre expression) were normal (Fig. 2D) and indistinguishable from controls (Fig. S2C).
Fig. 2.
Severe inflammation and airway mucus accumulation in lungs of Nedd4L conditional KO mice. (A–C) H&E staining of lungs of 10-day-old KO (i.e., Nedd4Lf/f;SPC-rtTA;teto-Cre) mice (A) or controls (Nedd4Lf/f;SPC-rtTA) mice (B), showing the whole lung (1× objective, Upper), or a higher magnification (20× objective, Lower) of a lung segment. Note large dense patches, revealing inflammation (neutrophil infiltration) in the Nedd4L KO lungs shown in A. (C) PAS staining of 10-day-old Nedd4L KO (Nedd4Lf/f;SPC-rtTA;teto-Cre) mice and control (Nedd4Lf/f;SPC-rtTA) mice revealing mucus accumulation (red color) in the airways of the knockout animals (original magnification 20×). (D) Normal lungs of E18.5 KO (Nedd4Lf/f;SPC-rtTA;teto-Cre) embryos just before birth (indicating that the defects shown in A–C developed postnatally). The pregnant mothers of these E18.5 embryos had been treated with Doxycycline (200 mg/kg in food plus 2 mg/mL in 5% sucrose solution in water) from E8 to E14 to maximize Cre expression.
Overall, the lung phenotype observed upon loss of Nedd4L appears remarkably similar to that seen in lungs of CF patients.
Knockout of Nedd4L in Lung Epithelia Leads to Increased ENaC Expression and Function.
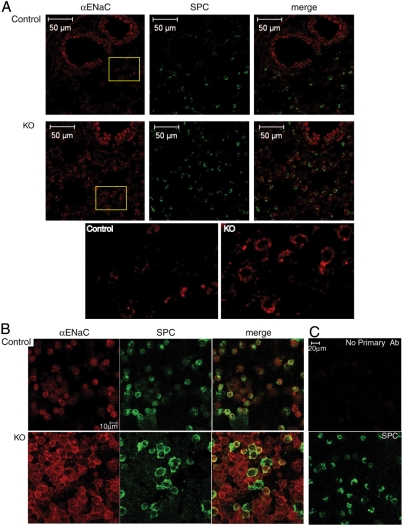
Because Nedd4L is known to inhibit ENaC when expressed in tissue culture cells or in Xenopus oocytes (11, 14, 15, 28), we tested using several approaches, whether loss of Nedd4L in type II and Clara cells leads to increased ENaC expression and function in vivo in the lung. First, we assessed levels of ENaC protein expression in lungs using immunostaining of lung sections and isolated type II cells under nonpermeablilizing condition (biochemical analysis using immunoblotting was not feasible due to insufficient number of type II cells that can be isolated from the mice). Fig. 3A shows an increase in levels of ENaC in knockout lung epithelia relative to controls as assessed by immunofluorescence of lung slices incubated with anti αENaC antibodies that recognize the ectodomain of this subunit. Likewise, an increase in cell surface αENaC in type II cells isolated from the knockout animals was also observed using these antibodies (Fig. 3B). These results suggest that ENaC protein levels, especially at the plasma membrane, are elevated in lung epithelia upon loss of Nedd4L.
Fig. 3.
Increased ENaC protein expression in lung epithelia and type II cells from Nedd4L conditional KO mice. (A) Immunostaining of cryo sections from lungs of 10-day-old Nedd4L KO (Nedd4Lf/f;SPC-rtTA;teto-Cre) and control (Nedd4Lf/f;SPC-rtTA) mice stained with anti αENaC antibodies (red) that are directed to the extracellular domain of αENaC. Tissues were then permeabilized and stained with anti-SPC antibodies (green). (Bottom) Enlargements of the insets indicated, revealing increased ENaC immunoreactivity in alveolar-type II cells of the KO lungs. (B) Immunostaining of isolated, nonpermeabilized type II cells with anti-αENaC antibodies followed by permeabilization and staining for SPC and analysis by confocal microscopy, as in A. Note the increase in membrane staining of ENaC in the KO type II cells relative to controls. (C) Immunostaining controls showing lack of staining of type II cells in the absence of primary anti-αENaC antibodies (Upper). (Lower) Depicts SPC staining of the same cells (after permeabilization). Immunostaining of lung sections and isolated type II cells was carried out using lungs from 4 KO and 7 control mice, with representative micrographs shown.
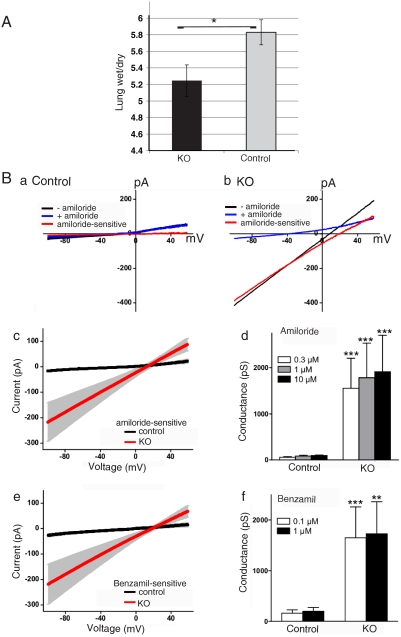
To assess the effect of loss of Nedd4L on ENaC function we utilized several approaches. First, we measured wet/dry ratio of newborn lungs (4 h postpartum), an indication of fluid absorption capacity (20). As seen in Fig. 4A, lungs of newborn Nedd4Lf/f;SPC-rtTA;teto-Cre mice were drier than controls (Nedd4Lf/f;SPC-rtTA or Nedd4Lf/f;teto-Cre), suggesting that the knockout mice are better able to absorb lung fluid at birth. Next, we tested fluid absorption in distal lung explants harvested from E16 embryos and grown in culture, as described previously (29). Fig. S4 A and B demonstrates poor explant expansion (drier explants) in Nedd4Lf/f;SPC-rtTA;teto-Cre explants relative to controls (Nedd4Lf/f;SPC-rtTA or Nedd4Lf/f;teto-Cre), likely due to increased fluid absorption. This dehydration was likely mediated by ENaC, because it was inhibited by low doses of the ENaC inhibitor amiloride (10 μM), which was added to the bathing medium upon seeding of the explant, to ensure luminal access of the drug. Thus, blocking ENaC function with amiloride rescued the hyperabsorption defect seen in the Nedd4Lf/f;SPC-rtTA;teto-Cre explants.
Fig. 4.
Increased ENaC function in Nedd4L conditional KO lungs and isolated type II cells. (A) Decreased wet/dry ratio of lungs harvested at 4 h postpartum from Nedd4L KO (Nedd4Lf/f;SPC-rtTA;teto-Cre) vs. Control (Nedd4Lf/f;SPC-rtTA or Nedd4Lf/f;teto-Cre) mice. Values are mean ± SD of 5–7 mice. P < 0.005 (Student’s t test). (B) Whole-cell patch-clamp recording to analyze ENaC activity in alveolar-type II cells isolated from lungs of 2-wk-old Nedd4L KO mice (Nedd4Lf/f;SPC-rtTA;teto-Cre) and controls (Nedd4Lf/f;SPC-rtTA). (A and B) Representative traces show the currents recorded during the voltage ramp from -100 to +60 mV before (−) and after (+) adding 10 μM amiloride in a control (A) cell and a KO (B) cell. The amiloride-sensitive difference currents are illustrated. (C) A plot showing average amiloride (10 μM) -sensitive currents in control (n = 19) and KO (n = 33) cells. The gray areas are ± 1 SEM for each point. (D) Bar graph showing average amiloride-sensitive (± SEM) conductance at the concentrations of amiloride tested (0.3, 1, or 10 μM) in control (n = 12) and KO (n = 24) cells. ***p < 0.001 compared with corresponding control group. (E) A plot showing average 1-μM benzamil-sensitive currents in control (n = 20) and KO (n = 26) cells. (F) Bar graph showing average benzamil-sensitive conductance at the concentrations of benzamil tested (0.1 or 1 μM) in control (n = 15) and KO (n = 25) cells. **p < 0.01, ***p < 0.001 compared with corresponding control group (Mann–Whitney U test). For all patch-clamp experiments, type II cells were isolated from 11 KO and 14 control mice, which originated from six different litters.
Finally, to directly investigate elevated ENaC activity, type II cells from P14 Nedd4Lf/f;SPC-rtTA;teto-Cre mice or controls (Nedd4Lf/f;SPC-rtTA) were isolated using a recently described protocol (30). These cells were subjected to whole-cell patch clamp recordings to analyze amiloride- and benzamil-sensitive currents. Fig. 4B(a–d) show that the amiloride-sensitive conductance in Nedd4Lf/f;SPC-rtTA;teto-Cre cells was significantly larger than in control cells (Nedd4Lf/f;SPC-rtTA) at all concentrations of amiloride tested (0.3, 1, or 10 μM). In contrast, in the presence of amiloride, the residual conductance in Nedd4Lf/f;SPC-rtTA;teto-Cre cells was similar to controls [Fig. 4B(a and b)]. Furthermore, we found that the conductance blocked by benzamil, an amiloride analogue highly specific for ENaC, was greater in Nedd4Lf/f;SPC-rtTA;teto-Cre cells than in control cells, at benzamil concentrations of 0.1 or 1 μM [Fig. 4B(e and f)]. Collectively, these findings indicate that ENaC activity was greatly increased in Nedd4L knockout cells.
Taken together, the above results show that deletion of Nedd4L in lung epithelial (SPC-expressing) cells leads to increased ENaC expression at the plasma membrane and enhanced channel function.
Rescue of the Lung Defects of Nedd4L Knockout Mice with Amiloride Treatment.
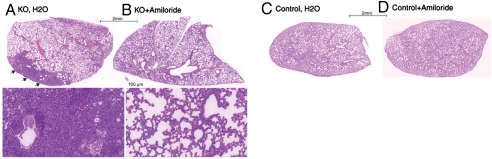
If knockout of Nedd4L in lung epithelia leads to CF-like lung disease due to excess ENaC function, then treatment of newborn pups with amiloride should ameliorate the lung disease. To test this, we administered amiloride (1 μL/g body weight of 10 mM solution) or water to young pups via nasal instillation (three times per day, from day 1 to 10), as recently described (31). As seen in Fig. 5A, whereas lungs harvested from 11-d-old Nedd4Lf/f;SPC-rtTA;teto-Cre mice treated with water exhibited the same defects as seen in untreated mice (Fig. 2A), mice treated with amiloride exhibited a markedly improved lung phenotype (architecture) with much reduced inflammation (Fig. 5).
Fig. 5.
Amelioration of lung defects of the Nedd4L conditional KO mice with amiloride treatment. Newborn mice were treated three times daily (from day 1 to 10) with amiloride, or water, and lungs of mice were sacrificed on day 11 analyzed by H&E staining. (A and B) KO (Nedd4Lf/f;SPC-rtTA;teto-Cre) mice treated with (A) H2O or (B) amiloride, showing whole lungs as well as a higher magnification of lung segments from each (Lower). Note the areas of inflammation in water-treated KO animals (arrows in A), not seen in amiloride-treated KO mice (B). (C and D) Control mice treated with (C) H2O or (D) amiloride. Results are representative of data from five to six mice per treatment.
These results therefore demonstrate that the CF-like lung disease caused by loss of Nedd4L results from an excessive function of ENaC.
Decreased Height of Airway Periciliary Liquid Layer and Hyperplasia of Airway Secretory Cells upon Loss of Nedd4L.
Excessive Na+ and fluid absorption leading to increased mucus formation in the airways of CF patients is expected to reduce ASL and the periciliary liquid (PCL) layer (4). To analyze PCL layers, we fixed trachea segments and assessed its thickness in Nedd4Lf/f;SPC-rtTA;teto-Cre lungs and controls (Nedd4Lf/f;SPC-rtTA) using electron microscopy. Our results reveal a decrease in average PCL height in Nedd4L knockout trachea relative to controls (Fig. S5 A and B). Interestingly, the epithelial cell layer lining the trachea, which is normally well organized, appears disorganized in the Nedd4L knockout lungs, with significant expansion of the nonciliated mucus secreting (mostly goblet) cells relative to ciliated cells (Fig. S5 C and D). Both reduced airway PCL and expansion of the secretory cell population are hallmark features of CF airways.
Discussion
Using a genetic approach we show here that ablation of Nedd4L specifically in epithelial alveolar type II and Clara cells in the airways leads to enhanced ENaC function and fluid absorption in the lung, causing accumulation of mucus in the airways, massive inflammation, and death due to airway obstruction and asphyxiation. These features recapitulate the defects seen in lungs of CF patients (1) and in transgenic mice that overexpress ENaC (32) and strongly support a role for ENaC in regulating the development of CF symptoms, as previously proposed (4, 33). Our results suggest that Nedd4L plays an important and beneficial role in opposing the elevated function of ENaC in CF patients, most likely by increasing ENaC ubiquitination and endocytosis, hence decreasing its levels and function.
Although we cannot currently exclude the possibility that Nedd4L targets other substrates in the lung in addition to ENaC, our ability to substantially ameliorate the lung defects with nasally instilled amiloride in the very young knockout mice strongly suggests that these lung defects were caused primarily by loss of suppression of ENaC due to ablation of Nedd4L. Our present work does not support the notion that Nedd4L directly ubiquitinates CFTR to target it for degradation, as suggested recently (34). If Nedd4L was the E3 ligase for CFTR that leads to its degradation, the putative excess CFTR accumulating upon loss of Nedd4L would not have caused CF-like disease seen here, because CF is caused by loss, not gain, of CFTR function. In addition, although Nedd4L was recently shown to regulate processing of SPC itself (35, 36), the phenotype of our mice is unrelated to the regulation of SPC, because the lungs appear normal just before birth and most of our knockout neonates survive well, suggesting no surfactant deficiency.
Recently, another Nedd4L knockout (floxed) model was generated, targeting a N-terminal region of the protein [in a region that is known to be subjected to splicing (21)]; these mice, when crossed to a Cre driver line that expresses Cre in the germ line (EIIa-Cre), developed salt-induced hypertension (28), but a lung phenotype was not described. Because their genetic background was similar to ours (although not identical), it is possible that those mice contain a shorter splice isoform of Nedd4L [suggested from a Nedd4L immunoblot (28)] that may help explain the milder phenotype. Another possibility is that because these mice lacked Nedd4L throughout embryonic development, other Nedd4 family E3 ligase(s) might have compensated for the loss of Nedd4L in the lung.
In conclusion, the present study shows that loss of Nedd4L in lung epithelia causes a CF-like disease, suggesting that normally this E3 ligase provides a protective function against the development of cystic fibrosis symptoms. Based on these findings, it is possible that in addition to a search for drugs that inhibit ENaC, a search for compounds that enhance Nedd4L activity could provide a useful approach to develop unique treatments for cystic fibrosis.
Materials and Methods
Generation of Floxed Nedd4L Mice.
The fragment of a bacterial artificial chromosome clone encoding the Nedd4L gene from the 13th to the 18th introns was cloned in the pL253 vector (37). One loxP site and another loxP site with a neomycin resistance cassette flanked by two FRT sites were integrated in 14th and 15th introns to flank the 15th exon (corresponding to a region located at the N terminus of the HECT domain; National Center for Biotechnology Information gene ID code 83814, CCDS#29305.1) by the recombineering method (37) (Fig. 1A). This approach leads to ablation of expression from Exon 15 and downstream residues (i.e., the HECT domain) due to frameshift. After transfection of the targeting vector into mouse ES cells derived from 129/Ola, properly recombined ES cells were selected with G418 (Invitrogen) and Ganciclovir (Roche). The genotypes of cloned ES cells were studied by Southern blotting after digesting the genomic DNA with BamHI (Fig. S1A). Two properly targeted clones were expanded and injected into blastocysts (38). Nedd4Lflox/+ (Nedd4Lf/+) were crossed with FLP-1 expressing deleter line (B6;SJL-Tg(ACTFLPe)9205Dym/J; Jackson Laboratory) to remove the Neo cassette. Southern blot analysis of genomic DNA of targeted embryonic stem cells was performed on purified genomic DNA digested with BamHI and separated by agarose gel DNA electrophoresis followed by transfer to the Hybond-N membrane (GE Healthcare) and hybridization with the radiolabeled probe. Knockout of Nedd4L in SPC-expressing lung epithelial cells was generated by crossing Nedd4Lf/f mice to SPCrtTA;teton-Cre mice. In the latter mice, Cre expression is stimulated by the docycycline-inducible reverse tetracycline transactivator (rtTA), which is expressed under the control of the SPC promoter (23). However, due to leakage of Cre (Fig. S1D), we performed all experiments (unless otherwise indicated) in the absence of Dox induction. Mice used for the experiments were on a mixed (C57BL/6;129;FVB/N) background. All animal work conformed to institutional guidelines. Genotyping of mice was carried out as described in the SI Text.
Histology and EM.
Pups were sacrificed by cervical dislocation, lungs inflated, fixed, sectioned (5 μm), and processed for H&E, PAS, or collagen (Trichrome) staining, as detailed in SI Text. EM analysis of trachea and some bronchi were processed as described (29).
Lung Wet/Dry Ratio, and Lung Explant Growth in Culture.
Wet/Dry weight ratio of lungs from 4-h-old newborns was carried out as described (29). Lung explant growth assay was performed on explants from E16 embryos in the presence/absence of amiloride (10 μM), as described (29) and detailed in SI Text.
Isolation of Alveolar Type II Cells.
Alveolar type II cells were isolated from lungs of ∼2-wk-old mice, as described (30), with minor modifications, as detailed in SI Text.
Double Immunofluorescence Staining.
For double immunostaining of αENaC and SPC, lung tissue cryo sections were first stained with anti αENaC rabbit polyclonal antibodies, which are directed to the extracellular domain of αENaC (1∶100) under nonpermeabilizing conditions, permeabilized, and then stained with goat anti-SPC antibodies (1∶200), as described in SI Text. The immunostained lung tissues and cells were analyzed by confocal microscopy (Zeiss LSM).
Patch Clamp Recording in Primary Cultured Alveolar Type II Cells.
Conventional whole-cell recordings were performed on isolated type II cells in the presence/absence of low doses of amiloride (0.3, 1 or 10 μM, Sigma), or the amiloride analog benzamil (0.1 or 1 μM, Sigma) that is highly specific for ENaC, as detailed in SI Text.
Amiloride Treatment of Young Pups.
Amiloride [1 μL/g body weight of 10 mM solution (i.e., 10 nmol/g)], or 1 μL/g body weight water, was administered to young pups via nasal instillation three times daily for 10 d (days 1–10), exactly as described (31). Mice were sacrificed on day 11, and lungs were analyzed by H&E staining, as above.
Supplementary Material
Acknowledgments.
We thank Dr. J. Whitsett (Cincinnati Children’s Hospital, Cincinnati, OH) for the SPC-rtTA;teto-Cre mice, Dr. G. Otulakowski, S. Gagnon, B. Hesse-Niessen, S. Wenger, S. Thiel, F. Benseler, I. Thanhäuser, D. Schwerdtfeger, and A. Zeuch for technical help, and Drs. E. Cutz, T. Gonska, M. Post, E. Herzog, and C. Bear for very helpful advice. This work was supported by the Canadian Institute of Health Research (D.R., W.Y.L., and M.S.), the Canadian CF Foundation (D.R. and W.Y.L.), the Max Planck Society (N.B.), and the German Research Foundation (Grant FZT103 to N.B.). H.K. is supported by the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad, the Yamanouchi Foundation for Research on Metabolic Disorders, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research. W.Z. is a recipient of University of Toronto Center for Study of Pain Clinician Scientist Trainee Award. D.R. and M.S. hold Canada Research Chair (Tier I) awards, and M.S. is an international Research Scholar of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010334108/-/DCSupplemental.
References
- 1.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 3.Guggino WB. Cystic fibrosis and the salt controversy. Cell. 1999;96:607–610. doi: 10.1016/s0092-8674(00)80570-x. [DOI] [PubMed] [Google Scholar]
- 4.Tarran R, et al. The CF salt controversy: In vivo observations and therapeutic approaches. Mol Cell. 2001;8:149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 5.Boucher RC. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 6.Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 7.Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canessa CM, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 9.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 10.Staub O, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 11.Kamynina E, Debonneville C, Bens M, Vandewalle A, Staub O. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J. 2001;15:204–214. doi: 10.1096/fj.00-0191com. [DOI] [PubMed] [Google Scholar]
- 12.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: Functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 13.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 14.Abriel H, et al. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle’s syndrome. J Clin Invest. 1999;103:667–673. doi: 10.1172/JCI5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic. 2007;8:1246–1264. doi: 10.1111/j.1600-0854.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- 16.Kabra R, Knight KK, Zhou R, Snyder PM. Nedd4-2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. J Biol Chem. 2008;283:6033–6039. doi: 10.1074/jbc.M708555200. [DOI] [PubMed] [Google Scholar]
- 17.Pradervand S, et al. A mouse model for Liddle’s syndrome. J Am Soc Nephrol. 1999;10:2527–2533. doi: 10.1681/ASN.V10122527. [DOI] [PubMed] [Google Scholar]
- 18.Smith DE, et al. Epithelial Na(+) channel (ENaC) expression in the developing normal and abnormal human perinatal lung. Am J Respir Crit Care Med. 2000;161(4, Pt 1):1322–1331. doi: 10.1164/ajrccm.161.4.9905064. [DOI] [PubMed] [Google Scholar]
- 19.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hummler E, et al. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 21.Itani OA, Campbell JR, Herrero J, Snyder PM, Thomas CP. Alternate promoters and variable splicing lead to hNedd4-2 isoforms with a C2 domain and varying number of WW domains. Am J Physiol-Renal. 2003;285:F916–929. doi: 10.1152/ajprenal.00203.2003. [DOI] [PubMed] [Google Scholar]
- 22.Itani OA, Stokes JB, Thomas CP. Nedd4-2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol-Renal. 2005;289:F334–346. doi: 10.1152/ajprenal.00394.2004. [DOI] [PubMed] [Google Scholar]
- 23.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 25.Perl AK, Zhang L, Whitsett JA. Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol. 2009;40:1–3. doi: 10.1165/rcmb.2008-0011ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan TZ, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 27.Cohen JC, Larson JE. Pathophysiologic consequences following inhibition of a CFTR-dependent developmental cascade in the lung. BMC Dev Biol. 2005;5:2. doi: 10.1186/1471-213X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi PP, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol-Renal. 2008;295:F462–470. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias N, et al. The role of alpha-, beta-, and gamma-ENaC subunits in distal lung epithelial fluid absorption induced by pulmonary edema fluid. Am J Physiol-Lung C. 2007;293:L537–545. doi: 10.1152/ajplung.00373.2006. [DOI] [PubMed] [Google Scholar]
- 30.Demaio L, et al. Characterization of mouse alveolar epithelial cell monolayers. Am J Physiol-Lung C. 2009;296:L1051–1058. doi: 10.1152/ajplung.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, et al. Preventive but not late amiloride therapy reduces morbidity and mortality of lung disease in betaENaC-overexpressing mice. Am J Respir Crit Care Med. 2008;178:1245–1256. doi: 10.1164/rccm.200803-442OC. [DOI] [PubMed] [Google Scholar]
- 32.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 33.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 34.Caohuy H, Jozwik C, Pollard HB. Rescue of DeltaF508-CFTR by the SGK1/Nedd4-2 signaling pathway. J Biol Chem. 2009;284:25241–25253. doi: 10.1074/jbc.M109.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotorashvili A, Russo SJ, Mulugeta S, Guttentag S, Beers MF. Anterograde transport of surfactant protein C proprotein to distal processing compartments requires PPDY-mediated association with Nedd4 ubiquitin ligases. J Biol Chem. 2009;284:16667–16678. doi: 10.1074/jbc.M109.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conkright JJ, et al. Nedd4-2-mediated ubiquitination facilitates processing of surfactant protein-C. Am J Respir Cell Mol Biol. 2009;42:181–189. doi: 10.1165/rcmb.2009-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.