Abstract
Sex differences in cocaine’s mechanisms of action and behavioral effects have been widely reported. However, little is known about how sex influences intracellular signaling cascades involved with drug-environment associations. We investigated whether ERK/CREB intracellular responses in the mesocorticolimbic circuitry underlying cocaine environmental associations are sexually dimorphic. We used a standard 4 day conditioned place preference (CPP) paradigm using 20mg/kg cocaine—a dose that induced CPP in male and female Fischer rats. In the nucleus accumbens (NAc) following CPP expression, cocaine treated animals showed increased phosphorylated ERK (pERK), phosphorylated CREB (pCREB) and ΔFosB protein levels. In the hippocampus (HIP) and caudate putamen (CPu), pERK and FosB/ΔFosB levels were also increased, respectively. Cocaine females had a larger change in HIP pERK and CPu ΔFosB levels than cocaine males; partly due to lower protein levels in saline female rats when compared to saline males. Prefrontal cortex (PfC) pCREB levels increased in cocaine males, but not females, whereas PfC pERK levels were increased in cocaine females, but not males. CPP scores were positively correlated to NAc pERK, HIP pERK and CPu FosB protein levels, suggesting that similar to males, the ERK/CREB intracellular pathway in mesocorticolimbic regions undergoes cocaine induced neuroplasticity in female rats. However, there seem to be intrinsic (basal) sexual dimorphisms in this pathway that may contribute to responses expressed after cocaine-CPP. Taken together, our results suggest that cellular responses associated with the expression of learned drug-environment associations may play an important role in sex differences in cocaine addiction and relapse.
Keywords: cocaine, CPP, ERK, CREB, FosB/ΔFosB, sex differences
1. Introduction
Addiction studies consistently show greater responses among females than males in various cocaine-related outcomes. As more attention is paid to sex-specific and hormonal effects on cocaine abuse, it is increasingly apparent that sex differences are present at all phases of drug abuse, from initiation through escalation of use and progression to addiction. For example, human females report a slower onset of the subjective effects of cocaine, undergo shorter periods of abstinence between cocaine use and experience cravings after cocaine use when presented with cocaine-associated cues more frequently than males (Anker and Carroll, 2011; Elman et al. 2001; Lynch, 2006; Quinones-Jenab and Jenab, 2010). Female rats learn to self-administer cocaine faster (Lynch and Carroll, 1999) and exhibit enhanced locomotor activity and behavioral sensitization to both acute and chronic cocaine administration compared to males (Chin et al. 2001; Craft and Stratmann, 1996; Festa et al. 2004; Sell et al. 2000; Van Haaren and Meyer, 1991). Still to be determined is the contribution of sex differences in central nervous system plasticity and cellular responses for the development of learned drug-environment associations that play an important role in addiction.
Sex differences in the mesocorticolimbic reward circuitry, including dopamine receptor (DAR) distribution, dopamine (DA) binding properties and intracellular signaling has been postulated to underlie sexually dimorphic responses to cocaine (Becker and Hu, 2008; Festa et al. 2006; Walker et al. 2006). Dopaminergic neurons of the ventral tegmental area (VTA) project to the nucleus accumbens (NAc) and form connections with the hippocampus (HIP), striatum (including the NAc and caudate putamen (CPu)) and prefrontal cortex (PfC) (Hyman and Malenka, 2001). Convergent glutamatergic circuitry is embedded within the reward system and overlap with the circuitry associated with learning and memory processes (Kelley, 2004). Conditioned place preference (CPP) behavioral models exploit this circuitry by using a Pavlovian conditioning procedure to determine the importance of environmental stimuli in cueing drug reward (Bardo and Bevins, 2000; Domjan, 2005). Female rats express cocaine-induced CPP in response to lower cocaine doses (Russo et al. 2003a; Zakharova et al. 2009) and after fewer cocaine-place pairings than males (Russo et al. 2003a). Differences in DAR sensitivity may underlie sex differences in cocaine CPP and the formation of cocaine environment associations. For example,Nazarian et al. (2004) found that regardless of sex, lower doses of a D1DAR antagonist blocked cocaine CPP acquisition, whereas higher doses of the antagonist only blocked CPP in males. However, the extent to which downstream molecular signaling in response to sexually dimorphic cocaine induced changes in DA activity contribute cocaine CPP remains to be determined.
Acute and chronic cocaine administration induces the phosphorylation of ERK, a signaling molecule of the mitogen activated protein kinase (MAPK) signal transduction family, in a DA dependent manner (Berhow and Nestler, 1996; Jenab et al. 2005; Sun et al. 2008). In male rats, ERK has been repeatedly implicated in the acquisition of psychostimulant-induced CPP and retrieval of cocaine-environment memories (Gerdjikov et al. 2004; Liu et al. 2011; Miller and Marshall, 2005; Pan et al. 2011; Valjent et al. 2006). Cocaine-induced ERK phosphorylation produces rapid increases in membrane excitability that after repeated exposure lead to long term changes in protein and gene expression associated with signaling reward (Lu et al. 2006; Nestler, 2001). Downstream of ERK, cAMP response element binding protein (CREB), FosB and ΔFosB are transcription factors associated with experience-dependent synaptic plasticity and long-term molecular neuroadaptations in response to cocaine (Carlezon et al. 2005; Larson et al. 2010; Marazziti et al. 2011; Zhang et al. 2006). In male rats, acute cocaine exposure increases striatal CREB phosphorylation and FosB protein levels after cocaine CPP expression and chronic cocaine exposure produces a persistent accumulation of NAc ΔFosB levels (Harris et al. 2007; McClung and Nestler, 2003; Nestler et al. 2001;Rawas et al. 2012; Tropea et al. 2008).
Sex differences in DA response to cocaine combined with the substantial evidence for the important role of ERK, CREB, and Fos proteins in cocaine CPP in males suggest that sex differences may underlie the formation of drug associated memories. However, to our knowledge, studies that have used the CPP paradigm to investigate molecular alterations involved with cocaine-context associations have only used male rats. We aimed to investigate the potential sex differences in the intracellular signaling molecules underlying the expression of cocaine environment associations. Specifically, we examined phosphorylated ERK (pERK), phosphorylated CREB (pCREB), FosB, and ΔFosB protein levels in mesocorticolimbic regions associated with reward, learning, and memory (NAc, CPu, PfC, and HIP), after cocaine CPP expression in male and female rats. We hypothesized that cocaine CPP expression would be associated with similar changes in protein levels in male and female cocaine treated rats. We also expected any sex differences in protein levels to be correlated with sex differences in the magnitude of CPP behavior.
2. Results
2.1. CPP behavior
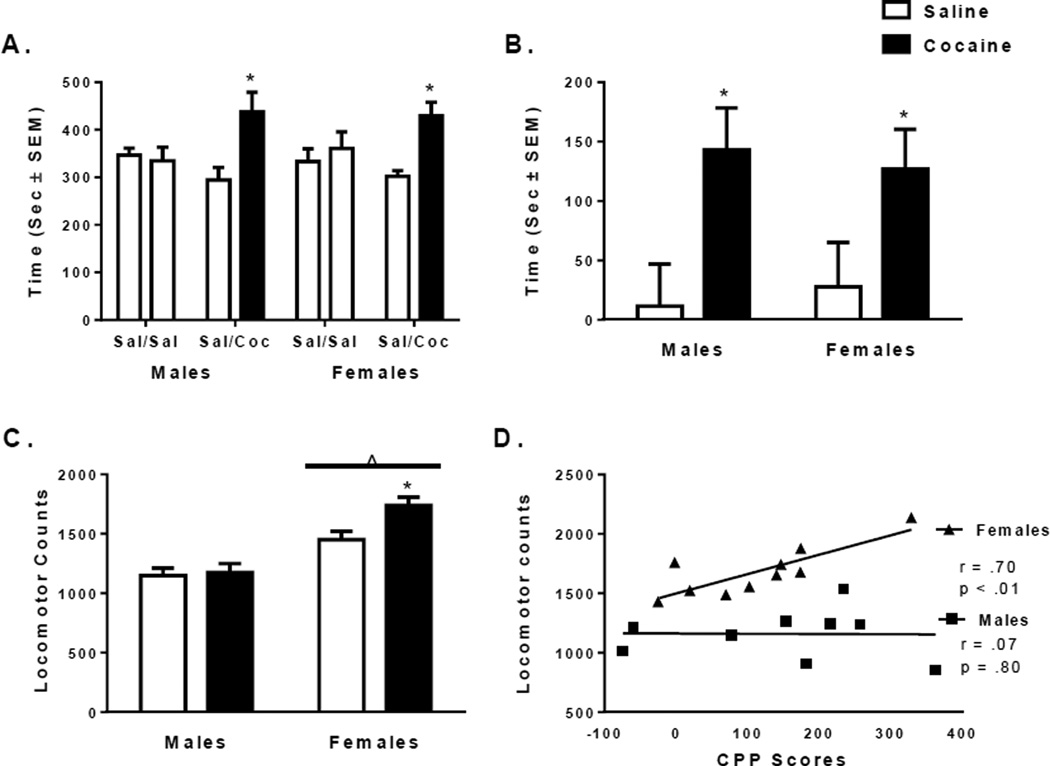
No differences in any treatment group were observed during the initial preconditioning test in the time spent in each chamber or in total locomotor behavior, confirming the unbiased nature of the CPP apparatus and testing protocol (data not shown). During CPP testing, cocaine treated rats spent significantly more time in the cocaine paired chamber than the saline paired chamber [male: t(8) = 2.55, p < 0.05; female: t(9) = 3.4, p < 0.05; Figure 1A]. Only cocaine treated female rats showed significant increases in explorations and entrances into the cocaine paired chamber compared to the saline paired chamber [explorations: t(9) = 3.7, p < 0.01; entrances: t(9) = 2.6, p < 0.05; Table 1]. A significant main effect of treatment was seen on the magnitude of CPP scores [F(1,35) = 30.15, p < 0.01; Figure 1B]. However, no sex differences in the magnitude of CPP scores were seen [F(1,35) = 1.49, p = 0.23].
Figure 1.
CPP and locomotor responses in male and female rats during testing after conditioning with 20mg/kg cocaine. (A) Average time spent (in seconds ± SEM) in the saline paired chamber compared to the cocaine paired chamber (B) CPP scores and (C) total locomotor activity in saline (white bars) and cocaine (black bars) treated males and females (n = 8–10 rats per group). *Indicates significant difference from saline controls of the same sex at p < 0.05. ^Indicates significant main effect of sex at p < 0.05. (D) Correlation between CPP scores and total locomotor activity during the CPP test in cocaine treated male (squares) and female (triangles) rats. The Pearson Correlation coefficients and p-values are displayed within the plot.
Table 1.
Cocaine effects on entrances and explorations during CPP test
| Entrances |
Explorations |
||||
|---|---|---|---|---|---|
| Saline-paired | Cocaine-paired | Saline-paired | Cocaine-paired | ||
| Male | Saline | 94.2±7.8 | 83.3±6.8 | 69.9±7.3 | 71.2±2.9 |
| Cocaine | 73.2±8.2 | 83.1±8.4 | 69.9±3.3 | 73.2±5.5 | |
| Female | Saline | 93.0±11 | 113±11.7 | 72.8±5.5 | 64.5±3.7 |
| Cocaine | 98.8±5.4 | 124.5±12.2* | 61.9±3.6 | 84.6±3.5* | |
Data are shown as mean (±SEM) number of entrances and explorations while in the saline-paired and cocaine-paired chambers during CPP testing (n = 8–10 animals per group).
Indicates significant difference from saline-paired chamber at p < .05.
A significant main effect of sex on total locomotor responses was observed [F(1,35) = 40.20, p < .01; Figure 1C]. Regardless of treatment, females were more active than males [p < 0.05 for all comparisons]. A significant interaction between sex and treatment was also seen [F(1,35) = 4.81, p < 0.05]. Cocaine treated females displayed more locomotor counts than males [F(1,35) = 34.58, p < 0.001] and female saline controls [F(1,35) = 11.01, p < 0.01; Figure 1C]. Additionally, total locomotor responses were significantly correlated to CPP scores in female rats [r = 0.70, p < 0.01] but not in males [r = 0.07, p = 0.80; Figure 1D].
2.2. ERK phosphorylation in the NAc, CPu, PfC, and HIP in male and female rats
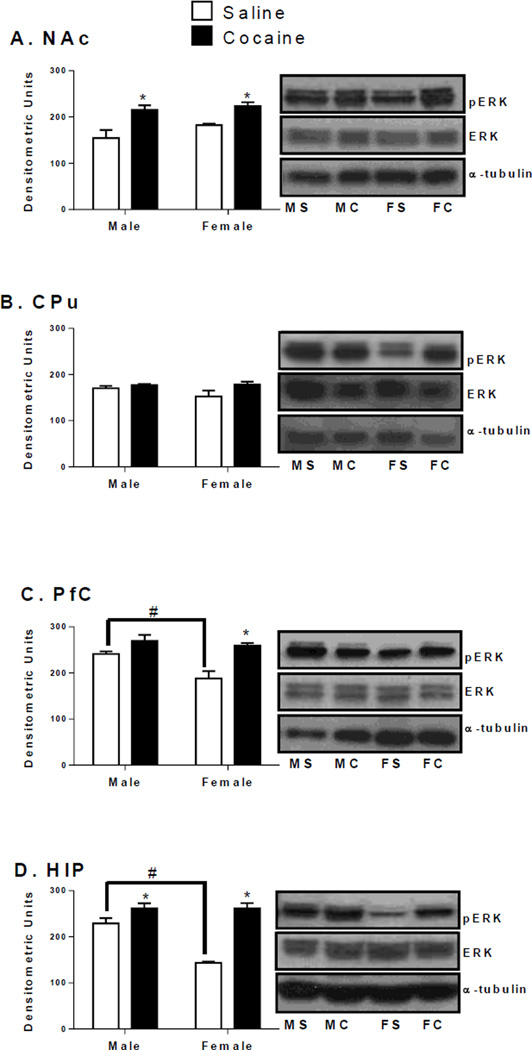
In the NAc, CPu, PfC and HIP no differences in total ERK or CREB protein levels were seen (Figure 2–4; Table 2). However, a main effect of treatment on NAc pERK protein levels was observed [F(1,12) = 15.83, p < 0.01; Figure 2A]. Regardless of sex, NAc pERK protein levels increased in cocaine treated rats [males: t(6) = 4.3, p < .01; females t(6) = 3.04, p < 0.05]. CPu pERK levels did not change based on sex or treatment (Figure 2B).
Figure 2.
Phosphorylated ERK1/2 protein levels (measured at 44/42 kDa) in the NAc (A), CPu (B), PfC (C), and HIP (D) of male and female rats after CPP testing. Phosphorylated protein levels are expressed as a ratio to their respective total protein levels (normalized to a tubulin, 55 kDa)(±SEM) (n = 4 animals per group). *Indicates significant difference from saline controls of the same sex at p < 0.05. #Indicates significant difference between male and female saline animals at p < 0.05.
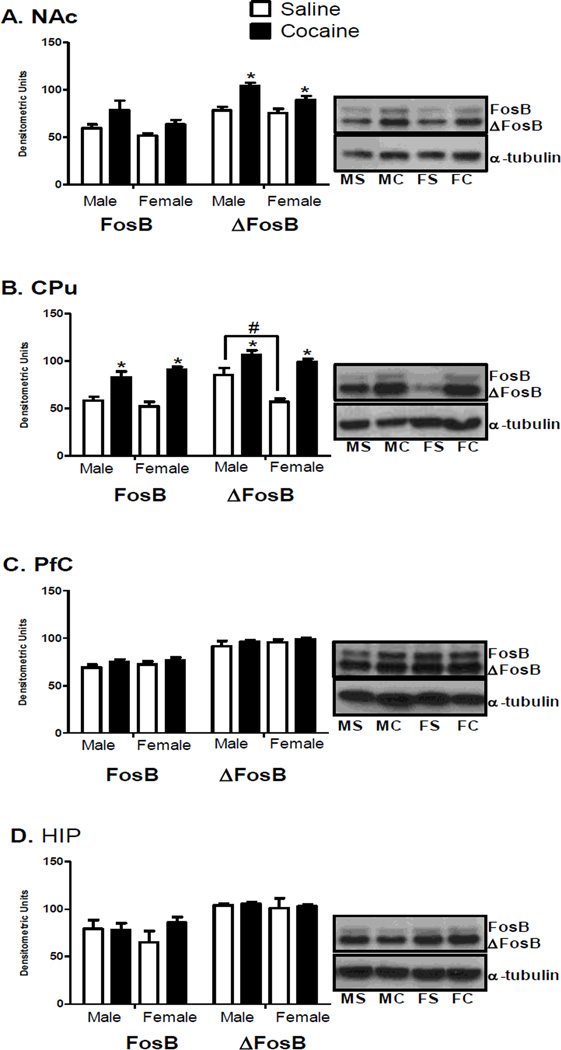
Figure 4.
FosB (left 4 bars, top band: 48 kDa) and ΔFosB (right 4 bars, bottom band: 38 kDa) protein levels in the NAc (A), CPu (B), PfC (C), and HIP (D) of male and female saline and cocaine rats after CPP testing. Protein levels are expressed as a ratio to their respective a tubulin levels (55 kDa) (±SEM) (n = 4 animals per group). *Indicates significant difference from saline controls of the same sex at p < 0.05. #Indicates significant difference between male and female saline animals at p < 0.05.
Table 2.
Total ERK and CREB protein levels after CPP expression
| Male | Female | ||||
|---|---|---|---|---|---|
| Protein | Saline | Cocaine | Saline | Cocaine | |
| ERK | |||||
| NAc | 87.2±2.9 | 89.4±4.7 | 88.0±3.6 | 84.8±1.9 | |
| CPu | 111.8±3.0 | 109.8±1.8 | 109.7±1.9 | 109.3± 3.4 | |
| PfC | 74.1±3.9 | 71.9±3.5 | 66.3±1.9 | 72.4±2.1 | |
| HIP | 75.4±2.2 | 82.9±6.3 | 81.4±3.5 | 77.5±3.1 | |
| CREB | |||||
| NAc | 94.8±8.4 | 85.2±5.1 | 77.8±4.9 | 76.4±5.7 | |
| CPu | 119.9±10 | 118.3±8.5 | 117.0±6 | 119.7±6.5 | |
| PfC | 112.2±5.9 | 104.2±3.4 | 100.8±2.3 | 109.1±3.3 | |
| HIP | 107.6±8.8 | 116.9±8.5 | 131.2±12.8 | 120.3±11.3 | |
Data are shown as mean arbitrary densitometric units (±SEM) normalized to α-tubulin (n = 4 animals per group). No significant differences were observed based on treatment or sex.
In the PfC, a significant main effect of sex on pERK levels was observed [F(1,12) = 8.2, p < 0.05; Figure 2C]. However, this was partly due to sex differences in saline controls; pERK levels in saline males were significantly higher than saline females [t(6) = 3.1, p < 0.05]. On PfC pERK levels, a significant main effect of treatment was also observed [F(1,12) = 20.2, p < 0.01; Figure 2C]. PfC pERK levels were higher in cocaine females than saline females [t(6) = 4.2, p < 0.01]. Likewise, cocaine females displayed a greater magnitude of change in PfC pERK levels than cocaine males [t(6) = 4.3, p < 0.01; Table 3].
Table 3.
pERK, pCREB, FosB and ΔFosB protein levels expressed as a percent of saline controls after CPP expression in cocaine-treated rats
| pERK | pCREB | FosB | ΔFosB | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| NAc | 112.8±1.6 | 122.9±3.8 | 118.1±3.7 | 118.4±5.3 | 112.8±7.4 | 110.9±13.7 | 138.2±11.1 | 17.8±4.2 |
| CPu | 103.9±2.8 | 124.3±3.1 | 104.6±8.8 | 94.2±4.7 | 142.0±10.3 | 173.3±6.1# | 124.9±5.4 | 173.8±6.2# |
| PfC | 111.7±5.4 | 138.1±3.0 | 122.6±6.6 | 104.3±3.1 | 108.1±4.2 | 106.0±3.8 | 105.6±1.3 | 103.2±1.9 |
| HIP | 122.0±3.2 | 166.9±8.3# | 100.8±8.5 | 108.1±8.6 | 98.4±9.5 | 118.6±17.1 | 101.2±2.1 | 101.7±2.3 |
Data are presented as densitometric units expressed as a mean percentage of saline controls of the respective sex which were set to 100% (± SEM) (n = 4 animals per group). Bold values indicate a significant difference between cocaine treated males and females (p < .05). Bold
indicates cases in which a significant (p < .05) difference between saline and cocaine animals was observed prior to expressing data as a percent of saline.
In the HIP, a significant interaction between sex and treatment on pERK protein levels was obtained [F(1,11) = 16.1, p < 0.01; Figure 2D]. HIP pERK levels were significantly higher in cocaine treated females and cocaine treated males than saline controls of their respective sex [males: F(1,11) = 5.16, p < 0.05; females: F(1,11) = 28.96, p < 0.01]. Furthermore, HIP pERK levels were significantly higher in saline treated males than saline treated females [F(1,11) = 23.03, p < 0.01; Figure 2D]. After cocaine CPP expression, sex differences were seen in the magnitude of change in HIP pERK levels; cocaine treated females had a larger magnitude of change from saline than cocaine males [t(6) = 5.04, p < 0.01; Table 3].
2.3. CREB phosphorylation in the NAc, CPu, PfC, and HIP of male and female rats
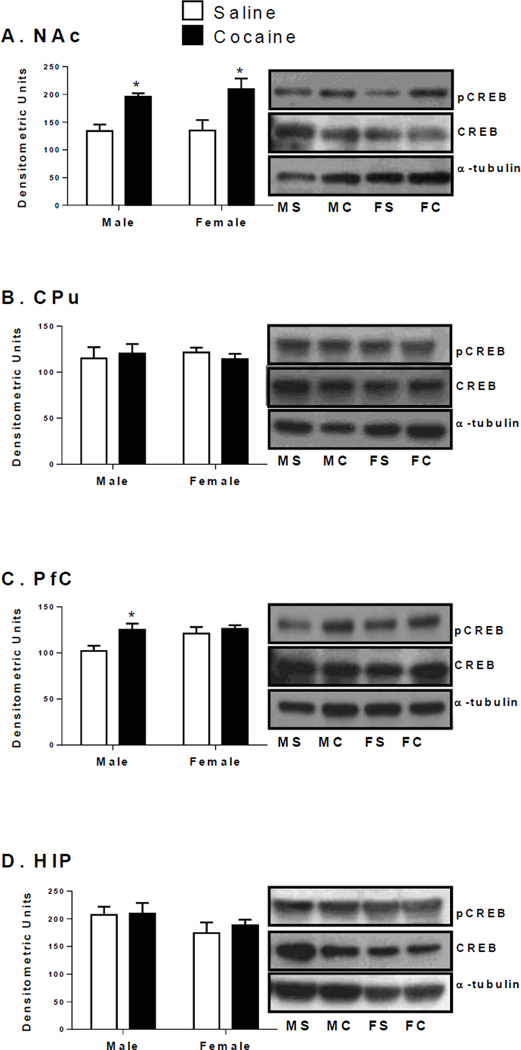
In the NAc, a significant main effect of treatment on pCREB levels was observed [F(1,12) = 20.44, p < 0.01; Figure 3A]; pCREB levels were higher in cocaine animals than saline controls [males: t(6) = 4.7, p < 0.01; females: t(6) = 2.7, p < 0.05]. Similarly, a significant main effect of treatment on PfC pCREB levels was observed [F(1,12) = 5.8, p < 0.05; Figure 3C]. However, only cocaine treated males had significantly higher PfC pCREB levels than saline males [t(6) = 2.6, p < 0.05]. The magnitude of change in PfC pCREB levels was greater in cocaine treated males than cocaine females, [t(6) = 2.5, p < 0.05; Table 3]. No changes were seen in CPu or HIP pCREB levels (Figure 3B and 3D).
Figure 3.
Phosphorylated CREB protein levels (measured at 46 kDa) in the NAc (A), CPu (B), PfC (C), and HIP (D) of male and female rats after CPP testing. Phosphorylated protein levels are expressed as a ratio to their respective total protein levels (normalized to a tubulin, 55 kDa)(±SEM) (n = 4 animals per group). *Indicates significant difference from saline controls of the same sex at p < 0.05.
2.4. Fos/AFosB levels in the NAc, CPu, PfC, and HIP in male and female rats
In the NAc, a significant main effect of treatment on ΔFosB levels was observed [F(1,11) = 22.8, p < 0.01; Figure 4A]. In both male and female cocaine treated rats, NAc ΔFosB levels were higher than their respective saline controls [males: t(6) = 4.9, p < 0.01; females: t(5) = 2.9, p < 0.05; Figure 4A]. NAc FosB levels did not significantly differ (Figure 4A).
In the CPu, a significant main effect of treatment was seen for FosB levels [F(1,12) = 46.44, p < 0.01; Figure 4B]. FosB levels were significantly increased in both cocaine males and females compared to saline control animals [males: t(6) = 3.4, p < 0.01; females: t(6) = 6.7, p < 0.01; Figure 4B]. The magnitude of change in CPu FosB levels in cocaine treated rats was significantly greater in females than males [t(6) = −2.6, p < 0.05; Table 3]. A significant main effect of treatment on CPu ΔFosB protein levels was observed [F(1,12) = 41.01, p < 0.01]. In both sexes, ΔFosB protein levels were increased compared to saline animals [males: t(6) = 2.5, p < 0.05; females: t(6) = 8.6, p < 0.01]. A significant main effect of sex on CPu ΔFosB levels was also observed [F(1,12) = 13.6, p < 0.01; Figure 4B]. Saline treated males had higher ΔFosB levels than saline females [t(6) = 3.6, p < 0.05]. The magnitude of change in ΔFosB protein levels in cocaine females was significantly greater than in cocaine treated males [t(6) = −5.9, p < 0.01; Table 3]. No changes were seen in PfC or HIP FosB/ΔFosB levels [Figure 4C and 4D].
2.5. Correlations between CPP behavior and phosphorylated protein levels
As shown in Table 4 (See Figure 5 for sample plot), CPP scores were significantly correlated to NAc pERK, PfC pERK and CPu FosB in male rats [NAc pERK: r = 0.79; PfC pERK: r = 0.86; CPu FosB: r = 0.76; p < 0.05 for all comparisons]. Similarly, CPP scores were significantly correlated to NAc pERK, PfC pERK and CPu FosB in female rats [NAc pERK: r = 0.81; PfC pERK: r = 0.76; CPu FosB: r = 0.76; p < 0.05 for all comparisons]. In female rats, significant correlations were also seen between CPP scores and levels of HIP pERK, NAc pCREB and CPu ΔFosB [HIP pERK: r = 0.70; NAc pCREB: r = 0.78; CPu ΔFosB: r = 0.77; p < 0.05 for all comparisons]. In males, CPP scores were significantly correlated with NAc ΔFosB levels [r = 0.76, p < 0.05]. No significant correlations were found between locomotor responses and phospho-protein levels changed by cocaine (See Table 5).
Table 4.
Pearson correlation coefficients (r) for CPP scores and phospho-protein levels
| pERK | pCREB | FosB | ΔFosB | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| NAc | 0.79 | 0.81 | 0.51 | 0.78 | 0.29 | 0.23 | 0.76 | 0.34 |
| CPu | 0.39 | 0.08 | −0.04 | −0.22 | 0.76 | 0.76 | 0.37 | 0.77 |
| PfC | 0.86 | 0.76 | 0.35 | 0.01 | −0.03 | 0.35 | −0.08 | 0.22 |
| HIP | 0.67 | 0.70 | −0.02 | 0.65 | −0.15 | −0.17 | 0.15 | 0.03 |
Correlations were assessed between CPP scores and protein levels normalized to saline controls of the respective sex (n = 4 animals per group). Bold values indicate a significant correlation at p < 0 .05. See Figure 5 for a representative plot.
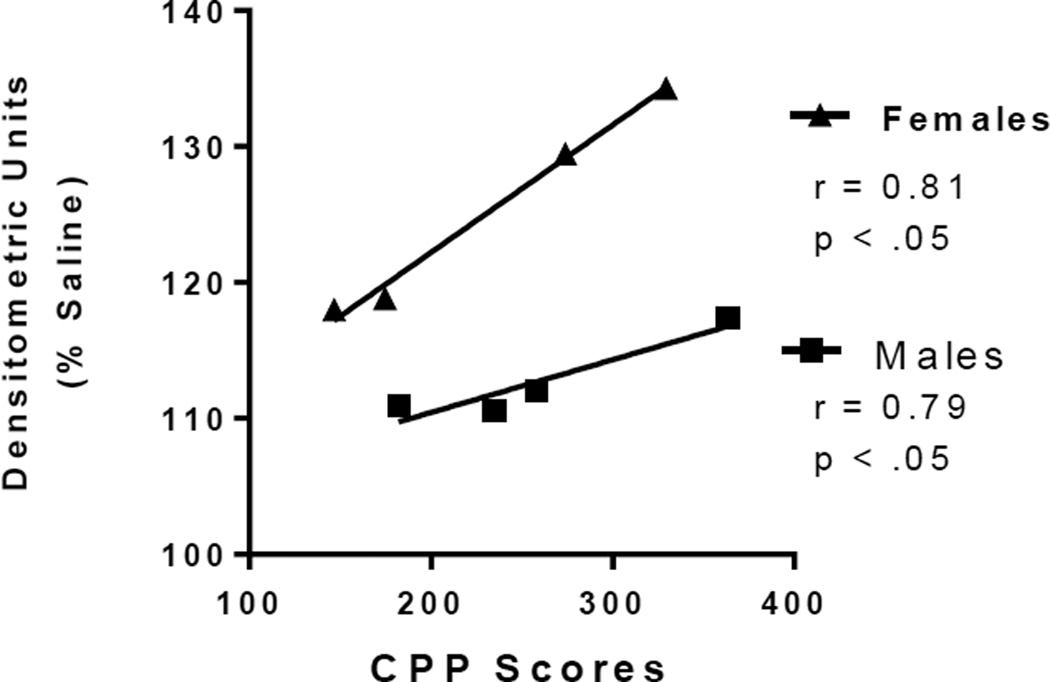
Figure 5.
Representative scatter plot of correlations between CPP scores and phosphoprotein levels. NAc pERK protein levels (expressed as a percentage of saline controls) were significantly correlated to CPP scores in both male (squares) and female (triangles) cocaine treated rats (n = 4 animals per group). The Pearson correlation coefficients and p-values are displayed within the plot.
Table 5.
Pearson correlation coefficients (r) for locomotor responses and phospho-protein levels
| pERK | pCREB | FosB | ΔFosB | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| NAc | 0.22 | −0.09 | 0.34 | 0.26 | −0.43 | −0.69 | 0.42 | −0.05 |
| CPu | 0.82 | 0.09 | −0.76 | −0.10 | 0.20 | −0.12 | 0.09 | −0.09 |
| PfC | 0.32 | 0.35 | −0.23 | 0.52 | −0.38 | −0.07 | −0.35 | −0.38 |
| HIP | −0.02 | −0.30 | −0.06 | 0.54 | −0.52 | 0.03 | −0.34 | −0.58 |
Correlations were assessed between total locomotor counts during the CPP test and protein levels normalized to saline controls of the respective sex (n = 4 animals per group). Bold values indicate a significant correlation at p < 0.05.
3. Discussion
Although both male and female rats displayed similar levels of CPP, some components of these behavioral responses were sexually dimorphic. Females displayed greater total locomotor responses during the CPP test than males, regardless of treatment. However, cocaine females exhibited greater motor responses, including entrances and explorations of the cocaine-paired chamber in addition to total locomotor activity, when compared to males and saline controls. We also show that similar to males, females undergo changes in ERK/CREB molecular signaling after cocaine CPP expression and that some response patterns on this pathway are sexually dimorphic. Specifically, we showed that sex affects: (1) the location of these changes and (2) the magnitude of the changes in this pathway. The degree to which alterations in protein levels are due to CPP and/or locomotor responses is still unclear. However, we observed positive correlations between protein levels and CPP scores, but not between protein levels and locomotor activity. Additionally, in regions where cocaine induced protein level increases in both males and females compared to saline controls, we saw no sex differences in phosphoprotein levels in cocaine rats. Instead, we observed region specific sex differences in phosphoprotein levels between saline treated males and females, resulting in sex differences in the magnitude of change from saline in cocaine rats. Therefore, we postulate that sex differences in basal levels in the ERK/ΔFosB cascade contribute to these changes.
The increased NAc pERK levels and positive correlation to CPP scores in cocaine treated males and females was expected and is consistent with previous literature showing that NAc ERK phosphorylation is associated with the expression of cocaine CPP (Miller and Marshall 2004, 2005; Valjent et al. 2000, 2004). Increases in NAc pERK corresponded with increased NAc pCREB and ΔFosB protein levels. Increased ΔFosB is consistent with previous research that shows ΔFosB accumulates in the NAc after cocaine exposure and enhances cocaine reward (Nestler, 2005; Rawas et al. 2012). It remains to be elucidated whether the observed increases in ΔFosB protein levels were in response to the cocaine associated context or were a lingering result of cocaine exposure. Due to the short time course of acute cocaine-induced phosphorylation of ERK, CREB, and FosB, we postulate that increases in pERK, pCREB, and FosB levels probably reflect conditioned responses to the cocaine paired environment in males and females (Girault et al. 2007; Harris et al. 2007; Kuo et al. 2007; Rawas et al. 2012; Sun et al. 2008). Since we did not test a cocaine treated control group in an unpaired context, we cannot rule out the possibility that these changes were due to cocaine alone and not to cocaine-environment associations. However, an overall limitation of the CPP paradigm is that even with the use of an un-paired control group, stimuli in the testing environment may potentially become indirectly associated with cocaine effects and therefore mask differences between paired and unpaired groups (Cunningham et al. 2011).
Studies that report cocaine induced CPP and increased locomotor responses during CPP testing have been shown with various cocaine doses (Bobzean et al. 2010; Russo et al. 2003a,b; Zakharova et al. 2009). In the present study, CPP scores were positively correlated to total locomotor responses in females, but not males. Previous research in male rats has reported conflicting evidence for a correlation between locomotor responses and cocaine CPP (Allen et al. 2007; Kosten and Miserendino, 1998), and others suggest that individual differences may account for differences in psychostimulant locomotor activity and CPP behavior (Mathews et al. 2010; Seymour and Wagner, 2008). Associations between activity and CPP may be more pronounced in females possibly due to individual differences and/or gonadal hormone levels, but this postulate requires further study. Although numerous studies report the effects of gonadal hormones on CPP (Reviewed in Anker and Carroll, 2011; Festa and Quinones-Jenab, 2004), it is unknown whether estrous cycle stage affects CPP behavior. Because we did not observe differences across groups in behavioral and locomotor responses prior to conditioning and our female behavioral and molecular data are no more variable than our male data, it is likely not the case that differences in estrous cycle stage are hiding or increasing the changes in behavior or protein phosphorylation reported here. However, some cocaine-induced phospho-protein levels have been shown to vary based on estrous cycle stage. For example, previous research shows that NAc pCREB increased 15 minutes after cocaine exposure in estrus females, but no basal differences in NAc or CPu pCREB or ΔFosB were seen based on cycle stage (Weiner et al. 2009). Therefore, our results are likely not due to estrous cycle effects, but more likely due to sex differences in neural organization utilizing sex specific intracellular responses.
Our results are consistent with Sato et al. (2011), in which chronic cocaine treatment resulted in more locomotor responses and a greater magnitude change in CPu FosB/ΔFosB in females than males. Contrary toSato et al. (2011), we saw no sex difference in cocaine induced NAc ΔFosB increase, which may be because of the difference in cocaine-treatment regimens used.Sato et al. (2011) report these differences in NAc and CPu ΔFosB levels after chronic cocaine administration, whereas animals in our study were only treated with cocaine twice. These results suggest region specific sexually dimorphic responses to cocaine after CPP expression. In males, cocaine induced ERK phosphorylation regulates ΔFosB accumulation underlying long-term changes in synaptic plasticity associated with addiction (Radwanska et al. 2006; Valjent et al. 2000). We report that although cocaine females’ displayed a greater increase in CPu ΔFosB levels than males, the resulting protein levels were similar in cocaine males and females. Although we did not demonstrate a direct correlation between locomotor behavior and CPu ΔFosB levels, locomotor responses and CPu ΔFosB levels were significantly correlated to CPP scores in cocaine females, but not in males. In addition to cocaine reward, increased ΔFosB has been associated with cocaine seeking behaviors and cocaine induced locomotor responses (Kelz et al. 1999). It is therefore reasonable to postulate that our observation of females enhanced responding during the CPP test may be related to their larger increase in CPu ΔFosB levels. To what extent differences in locomotor behavior during CPP testing are a component of the conditioned response to cocaine and thus a sex difference in cocaine-induced conditioned activity is yet to be determined.
In addition to enhanced locomotor activity,Nazarian et al. (2009) found that in female rats, cocaine-induced CREB phosphorylation was greater, but shorter lasting than in males, providing evidence that females’ enhanced locomotor response to cocaine may be due to a rapid increase and drop in pCREB. Our observed sex differences in protein levels could also reflect temporal sex differences in protein phosphorylation. Similarly, we found that PfC pERK levels were only increased in cocaine females and not in cocaine males, whereas PfC pCREB levels were increased in cocaine males, but not females. It is therefore possible that ERK phosphorylation in response to a cocaine-associated context may be faster acting in males than females, leading to a more rapid increase in pCREB levels, indicating potential sex differences in the time course of protein phosphorylation that underlie cocaine-cue-induced behaviors (Radwanska et al. 2006). PfC pERK and pCREB levels were positively correlated to CPP scores in males and females, an indication that in the PfC ERK/CREB responses induced after cocaine CPP expression are sexually dimorphic. Likewise, sexually dimorphic changes in DA activity and downstream molecular events have previously been seen in PfC responses to cocaine (Sun et al. 2010). Additionally, when HIP pERK and PfC pERK levels were lower in saline females than saline males, cocaine-induced increases in FosB/ ΔFosB were not observed. Thus, males and females show region specific variations in ERK/ΔFosB signaling related to cocaine/cocaine-cue induced behaviors associated with addiction (Becker et al. 2012).
3.1 Conclusions
The findings of the present study are consistent with most of the previous literature on sex differences in cocaine induced CPP behaviors. Our molecular results are also consistent with most of the previous literature associating changes in protein phosphorylation with cocaine CPP. Similar to males, we demonstrate that in female rats the ERK/CREB signaling cascade is modified after the expression of cocaine induced CPP. However, the NAc was the only brain region in which we observed corresponding increases in pERK, pCREB, and ΔFosB, regardless of sex. Therefore, results of studies that associate CPP behaviors in males with molecular or synaptic changes outside of the NAc should be cautious when extending these results to females. The changes in ERK/CREB/ΔFosB protein levels reported here reflect changes in signaling molecules associated with neuroplastic changes in mesolimbic circuitry. Our observation of the lack of sex differences in the NAc are in accordance with human fMRI studies showing similar patterns of NAc activation in cocaine dependent males and females in response to cocaine conditioned cues, while cocaine-cue induced activation of frontal regions differed based on sex (Kilts et al. 2004). Overall, our results suggest novel sexual dimorphisms in molecular alterations observed after cocaine CPP expression, likely due to sex differences present prior to cocaine exposure.
4. Methods
4.1 Animals
8 week old male and female Fischer rats (Charles River, Raleigh, NC) were maintained on a 12 hour light/dark cycle and individually housed with ad libitum access to food and water. Rats were randomly assigned to a saline only control group or a saline/cocaine treatment group for conditioning (n = 8–10 per group). Estrous cycle stage was not monitored because vaginal lavages attenuate cocaine induced locomotor behavior, produce CPP (Walker at al. 2002) and increase NAc ΔFosB (Reviewed in Robison and Nestler, 2011). Animal care and use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, Bethesda, MD, USA) and approved by the Hunter College, CUNY, Institutional Animal care and Use Committee.
4.2 CPP Apparatus
The CPP apparatus was purchased from Med Associates (Georgia, VT) and consisted of a rectangular cage divided into 3 chambers with different tactile and visual cues (2 conditioning chambers separated by a smaller, neutral chamber in the middle). The two conditioning chambers consisted of squares with 28cm in length walls. One had a stainless steel mesh floor with white walls and the other had a stainless steel grid rod floor with black walls. The middle chamber had grey walls and a smooth PVC floor and was 12cm long and 4cm wide. The walls of the middle chamber (and one wall of each conditioning chamber) had computer-automated guillotine doors that allowed free access to all three chambers when opened during preference testing. Chambers were equipped with a computerized photo-beam system that runs with MED PC software that recorded time spent in each chamber, total locomotor behavior (the sum of all horizontal counts), the total number of entrances to each chamber (multiple beams broken as the rat enters a chamber) and exploratory behavior (a single broken beam in the adjacent chamber without actually entering the chamber).
4.3 CPP procedure
The day before conditioning, rats were placed into the neutral middle chamber and allowed to explore all three chambers for 20 minutes. Rats were conditioned with 20 mg/kg cocaine, i.p., using a standard unbiased and counterbalanced 4-day CPP procedure consisting of alternating saline/cocaine injections over 4 days. On odd numbered days, rats were administered saline and confined to one of the conditioning chambers for 30 minutes. On even numbered days, rats were treated with cocaine and confined to the opposite chamber for 30 minutes. Control rats were administered saline in both chambers on alternate days. CPP testing was conducted 24 hours after the last conditioning session (drug-free), following the same procedure as the preconditioning test. Preliminary testing in our lab has shown that this paradigm produces equivalent CPP scores between sexes as compared to Russo et al. (2003a). The 2 major differences between our paradigm andRusso et al. (2003a) are: (1) animals were not handled daily for 30 minutes for a week prior to our testing; and (2) we did not begin the CPP test with a 5 minute acclimation period in the test apparatus before a 15 minute test. Pre-handling of rats has been shown to affect overall CPP scores in male rats (Cunningham et al. 2006; 2011).
4.4 Protein preparation
After the 20 minute CPP test (24 hours and 20 minutes after the last cocaine treatment), rats were briefly exposed to CO2 and rapidly decapitated. Brains were removed and flash frozen in 2methylbutane and stored at −80 °C. Tissue punches of the PfC, NAc, CPu, and HIP were simultaneously dissected on a cold glass plate. Tissue was homogenized with a Polytron handheld homogenizer (Kinematica, Luzern, Switzerland) in lysis buffer (50mM Tris-HCl, 150 mMNaCl, 2 mM EDTA, 10% Glycerol, 1% Triton X-100, 1% sodium deoxycholic acid) containing a phosphatase inhibitor cocktail. Homogenates were incubated for 30 minutes and centrifuged for 15 minutes (13,000 rpm, 4°C). Supernatants were stored at −80°C until used for western blot analysis.
4.5 Drugs and antibodies
Cocaine hydrochloride was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Primary antibodies for total ERK (#9102) and pERK (#9101), FosB (#2251) and total CREB (#9197) were purchased from Cell Signaling Technologies (Beverly, MA). The pCREB antibody (#06–519) was purchased from Millipore (Billerica, MA, USA). The Antibody for a-tubulin (#sc-8035) was purchased from Santa Cruz Technologies (Santa Cruz, CA). Horseradish peroxidaseconjugated anti-rabbit IgG (#NA-934) and anti-mouse (#NA-931) were obtained from Amersham Phamacia (Piscataway, NJ).
4.6 Protein measurement and Western Blot analysis
The total protein content was found using a Bradford kit from Bio-Rad laboratories (Hercules, CA). 30–50ug of protein extracts were boiled for 5 minutes in Lammeli buffer that contained 1% Beta-mercapthoethanol before being electrophoresed onto 10–12% Tris-HCl SDSPAGE gels and transferred onto PVDF membranes. Membranes were blocked at room temperature for 1 hour with 5% nonfat dry milk and Tris-Tween-20 Buffer (TBST; pH = 7.4). After three washes with TBST, membranes were incubated overnight individually at 4°C with the primary antibody for pCREB, FosB, or pERK (1:3000). Membranes were then washed with TBST 3 more times and incubated for 1 hour at room temperature with the appropriate secondary antibody (1:1000). Membranes were again washed in TBST 3 times before a chemiluminescence kit (ECL; Amersham Pharmacia, Piscataway, NJ, USA) was used to detect antibody binding. Membranes were then re-probed for a-tubulin and their respective total proteins (1:3000 or 1:500).
4.7 Data analysis
Paired-samples t-tests were used to assess differences in entrances, explorations, and the time spent in the cocaine and saline paired chambers for each group during CPP testing. Significant CPP behavior was defined as spending significantly more time in the cocaine paired chamber than the saline paired chamber during preference testing. To measure differences in the magnitude of CPP, preference scores were calculated by subtracting the time spent in the saline paired chamber from the time spent in the cocaine paired chamber during testing. Due to the unbiased conditioning procedures used in this study, we did not compare the time spent in the cocaine paired chamber to the time spent in that chamber during the pretest (Cunningham et al. 2006). Changes in the magnitude of CPP scores and total locomotor activity were assessed with 2 × 2 (sex × treatment) ANOVAs. To assess the relationship between CPP scores and locomotor activity during the CPP test, we conducted a Bivariate Pearson correlation analysis between CPP scores and locomotor activity in cocaine males and females (separately).
Films were scanned on an Epson Perfection v700 desktop scanner and Western blot band intensities were quantified with imageJ molecular quantifying software (NIH). Levels of pERK1/2 were quantified together. Total protein levels were normalized to their respective atubulin levels (as a loading control), and total protein levels were used for the normalization of phosphorylated protein levels (where appropriate) and expressed as arbitrary densitometric units. Changes in protein levels were assessed with 2 × 2 (sex by treatment) ANOVAs. Significant main effects were compared using unpaired t-tests with Bonferonni corrections (where appropriate) and significant interactions were followed by pairwise comparisons of simple main effects. To account for differences in protein phosphorylation between saline treated males and females, in a separate analysis, we assessed sex differences in the magnitude of change in protein phosphorylation from saline in cocaine treated rats by normalizing raw data to saline controls of the respective sex (arbitrarily set to 100%). In cases where a cocaine-induced increase in proteinlevels was observed in male or female cocaine treated rats compared to their respective saline controls, independent samples t-tests were then conducted on percentage control data between male and female cocaine treated rats. Additionally, Bivariate Pearson Correlations were run on normalized data for male and female cocaine treated animals (separately) to identify relationships between CPP scores, locomotor activity and phosphorylated protein levels. Correlation analysis between CPP scores and protein levels were performed using data obtained from the animals used for molecular analysis.
Highlights.
No sex differences were observed in the expression or magnitude of cocaine CPP
Cocaine females were more active than males and saline controls during CPP testing
Cocaine males and females showed robust increases in NAc pERK, pCREB and ΔFosB
Acknowledgments
This work was supported by NIDA DA12136, RCMI RR-03037, ULI-RR024996 and PSCCUNY to S.J/V.Q.J.
Abbreviations
- CPP
conditioned place preference
- CPu
caudate putamen
- CREB
cAMP response element binding protein
- DA
dopamine
- DAR
dopamine receptor
- ERK
extracellular regulated kinase
- HIP
hippocampus
- MAPK
mitogen activated protein kinases
- MEK
mitogen activated extracellular-regulated protein kinase
- NAc
nucleus accumbens
- pCREB
phosphorylated CREB
- pERK
phosphorylated ERK
- PfC
prefrontal cortex
- VTA
ventral tegmental area
Footnotes
Author contributions: S.K.N., V.Q.J., and S.J. designed experiments; S.K.N. performed experiments, statistical analysis, and wrote the manuscript. A.K., R.H. and S.J. helped perform behavioral testing, sacrificing, and tissue preparation. M.H.E., A.C., and J.C.B. ran and analyzed western blots, and helped edit the manuscript.
Contributor Information
Anthony Klambatsen, Email: anthonyklambatsen@yahoo.com.
Ruhal Hazim, Email: swamijie@gmail.com.
Mohamed H. Eltareb, Email: meltareb@gmail.com.
Jeff C. Blank, Email: blank.jeff@gmail.com.
Anna J. Chang, Email: ajc473@gmail.com.
Vanya Quinones-Jenab, Email: vaquinon@hunter.cuny.edu.
Shirzad Jenab, Email: sjenab@hunter.cuny.edu.
References
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. In: Neill JC, Kulkarni J, editors. Biological Basis of Sex Differences in Psychopharmacology. Berlin Heidelberg: Springer-Verlag; 2011. pp. 74–96. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front NeuroEndo. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol. Sex. Diff. 2012;3(14):1–35. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhow MT, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase) part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J. Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean SA, Dennis TS, Addison BD, Perrotti LI. Influence of sex on reinstatement of cocaine-conditioned place preference. J. Neurochem. 2010;114:530–541. doi: 10.1016/j.brainresbull.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu WH, Fletcher H, Perotti LI, Jenab S, Quinones-Jenab V. Sex differences in cocaine induced behavioral sensitization. Cell Mol Biol. 2001;47:1089–1095. [PubMed] [Google Scholar]
- Craft RM, Stratmann JA. Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend. 1996;42:27–37. doi: 10.1016/0376-8716(96)01259-8. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Groblewski PA, Voorhees CM. Place conditioning. In: Olmstead MC, editor. Animal Models of Drug Addiction, Neuromethods. Springer Science + Business Media LLC; 2011. pp. 167–189. [Google Scholar]
- Domjan M. Pavlovian conditioning: A Functional perspective. Annu. Rev. Psyhol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt K, Gastfriend DR. Gender differences in craving, mood and stress among non-treatment seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Hormones and Behavior. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin S, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Festa ED, Jenab S, Weiner J, Nazarian A, Niyomchai T, Russo SJ, Kemen LM, Akhavan A, Wu HBK, Quinones-Jenab V. Cocaine-induced sex differences in D1 receptor activation and binding levels after acute cocaine administration. Brain Res. Bull. 2006;68:277–284. doi: 10.1016/j.brainresbull.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK p38 MAP kinases in rats. Behav. Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: A Logical AND gate critical for drug-induced plasticity? Curr. Opin. Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Harris GC, Hummel M, Wimmer M, Mague SD, Aston-Jones G. Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience. 2007;147:583–591. doi: 10.1016/j.neuroscience.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Mol. Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nesterl EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ. Dissociation of novelty- and cocaine- conditioned locomotor activity from cocaine place conditioning. Pharmacol. Biochem. Behav. 1998;60:785–791. doi: 10.1016/s0091-3057(97)00388-2. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Liang KC, Chen HH, Cherng CG, Lee HT, Lin Y, Huang AM, Liao RM, Yu L. Cocaine-but not methamphetamine-associated memory requires de novo protein synthesis. Neurobiol. Learn. Mem. 2007;87:93–100. doi: 10.1016/j.nlm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW. Striatal regulation of ΔFosB, FosB, and cFos during cocaine self-administration and withdrawal. J. Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zheng D, Peng XX, Cabeza de Vaca S, Carr KD. Enhanced cocaine-conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluA1 in food-restricted rats. Brain Res. 2011;1400:31–41. doi: 10.1016/j.brainres.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp. Clin. Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Morrissey MD, McCormick CM. Individual differences in activity predict locomotor activity and conditioned place preference to amphetamine in both adolescent and adult rats. Pharmaco, Biochem, Behav. 2010;95:63–71. doi: 10.1016/j.pbb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Marazziti D, DiPietro C, Mandillo S, Golini E, Matteoni R, Tocchini-Valentini GP. Absence of the GPR37/PAEL receptor impairs striatal Akt and ERK2 phosphorylation, ΔFosB expression, and conditioned place preference to amphetamine and cocaine. FASEB J. 2011;25:2071–2081. doi: 10.1096/fj.10-175737. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat. Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J. Neurosci. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V. The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull. 2004;63:295–299. doi: 10.1016/j.brainresbull.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Sun WL, Zhou L, Kemen LM, Jenab S, Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology. 2009;203:641–50. doi: 10.1007/s00213-008-1411-5. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Total recall: The Memory of addiction. Science. 2001;292:2266–2268. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: A Sustained molecular switch for addiction. Proc. Acad. Sci. (USA) 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Sci. Pract. Perspect. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J. Neurosci. 2011;3:11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm. Behav. 2010;58(1):22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Radwanska K, Valjent E, Trzaskos J, Caboche J, Kaczmarek L. Regulation of cocaine-induced activator protein 1 transciption factors by the extracellular signal-regulated kinase pathway. Neurosci. 2006;137:253–264. doi: 10.1016/j.neuroscience.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Rawas ER, Klement S, Salti A, Fritz M, Dechant G, Saria S, Zernig G. Preventive role of social interaction for cocaine conditioned place preference: correlation with FosB/DeltaFosB and pCREB expression in rat mesocorticolimbic areas. Front. Behav. Neurosci. 2012;6:8. doi: 10.3389/fnbeh.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain. Res. 2003a;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian S, Gazi F, Kraish M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003b;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Sato SM, Wissman AM, McCollum AF, Woolley CS. Quantitative mapping of cocaine-induced ΔFosB expression in the striatum of male and female rats. PLoS ONE. 2011;6(7):e21783. doi: 10.1371/journal.pone.0021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J. Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Seymour CM, Wagner JJ. Simultaneous expression of cocaine-induced behavioral sensitization and conditioned place preference in individual rats. Brain Res. 2008;1213:57–68. doi: 10.1016/j.brainres.2008.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of dopamine and NMDA receptors of cocaine-induced Fos expression in the striatum of Fischer rats. Brain Res. 2008;1243:1–9. doi: 10.1016/j.brainres.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Festa ED, Jenab S, Quinones-Jenab V. Sex differences in dopamine D2-like receptor-mediated G-protein activation in the medial prefrontal cortex after cocaine. Ethn. Dis. 2010;20:88–91. [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J. Neurochem. 2008;106:1780–1790. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in the mouse brain. EurJNeurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during re-exposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacology, Biochemistry, and Behavior. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine stimulated activity and establishes place preference in rats. Pharmacol. Biochem. Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;3:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Weiner J, Sun WL, Zhou L, Kreiter CM, Jenab S, Quinones-Jenab V. PKA-mediated responses in females’ estrous cycle affect cocaine-induced responses in dopamine-mediated intracellular cascades. Neuroscience. 2009;161:865–876. doi: 10.1016/j.neuroscience.2009.03.071. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. C-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J.Neuroscience. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]