Perivascular adipose tissue (PVAT) reduces vasoconstriction to norepinephrine (NE); thus, we tested the hypothesis that PVAT has a NE uptake mechanism. Functional NE uptake assays, fluorescence imaging, and PCR of mesenteric and aortic PVAT revealed the potential role of organic cation transporter 3 in NE uptake.
Keywords: 4-[4-(dimethylamino)-styrl]-N-methylpyridinium iodide, norepinephrine uptake, high-performance liquid chromotography, catecholamines, perivascular adipose tissue, adipocytes
Abstract
Perivascular adipose tissue (PVAT) reduces vasoconstriction to norepinephrine (NE). A mechanism by which PVAT could function to reduce vascular contraction is by decreasing the amount of NE to which the vessel is exposed. PVATs from male Sprague-Dawley rats were used to test the hypothesis that PVAT has a NE uptake mechanism. NE was detected by HPLC in mesenteric PVAT and isolated adipocytes. Uptake of NE (10 μM) in mesenteric PVAT was reduced by the NE transporter (NET) inhibitor nisoxetine (1 μM, 73.68 ± 7.62%, all values reported as percentages of vehicle), the 5-hydroxytryptamine transporter (SERT) inhibitor citalopram (100 nM) with the organic cation transporter 3 (OCT3) inhibitor corticosterone (100 μM, 56.18 ± 5.21%), and the NET inhibitor desipramine (10 μM) with corticosterone (100 μM, 61.18 ± 6.82%). Aortic PVAT NE uptake was reduced by corticosterone (100 μM, 53.01 ± 10.96%). Confocal imaging of mesenteric PVAT stained with 4-[4-(dimethylamino)-styrl]-N-methylpyridinium iodide (ASP+), a fluorescent substrate of cationic transporters, detected ASP+ uptake into adipocytes. ASP+ (2 μM) uptake was reduced by citalopram (100 nM, 66.68 ± 6.43%), corticosterone (100 μM, 43.49 ± 10.17%), nisoxetine (100 nM, 84.12 ± 4.24%), citalopram with corticosterone (100 nM and 100 μM, respectively, 35.75 ± 4.21%), and desipramine with corticosterone (10 and 100 μM, respectively, 50.47 ± 5.78%). NET protein was not detected in mesenteric PVAT adipocytes. Expression of Slc22a3 (OCT3 gene) mRNA and protein in PVAT adipocytes was detected by RT-PCR and immunocytochemistry, respectively. These end points support the presence of a transporter-mediated NE uptake system within PVAT with a potential mediator being OCT3.
NEW & NOTEWORTHY
Perivascular adipose tissue (PVAT) reduces vasoconstriction to norepinephrine (NE); thus, we tested the hypothesis that PVAT has a NE uptake mechanism. Functional NE uptake assays, fluorescence imaging, and PCR of mesenteric and aortic PVAT revealed the potential role of organic cation transporter 3 in NE uptake.
perivascular adipose tissue (PVAT) closely envelops many blood vessels of the body (65). This relationship between PVAT and the blood vessel has earned PVAT its place as the fourth layer of the blood vessel, the “tunica adiposa” (14). Beyond providing structural support, PVAT has many roles in modulating blood vessel function (68). The release of vasoactive molecules from PVAT influences vascular function by altering the proliferation, migration, inflammation, and contraction of vascular smooth muscle (9, 20–22, 24, 46, 68, 72). Interestingly, a releasable pool of catecholamines is present in PVAT (5, 67). Although both contractile and anticontractile substances can be released from PVAT (9, 22, 24, 25, 72), the presence of PVAT on blood vessels generally reduces vessel contraction in response to various agonists, including norepinephrine (NE) (64). Knowledge on how these mechanisms interact to influence the anticontractile properties of PVAT in NE-induced contraction is not complete (36).
The anticontractile effect of PVAT is lost in obesity and hypertension, implicating PVAT as an integral link between both of these diseases (3). Over one-third of all adults in the United States are hypertensive (13), a condition that significantly increases the risk of death from myocardial infarction or stroke (42). A major risk factor for hypertension is obesity (29). Globally, 13% of adults are obese (have a body mass index of ≥30) (73a), and in the United States, the number is higher, with 34.9% of adults classified as obese (53). In obesity, dysfunction of the anticontractile effect of PVAT is observed along with overall changes in adipocyte function (3). Thus, the relationship between the adipocytes within PVAT and blood vessel function is of interest.
A dynamic adrenergic system that affects blood vessel contraction exists in PVAT (5). Catecholamines are released from PVAT upon the addition of tyramine, a sympathomimetic drug, leading to contraction of the rat aorta and superior mesenteric artery (5). Moreover, pharmacological inhibition of NE transporters (NET) reduces the PVAT-dependent vascular contraction to tyramine (5). Soltis and Cassis (64) discovered that inhibition of NE uptake in the rat aorta abolished the anticontractile effect of PVAT. Collectively, this work led us to investigate the presence of a NE uptake system in PVAT.
The present study tested the hypothesis that PVAT takes up NE through molecular transporters and aims to identify the transporters that transport NE in PVAT. Our interest in studying mesenteric PVAT is guided by the knowledge that contraction in mesenteric resistance arteries increases peripheral resistance, a contributing event toward the elevation of blood pressure. Furthermore, this adipose depot is important for cardiovascular risk. Individuals with large masses of visceral fat have a higher risk of cardiovascular disease than individuals with large masses of subcutaneous fat (40).
PVAT of mesenteric resistance arteries most closely resembles white adipose tissue in that it contains adipocytes that have large unilocular lipid droplets (10). We focused on adipocytes from normal rats as studies of NE transport in adipocytes are sparse and none have been performed on PVAT adipocytes specifically. To test our hypothesis, we used PVAT from normal male Sprague-Dawley rats for HPLC measures of NE in PVAT and isolated adipocytes. We also measured uptake of NE and used pharmacological inhibitors to transporters to reveal the main transporters that transport NE in PVAT. Confocal microscopy of PVAT was used using the fluorescent NE transport substrate dye methylpyridinium ASP+ (61) in addition to immunohistochemistry, immunocytochemistry, and gene expression analysis of mesenteric and aortic PVAT to reveal the role of organic cation transporter 3 (OCT3).
MATERIALS AND METHODS
Chemicals.
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO). The pharmacological inhibitors nisoxetine (inhibits NET), citalopram [inhibits the 5-hydroxytryptamine (serotonin) transporter (SERT)], corticosterone (inhibits OCT3), and desipramine [inhibits NET and SERT at the concentration used (10 μM)] were purchased from Bio-Techne (Minneapolis, MN). Pargyline (a monoamine oxidase inhibitor), Ro 41-0960 (a catechol-o-methyltransferase inhibitor), and NE were purchased from Sigma-Aldrich. ASP+ was synthesized and provided by James N. Wilson (University of Miami, Miami, FL) (73).
Animals.
Male Sprague-Dawley rats (225–275 g or ∼8–10 wk of age, Charles River, Indianapolis, IN) were used. All protocols were approved by the Institutional Animal Care and Use Committee of Michigan State University and followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (8th ed., 2011). Rats were anesthetized with pentobarbital sodium (60–80 mg/kg ip). Anesthesia was verified by lack of paw pinch and eye blink reflexes. Death was assured by pneumothorax and exsanguination, after which tissues were removed for one of the following protocols.
NE uptake.
Mesenteric and aortic PVATs were dissected, and 20–100 mg of tissue were placed in microcentrifuge tubes containing physiological salt solution (PSS) [containing (in mM) 130 NaCl, 4.7 KCl, 1.8 KH2PO4, 1.7 MgSO4·7H2O, 14.8 NaHCO3, 5.5 dextrose, 0.03 CaNa2 EDTA, and 1.6 CaCl2 (pH 7.2)] within 30 min of tissue removal from the rat. Pargyline (10 μM) and Ro 41-0960 (1 μM) were added to the PSS to inhibit NE metabolism. Vehicle or a transporter inhibitor [nisoxetine (1 μM), citalopram (100 nM), corticosterone (100 μM), citalopram (100 nM) with corticosterone (100 μM), desipramine (10 μM), or corticosterone (100 μM) with desipramine (10 μM)] was added for 30 min at 37°C. The concentrations were selected based on their specificity for the transporter in question. NE (10 μM) or vehicle (either H2O or ethanol) was added for another 30 min. Tissues were rinsed four times in drug-free PSS and then three times in tissue buffer (0.05 mM sodium phosphate and 0.03 mM citric acid buffer, pH 2.5, in 15% methanol). Samples were saved in tissue buffer and kept at −80°C until assay. The day of the assay, samples were thawed and sonicated for 3 s. Samples were centrifuged at 18,000 g for 15 min at 4°C, and the supernatant was transferred to new tubes for HPLC analysis. Tissue pellets were dissolved in 1.0 N NaOH and assayed for protein using a Bicinchoninic Acid Protein Assay Kit (catalog no. BCA1, Sigma-Aldrich).
ASP+ uptake.
The mesenteric arcade was dissected from Sprague-Dawley rats and stored in PSS without calcium [containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2·7H2O, 10 HEPES, and 10 glucose; pH 7.4] at 4°C until use, for up to 5 h. Immediately before experiments, mesenteric resistance arteries with associated PVAT were dissected and pinned onto the Sylgard-coated bottom of an imaging chamber (volume = 1 ml) with the use of a stereomicroscope. Experiments were performed in the dark or under safe lights at 37°C. The tissue was superperfused with PSS with calcium (1.8 mM CaCl2·2H2O) and allowed to equilibrate to temperature for 15 min, after which a background image was captured. For the ASP+ concentration uptake experiment, the tissue was superfused with PSS containing ASP+ (1 nM–10 μM) for 10 min and imaged. To test each concentration, a new section of tissue was used from the same animal and the order in which the concentrations were tested was randomized. Each tissue was only used for one condition. For ASP+ uptake experiments in which inhibitors or NE were used, the tissue was superfused with an inhibitor of transport, NE (1 mM), or vehicle in PSS for 10 min, and an image was captured to assess background fluorescence. ASP+ (2 μM) was added for 10 min, and the tissue was imaged again. For the ASP+ concentration uptake experiments and ASP+ uptake experiments where nisoxetine or citalopram were used, tissue imaging was performed with a Leica DMLFSA confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Yokogawa CSU10 spinning disk confocal head (Yokogawa, Tokyo, Japan) coupled to a XR-Mega10 intensified charge-coupled device (Stanford Photonics, Palo Alto, CA) using a ×40 water-immersion objective. Illumination was provided by an X-cite Exacte Illuminator (Excelitas Technologies, Waltham, MA). Images were recorded with Piper-Control (Stanford Photonics) and analyzed using ImageJ (NIH). For the corticosterone, citalopram with corticosterone, desipramine, desipramine with corticosterone, and NE experiments, the protocol was the same as above except that a solid-state 488-nm laser was used for illumination and a TurboEX ICCD camera (Stanford Photonics) controlled by μManager (66a) was used for image acquisition. Images were captured as stacks of 50 TIFF (16-bit) images, which were then combined with the average z-projection function in ImageJ. Fluorescence intensity was quantified in relative fluorescent units.
Sample preparation of the mesenteric PVAT, mesenteric resistance vessels, adipocytes, and stromal vascular fraction.
Mesenteric PVAT and mesenteric resistance vessels were dissected in a Sylgard-coated petri dish in PSS with the use of stereomicroscope. Images of the whole mesentery were captured with a Lumix DMC-ZS25 camera (Panasonic, Osaka, Japan) and processed using Adobe Photoshop CC 2014 (Adobe Systems, San Jose, CA). PVAT was either flash frozen for whole PVAT measurements or digested to obtain separate cellular fractions by the following protocol. PVAT was added to 1 ml PSS with 1 mg/ml collagenase from Clostridium histolyticum type IA (catalog no. C9891, Sigma-Aldrich) and incubated at 37°C with slow rotation until fully digested (∼1 h). PVAT was centrifuged at 200 g for 5 min, and the stromal vascular fraction (SVF), which pellets to the bottom, was transferred to a separate tube. Adipocytes and the SVF were washed three times with PSS and centrifuged at 200 g for 10 min. For immunocytochemistry, mesenteric PVAT adipocytes were resuspended in PSS and centrifuged onto CellTak (catalog no. 54240, BD Biosciences, Bedford, MA)-coated slides using a Cytospin 4 cytocentrifuge (700 g for 2 min), and an aliquot was saved to assess purity using a hemacytometer. For Western blots, the mesenteric PVAT, mesenteric resistance vessels, adipocytes, and SVF were added to RIPA buffer solution (catalog no. R3792, Teknova, Hollister, CA) with protease inhibitors (0.5 mM PMSF, 1 mM orthovanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) into a 2-ml bead tube (Omni, Kennesaw, GA). Tissues were homogenized using the Omni Bead Ruptor Homogenizer (Omni) and centrifuged for 15 min at 18,000 g, and supernatants were saved for Western blot analysis. Supernatants were quantified for protein content using a Bicinchoninic Acid Protein Assay Kit (catalog no. BCA1, Sigma-Aldrich). For mRNA isolation and HPLC analysis, the adipocytes and SVF were placed into separate tubes with PSS and centrifuged 200 g for 10 min, after which the supernatant was removed. The tissue was then flash frozen in liquid nitrogen and saved at −80°C until assay. Images of the isolated adipocytes were taken on a Nikon TE2000 inverted microscope with MMI Cell Tools (Molecular Machines & Industries, Zurich, Switzerland).
Western blot analysis for NET.
Fifty micrograms of protein from the mesenteric PVAT, mesenteric resistance vessels, adipocytes, SVF, and vena cava (positive control) were separated on a 10% SDS gel and transferred to a polyvinylidene difluoride membrane. The membrane was blocked in 4% (wt/vol) chicken egg ovalbumin in Tris-buffered saline and Tween 20 (TBST) for 3 h at 4°C and then incubated with primary antibody [mouse anti-NET (1:500, NET05-2, MAb Technologies, Stone Mountain, GA) and mouse anti-β-actin (1:2,000, A3854, Sigma)] diluted in blocker overnight at 4°C. The blot was washed with TBST (10 min each, three times) and then incubated with IRDye anti-mouse secondary antibody (1:1,000, no. 926-32210, Li-Cor, Lincoln, NE) diluted in Odyssey Blocking Buffer (no. 927-40000, Li-Cor) for 1 h at 4°C. The blot was washed with TBST (10 min each, three times) and developed on the Li-Cor Odyssey. Densitometric analysis was done using ImageJ.
Preparation of aortic PVAT for PCR and immunohistochemistry.
To obtain samples of aortic PVAT, the thoracic aorta was removed from the rat and placed into PSS. PVAT was then dissected from the aorta on a Sylgard-coated petri dish with the use of a stereomicroscope. PVAT was removed and snap frozen in liquid nitrogen for RNA extraction. The Investigative Histopathology Laboratory at Michigan State University prepared the fresh frozen rat aorta slides.
Real-time PCR.
Tissue was homogenized using an Omni Bead Ruptor (Omni). RNA was extracted with the Quick RNA MiniPrep kit (catalog no. R1054, Zymo Research, Irving, CA), and purity (260-to-280 and 260-to-230 ratios ≥ 1.8) was verified using a Nanodrop 2000C spectrophotometer (Thermoscientific, Wilmington, DE). mRNA was reverse transcribed with qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). RT-PCR was performed using FAST SYBR Green MasterMix (catalog no. 4385612, Applied Biosystems, Foster City, CA) on the ABI 7500 Fast Real Time PCR system (Life Technologies, Carlsbad, CA) using the following parameters: 95°C for 20 s, 95°C for 1 s, and 60°C for 20 s for 40 cycles. The primer sequences for Slc22a3 (OCT3 gene) amplification were as follows: forward 5′-TATGCAGCGGACAGATACGG-3′ and reverse 5′-AAAATTCGGTGCAAACGCCA-3′ (Integrated DNA Technologies, Coralville, IA). Measures were normalized to β2-microglobulin (RT2 qPCR Primer Assay, catalog no. PPR42607A, Qiagen, Valencia, CA). A melt curve was performed to verify the presence of one PCR product after the amplification. Data were analyzed using the 2−ΔΔCT method (where CT is threshold cycle) (45).
Immunocyto/histochemistry.
Fresh frozen 8-μm aortic tissue sections and adipocyte slides (described above) were fixed in acetone and immunostained using a VECTASTAIN ABC kit (rabbit: catalog no. PK-4001, Vector Laboratories, Burlingame, CA) and an Avidin-Biotin Blocking Kit (SP-2001, Vector Laboratories). Slides were incubated for 24 h with anti-OCT3 antibody (1:100, catalog no. orb107605, Biorbyt, San Francisco, CA) or without primary antibody at 4°C. Slides were developed using 3,3-diaminobenzidine (catalog no. SK-4100, Vector Laboratories) and counterstained with hematoxylin (catalog no. H-3401, Vector Laboratories) for 30 s. Imaging was performed on a Nikon TE2000 inverted microscope with MMI Cell Tools (Molecular Machines & Industries).
HPLC.
PVATs were weighed and homogenized in four times their weight of 0.1 M perchloric acid and centrifuged at 15,000 g for 10 min, and the supernatant was analyzed by HPLC. Supernatants from uptake experiments were diluted 1:10 in tissue buffer before analysis. The HPLC system consisted of a Coulochem III electrochemical detector set at −350 mV with a HR-80 reverse-phase column with Cat-A-Phase II mobile phase (Thermoscientific). The separation column was maintained at 35°C with a flow rate of 1.1 ml/min. Quantification was performed by comparing sample area measurements with a calibration curve. Standards were run every fifth sample to verify the identity of our peaks of interest on the chromatogram. The limit of detection was 0.1 ng/ml, and NE content was either expressed per weight or by protein content.
Data analysis.
Statistical analyses were performed using GraphPad Prism 6.0 (La Jolla, CA). When two groups were compared, either an unpaired Student's t-test was used with similar variances and the Mann-Whitney test was used when the variances were different (as verified by the F-test). When more than two groups were compared, ANOVA with a Newman-Keuls test was used. With non-normally distributed data, Kruskal-Wallis ANOVA was used followed by a Dunn's test for multiple comparisons. The tests were unpaired. P values of <0.05 were considered statistically significant. Means ± SE are reported where appropriate. To calculate percent NE uptake, the concentration of NE in tissues incubated with NE and the pharmacological inhibitor were divided by the concentration of NE in tissue incubated with NE and the vehicle. Image contrast for the ASP+ experiments was normalized to the brightest image recorded in the data set. Pseudocolorization of ASP+-stained images was performed using the “Fire” lookup table and constructed surface plots by applying the “surface plot” function in ImageJ (version 1.48). All image adjustments in brightness and contrast were made to the whole panel of an image, not a portion. To calculate percent ASP+ uptake, the fluorescence intensity ratio (F/F0) was used, where F is the fluorescence intensity after incubation with the inhibitor and ASP+ minus the background intensity and F0 is the fluorescence intensity of ASP+ incubated with vehicle minus the background intensity.
RESULTS
NE is present in rat mesenteric PVAT.
A large proportion of NE found in PVAT is in the adipocyte fraction (Fig. 1A). Measures of NE by HPLC in mesenteric PVAT and isolated mesenteric PVAT adipocytes were similar (P > 0.05). Representative images of each sample are shown in Fig. 1A, bottom left and bottom right. The white box in Fig. 1A, bottom left, highlights a representative area of tissue that was used. One mechanism by which NE could localize to adipocytes in PVAT is through transporter-mediated uptake of extracellular NE; thus, we investigated this further.
Fig. 1.
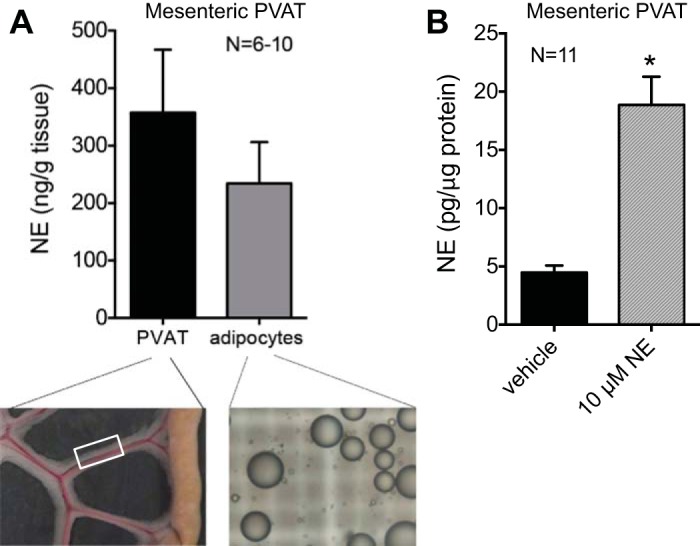
Norepinephrine (NE) is present in perivascular adipose tissue (PVAT) adipocytes, and PVAT can take up extracellular NE. A: mesenteric PVAT adipocytes were isolated, and NE content was measured by HPLC. Measures were normalized to tissue weight. Bottom left, representative image of the mesenteric PVAT used. The white box highlights the portion of PVAT used in the experiments in this study. Bottom right, representative image of adipocytes isolated from mesenteric PVAT. B: addition of NE (10 μM) to PVAT in physiological saline solution (PSS) for 30 min increased NE accumulation, as measured by HPLC and normalized to protein content (*P < 0.05). Values are means ± SE for the number of animals (N) shown.
NE uptake occurs in PVAT.
We used a pharmacological approach to test the hypothesis that uptake of NE into PVAT is mediated by transporters. First, we established that NE uptake occurs in PVAT (Fig. 1B). PVAT was then incubated with NE (10 μM) or vehicle in PSS for 30 min, the tissue was washed to remove excess NE, and NE in the tissue was measured by HPLC. As shown in Fig. 1B, mesenteric PVAT NE content was significantly increased after the addition of NE (10 μM) compared with the addition of vehicle (18.87 ± 2.42 vs. 4.48 ± 1.98 pg/μg protein, respectively, P < 0.05). This NE uptake could be reduced by the inhibition of NE transport by preincubation with inhibitors of NET, SERT, and OCT3: nisoxetine (1 μM), citalopram (100 nM) with corticosterone (100 μM), and desipramine (10μM) with corticosterone (100 μM). Desipramine, citalopram, or corticosterone alone did not significantly reduce NE uptake compared with vehicle (Fig. 2). These data support transporter-mediated uptake of NE in mesenteric PVAT through NET, SERT, and OCT3.
Fig. 2.
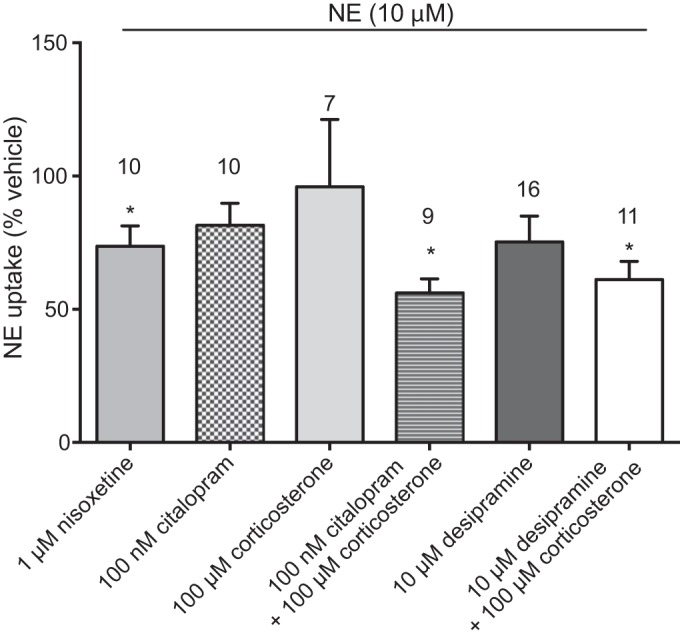
PVAT accumulates NE through transporter-mediated uptake. Mesenteric PVAT was incubated for 30 min with transporter inhibitors before the addition of 10 μM NE. Data are reported as percent uptake from vehicle. Values are means ± SE. Numbers above the bars are N values for each inhibitor. *P < 0.05 vs. vehicle.
ASP+ fluorescently labels monoamine transporters on PVAT adipocytes.
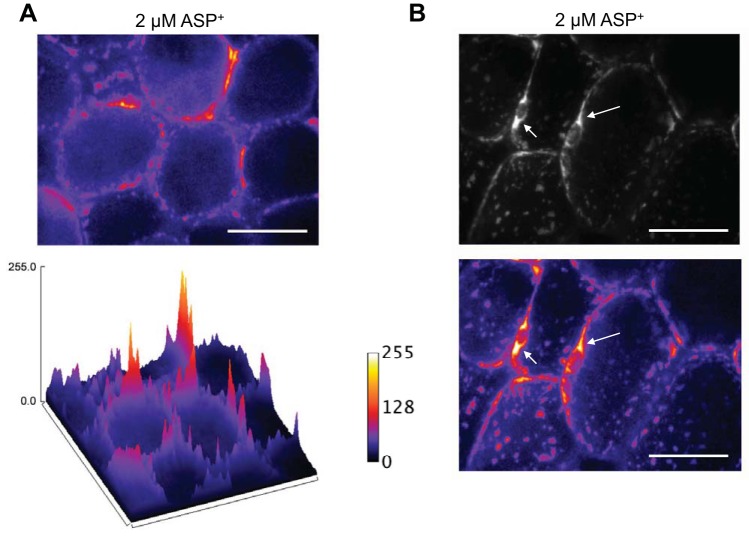
The transporter substrate ASP+ was used to identify the presence of NET on PVAT adipocytes. ASP+ fluoresces upon binding and is transported and accumulates in mitochondria (61). To determine if ASP+ would bind to PVAT adipocytes, we added increasing concentrations of ASP+ to mesenteric PVAT and imaged the tissue by fluorescence microscopy, and a graph of the concentration-fluorescence intensity relationship was constructed (Fig. 3A). Quantification of the intensity of ASP+ fluorescence and comparison of fluorescence intensity between vehicle (water) and each concentration revealed a significant increase in fluorescence from vehicle starting at 1 μM ASP+. Fluorescence intensity saturated the camera at 10 μM ASP+ (Fig. 3B). A concentration of 2 μM ASP+ was chosen for subsequent experiments to achieve a detectable fluorescence signal while avoiding camera saturation. By imaging the adipocyte at the focal plane that transverses the adipocyte, ASP+ fluorescence was localized to the periphery of the adipocyte, where the cytoplasm is located (Fig. 4A, top). The surface plot image of the fluorescence intensity levels with pseudocolorization showed this more clearly (Fig. 4A, bottom). Confocal imaging at the level of the adipocyte nucleus revealed intense staining around the adipocyte nuclei, an area rich in mitochondria in white adipose tissue adipocytes (Fig. 4B, with the top showing an image without color information and the bottom showing the image with pseudocolor; arrows point to perinuclear staining) (19). Perinuclear ASP+ fluorescence in PVAT adipocytes suggests transport of ASP+ into the adipocyte as opposed to only surface binding.
Fig. 3.
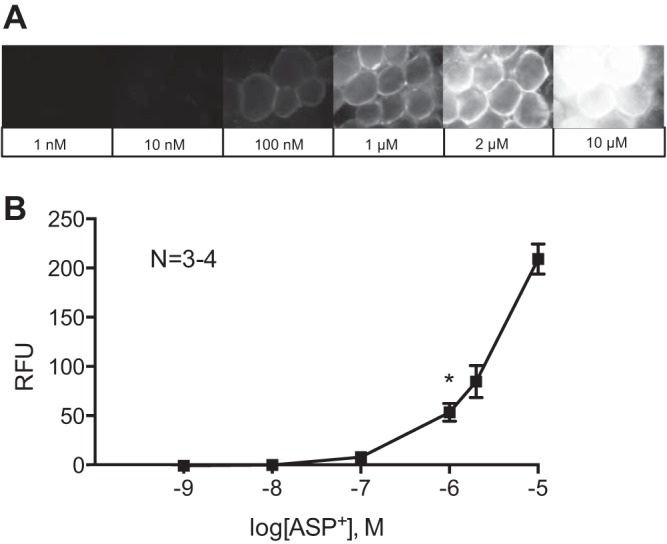
ASP+, a fluorescent substrate of cation transporters, binds to mesenteric PVAT adipocytes. ASP+ fluorescence in PVAT was tested by adding ASP+ at different concentrations for 10 min and then imaging by fluorescence microscopy. A: representative images of ASP+ at each concentration tested. B: quantification of ASP+ fluorescence intensity at each concentration. *P < 0.05 indicates the first concentration with significantly increased fluorescence vs. background. The fluorescence intensity was expressed in relative fluorescence units (RFU). Values are means ± SE for the number of animals (N) shown. Samples were imaged with a ×40 objective.
Fig. 4.
ASP+ is transported into mesenteric PVAT adipocytes. ASP+ (2 μM) was added to mesenteric PVAT for 10 min in PSS and imaged by confocal microscopy. A: pseudocolored representation of the fluorescence intensity of ASP+ (2 μM) binding revealing that ASP+ binds along on the periphery of the adipocyte in a punctate pattern (top). The surface plot of the same image shows this more clearly (bottom). Refer to the RFU scale to the right. Lighter (white-yellow) colors indicate higher fluorescence intensities as measured by RFU, and darker (violet-black) colors indicate lower fluorescence intensities. B, top: image of a PVAT adipocyte imaged at the level of the nucleus. Arrows point to areas of perinuclear staining. Pseudocolorization of the images (bottom) shows that intense staining was present in the perinuclear region. Images are representative of six animals. Scale bars = 50 μm. Samples were imaged with a ×40 objective.
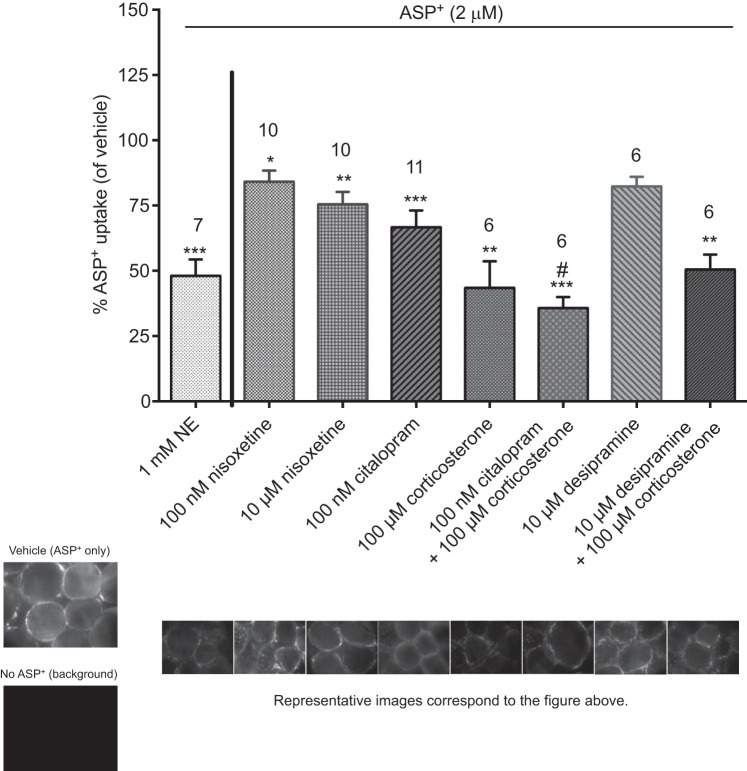
Preincubation of PVAT with an excess of NE (1 mM) reduced ASP+ uptake (Fig. 5). Any residual binding of ASP+ in the presence of this saturating concentration of NE can be considered nonspecific to NE transport mechanisms. To identify which transporters were involved in ASP+ uptake, mesenteric PVAT was incubated at 37°C in PSS containing an inhibitor of transport or vehicle for 10 min followed by ASP+ (2 μM) and imaged (Fig. 5). Representative images are shown of the fluorescence obtained with vehicle only with ASP+ (2 μM), background (no ASP+), and each experiment after preincubation with NE or an inhibitor of transport (Fig. 5). ASP+ uptake was significantly reduced by inhibition of NET by nisoxetine (100 nM and 10 μM), SERT by citalopram (100 nM), OCT3 by corticosterone (100 μM), SERT and OCT3 by citalopram with corticosterone (100 nM and 100 μM, respectively), and NET and OCT3 by desipramine with corticosterone (10 and 100 μM, respectively). However, uptake was not significantly reduced by desipramine alone (10 μM). Binding and transport of ASP+ in PVAT adipocytes thus may be mediated by NET, SERT, and OCT3, consistent with our experiments of NE transport into PVAT (Fig. 2).
Fig. 5.
ASP+ uptake is reduced by transporter inhibitors. Mesenteric PVAT was incubated with inhibitors of transport or vehicle for 10 min followed by the addition of ASP+ (2 μM). Data are reported as percent uptake from vehicle. Numbers above the bars indicate numbers of animals (N) used in each experiment. Values are means ± SE. *P < 0.05, **P < 0.005, and ***P < 0.001 vs. vehicle; #P < 0.05 vs. corticosterone. Representative images from each experiment are located beneath the corresponding bar.
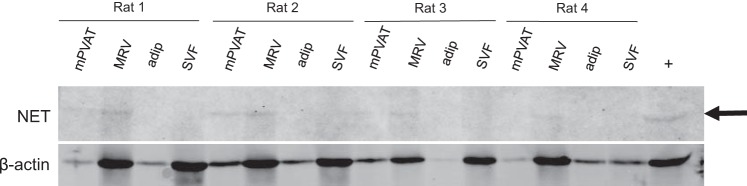
NET is not present in mesenteric PVAT adipocytes.
The presence of NET in the mesentery was investigated because of the modest effect of nisoxetine on ASP+ uptake. Protein from mesenteric PVAT, mesenteric resistance vessels, mesenteric PVAT adipocytes, and mesenteric PVAT SVF were assayed for NET by Western blot analysis. We did not observe bands for NET in adipocytes or the SVF but did observe some faint bands for NET in the mesenteric PVAT, resistance vessels, and vena cava, our positive control (Fig. 6), indicating that the NET is most likely not the main transporter that is mediating uptake of NE in PVAT. Corticosterone and corticosterone with citalopram caused the greatest reduction in ASP+ uptake (Fig. 5); therefore, we focused on OCT3 for the rest of the experiments.
Fig. 6.
NE transporter (NET) is not located in mesenteric PVAT (mPVAT) adipocytes. Whole PVAT was assayed for NET by Western blot along with the mesenteric resistance vessels (MRV), PVAT adipocytes (adip), and PVAT stromal vascular fraction (SVF). The arrow points to the band of interest. β-Actin was used as the loading control, and the vena cava was used as a positive control. N = 4.
OCT3 is present in PVAT adipocytes.
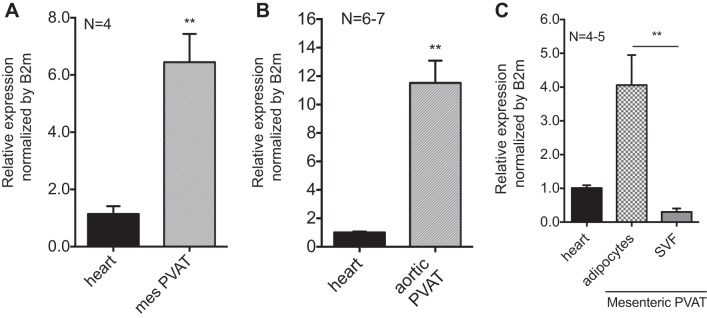
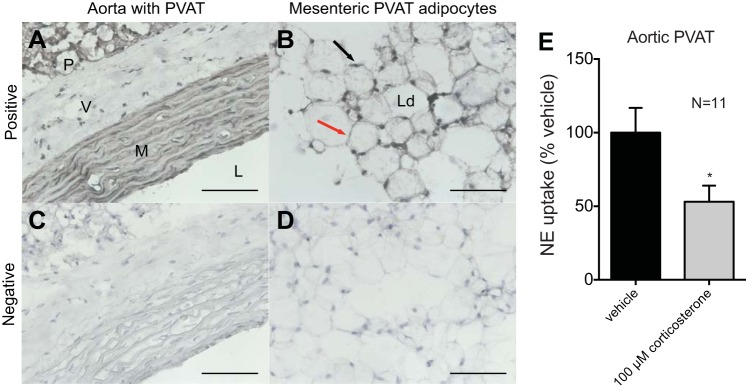
PVAT expression of Slc22a3 (the gene for OCT3) was compared with its relative expression in the heart, a positive control (15) (Fig. 7A). Mesenteric and aortic PVATs expressed Slc22a3 at higher relative expression than in the heart (Fig. 7, A and B). Mesenteric PVAT was separated into adipocytes and the SVF to allow for the assay of Slc22a3 expression in each fraction. Relative expression of Slc22a3 was higher in adipocytes than in the SVF (Fig. 7C). We used the reference gene β2-microglobulin because it gave us the most similar expression among all of the sample types compared with other housekeepers we assayed (data not shown). The cycles at which each sample reached threshold for β2-microglobulin were similar between the heart and mesenteric PVAT (CT: 20.0 and 19.4, respectively) as well as the heart and aortic PVAT (CT: 17.8 and 17.5, respectively) but were dissimilar for the heart, adipocytes, and SVF (CT: 18.4, 16.7, and 19.9, respectively). This would affect our calculations for relative expression for the last group (CT for Slc22a3: 26.9 in the heart, 23.0 in adipocytes, and 30.7 in the SVF). Immunostaining revealed OCT3 protein in aortic PVAT (Fig. 8A) and mesenteric PVAT adipocytes (Fig. 8B) using the aorta as a positive control for OCT3 (70). Aortic staining for OCT3 was located to the tunica media (labeled M in Fig. 8A) but not the tunica adventitia (labeled V in Fig. 8A). Immunostaining for OCT3 was present on the periphery of the adipocytes in both aortic and mesenteric PVATs (Fig. 8, A and B). Immunostaining was not present when the primary antibody was excluded (Fig. 8, C and D). Inhibition of OCT3 with corticosterone (100 μM) reduced NE uptake in aortic PVAT by 47.0 ± 11.0% (Fig. 8E). These data support the presence of OCT3 on adipocytes and the potential for OCT3 to transport NE in PVAT.
Fig. 7.
Slc22a3 mRNA is expressed in mesenteric and aortic PVAT. A: relative mesenteric (mes) PVAT expression of Slc22a3 mRNA was measured and compared with the heart as a positive control. B: whole aortic PVAT relative expression of Slc22a3 was measured and compared with the heart as a positive control. C: PVAT adipocyte relative expression of Slc22a3 mRNA was higher than that in the SVF (**P < 0.005) compared with the heart as a positive control. Measures were normalized to B2M. Values are means ± SE for the number of animals (N) shown. **P < 0.005.
Fig. 8.
Immunohistochemical and immunocytochemical staining revealing the presence of organic cation transporter 3 (OCT3) in aortic PVAT and mesenteric PVAT adipocytes and that aortic PVAT NE uptake is reduced by inhibition of OCT3. A: the aorta was used as the positive control for OCT3. Aortic staining for OCT3 protein was evident in the media (M) but not in the adventitia (V). PVAT (P) around the aorta also stained for OCT3. L, lumen. B: mesenteric PVAT adipocytes were isolated and stained for OCT3 protein. The black arrow points to the location of adipocyte nuclei, and the red arrow points to the location of the cytoplasm of the adipocyte. Ld, lipid droplet. C: the aorta with PVAT was stained without the inclusion of the primary antibody to serve as a negative control. D: mesenteric PVAT adipocytes were stained without the inclusion of the primary antibody as a negative control. Adipocyte images are representative of four animals. Samples were imaged with a ×40 objective. Scale bar = 50 μm. E: NE uptake of aortic PVAT was assayed after incubation with vehicle or corticosterone (100 μM). Data are reported as percentages from vehicle, and measures are expressed as NE concentration to protein content. Values are means ± SE for the number of animals (N) shown. *P < 0.05.
DISCUSSION
PVAT modulates blood vessel function (5, 9, 14, 67, 68), and, in the current study, we present evidence that at least part of this can occur through direct NE uptake. The novel discovery of NE uptake by PVAT could present a mechanism by which adipose tissue reduces the local concentration of NE, thereby reducing the ability of NE to interact with vascular smooth muscle to induce contraction and vasoconstriction. This could be a physiologically relevant mechanism by which PVAT modulates vascular tone. In the present study, we discuss NE uptake in PVAT with the consideration that most NE that the blood vessel is exposed to is not circulating NE but rather NE released from sympathetic nerve boutons in and around the blood vessel. Autonomic nervous system interactions with PVAT and its effects on the blood vessel have been recognized (12). NE released from the nerves that innervate PVAT and the vascular smooth muscle cell could be taken up by PVAT, thus reducing vascular contraction. In other words, PVAT might serve as a sink or source of NE.
PVAT can take up NE.
An adrenergic system exists in adipose tissue, as evidenced by the discovery that mesenteric adipose tissue adipocytes synthesize NE and serotonin (66, 69). We previously demonstrated that PVATs possess measurable catecholamines (5). The source of these catecholamines may be from the adipocyte fraction (Fig. 1A). Other sources of NE could be found in the SVF of PVAT, such as macrophages (11), lymphocytes (58), and neurons. The catecholamines present in PVAT are releasable by tyramine and support contraction in the rat superior mesenteric artery independent of sympathetic innervation (5). PVAT-dependent contraction to tyramine was reduced by the NET inhibitor nisoxetine (5), directing us further into the investigation of NE transport in PVAT. In the present study, we used the transporter inhibitors desipramine [10 μM, inhibitory constant (ki) for NET: 7.36 nM (55) and for SERT: 228 nM (16)], nisoxetine [1 μM, ki for NET: 0.46 nM and for SERT: 158 nM (16)], and corticosterone [100 μM, OCT3 IC50: 120–290 nM (39)]. Desipramine, a NET inhibitor, at higher concentrations can also inhibit SERT [ki: 129.00 nM (54)] and OCT3 [IC50 of 700 nM (75)]. Since our experimental samples were intact tissues, not isolated transporters or membranes, each inhibitor was used at a concentration that was ∼50–100 times above the ki values to assure the inhibitors reached their target, considering they can be metabolized or bound.
Uptake of NE was reduced upon the addition of nisoxetine, citalopram with corticosterone, and desipramine with corticosterone. While the high concentration of nisoxetine reduced uptake on its own, there are two possibilities for why inhibition with two drugs (desipramine/corticosterone and citalopram/corticosterone), as opposed to either of them alone, was necessary to observe a reduction in uptake. First, redundancy of NE transport may exist through different transporters in PVAT. In the brain, the uptake-2 system (another term for OCT3-mediated transport) has been implicated in limiting the reduction of NE, dopamine (DA), and serotonin uptake by specific inhibitors (30). Therefore, in our system, to significantly reduce NE uptake in PVAT, multiple transporters may have to be targeted. Second, it is possible that transporters on adipocytes heteroligomerize (18, 32, 37). NET and SERT can heteroligomerize, but it is debated whether heteroligomerization affects their function. Less is known about oligomerization of OCT3. Homodimerization of rat OCT1 and human OCT2, the other OCT isoforms, is required for the transporter to be placed on the plasma membrane (38). While oligomerization would be interesting to study, it is not the focus of this work. It is a possibility that different PVAT depots may contain a different distribution of transporters of NE. Therefore, the applicability of these findings to other PVAT depots is not known outside of rat aortic and mesenteric PVATs.
The cation transporter substrate ASP+ is taken up by PVAT adipocytes in a NET-, SERT-, and OCT3-dependent manner.
ASP+ is a useful experimental tool for probing NE transport, as previously validated in NE uptake assays using radiolabeled 3[H]NE (28). ASP+ fluorescence detection permits its use for live cell imaging to identify potential transporters of NE. Confocal imaging of ASP+-stained PVAT adipocytes allowed us to visualize ASP+ taken into the adipocyte via the observation of bright halos around the adipocyte nuclei. This pattern of perinuclear ASP+ fluorescence was observed in all six of the tissues imaged. The punctate pattern of ASP+ fluorescence in the adipocyte is strikingly similar to that observed when adipocyte mitochondria were stained using rhodamine 123 (19). This previous study reported nuclear “haloing” when mitochondria were stained using the fluorescent dye similar to what we observed when we applied ASP+ to PVAT. Furthermore, ASP+ accumulation in mitochondria after transport into the cell has been shown previously (8), additionally supporting our observation that ASP+ was able to bind and be transported into the cell. The finding that ASP+ was citalopram sensitive is in line with the finding that adipocytes express functional SERT (66). Moreover, a study by Pizzinat et al. (57) found that [3H]NE uptake in isolated human adipocytes obtained from abdominal or mammary lipectomies could be reduced by inhibiting the uptake-2 system (OCT3) with disprocynium 24. In our study, nisoxetine reduced ASP+ uptake at both concentrations tested, and it inhibited NE uptake in PVAT at 1 μM, a concentration that would be nonspecific for NET. Although convincing evidence that NET is present on mesenteric PVAT adipocytes was not found (Fig. 6), it is possible that ASP+ is a more sensitive tool for the detection of NET. We did not investigate the presence of DA transporters (DAT) further due to the finding that GBR-12935 [100 nM, a DAT inhibitor, ki: 3.7 nM (59)] did not reduce ASP+ fluorescence, and mRNA for DAT could not be detected by PCR in mesenteric PVAT in 40 cycles (data not shown). Therefore, it is unlikely that DAT plays a role in NE uptake in PVAT.
The “anticontractile” effect of PVAT to NE in the rat thoracic aorta is attenuated by desipramine plus deoxycorticosterone (64), and this observation was the impetus to study PVAT NE transport. Since the anticontractile effect of PVAT due to NE transport at least in the rat aorta has already been shown (64), we did not pursue these experiments in the present study. Instead, we set out to find the mechanism by which PVAT can take up NE. We also used desipramine and corticosterone (similar to deoxycorticosterone in that it inhibits OCT3) in this study to investigate transport. Desipramine alone did not have an effect on ASP+ or NE uptake, but, when added in conjunction with corticosterone, we observed a significant reduction in both assays. We observed similar general patterns of inhibition in NE uptake and ASP+ experiments. Interestingly, in contrast to our NE uptake experiment, which required both desipramine and corticosterone or citalopram and corticosterone to reduce uptake, ASP+ fluorescence was reduced by preincubation with corticosterone or citalopram alone. This could be due to a difference in transporter affinity for ASP+ versus NE. ASP+ has been used as a surrogate for [3H]NE, due to the similarity in their pharmacological profiles, but there are differences in their affinity to certain transporters (28). This was also evidenced by the observation that adding a high concentration of NE to saturate NET failed to abolish ASP+ staining, indicating the presence of ASP+ fluorescence nonspecific to NE transport mechanisms. Schwartz et al. (61) observed nonspecific fluorescence of ASP+ in experiments using human embryonic kidney cells. Therefore, care needs to be taken when interpreting findings from ASP+-binding studies to mechanisms of specific NE transport. Both ASP+ and NE uptake experiments pointed to OCT3 as being important, and, thus, the was the focus in our final experiments.
OCT3 is highly expressed in mesenteric and aortic PVATs.
OCT3 is a low-affinity, high-capacity uptake transporter, formally known as extraneuronal monoamine transporter and also termed as the uptake-2 system, and is broadly expressed in non-neuronal cells (51). OCT3 expression on PVAT adipocytes has not yet been investigated. We were surprised to find a higher expression of OCT3 in mesenteric and aortic PVATs than in the heart. It is interesting to speculate on the function NE transport molecules on adipocytes when NE activates lipolysis. Adipocytes contain levels of monoamine oxidase activity that are comparable to that of the liver, an organ high in monoamine metabolizing activity (57). This would support the idea that the function of NE transporters is to deliver NE into the cell to be metabolized.
We observed high expression of OCT3 in aortic PVAT and inhibition of OCT3 reduced NE uptake. The presence of OCT3 in the aorta, a conduit artery, would lead one to question the role of OCT3 in blood pressure regulation. Ultimately, the main role of OCT3 in PVAT is unknown. Uptake of NE by aortic PVAT was reduced by inhibition of OCT3 with corticosterone. Thus, it could serve to remove excess NE (57), but there may be other physiological roles for OCT3 that may not involve blood pressure regulation, such as polyamine transport (60) and clearance of toxins (35). Therefore, OCT3 may be more necessary in the aortic PVAT due to its other roles versus mesenteric resistance PVAT. This would have to be investigated further.
Limitations.
Although in vitro NE transport influences the contractility of blood vessels to NE (5, 64), there is no confirmation that this occurs in vivo. The mesentery and omentum are considered “visceral fat” (33, 52), and the adipose tissue around mesenteric resistance arteries is most specifically referred to as mesenteric PVAT. We showed an image of mesenteric PVAT in Fig. 1 to clarify this point. Mesenteric PVAT is a common PVAT depot that is studied for its relevance to alterations in vascular response and blood pressure (5, 23, 43, 49, 71, 72), whereas PVAT around skeletal muscle arteries is more associated with mechanisms of insulin resistance (7, 27, 47, 48, 74). Therefore, mesenteric PVAT was the most relevant PVAT for us to study.
Aortic PVAT was included in the immunohistochemistry, NE uptake, and RT-PCR analyses as this is the most discrete and widely studied PVAT depot and was therefore useful when we compared our findings with those of other studies. Nonetheless, it should be noted that mesenteric PVAT mechanisms of NE uptake are more physiologically relevant with regard to blood pressure regulation. The lack of specific antibodies against NET and SERT in which we have confidence for use in adipose tissue has been an experimental limitation and why a pharmacological approach using well-characterized transporter inhibitors was used to characterize monoamine transporters. The Western blot analysis for NET lacked strong reactivity to the positive control (Fig. 6), indicating that a lack of signal in our samples could be attributed to the low affinity of the antibody and not that the transporter is not present. This was the best NE antibody that we had available. Studies using knockout animals and/or small interfering RNA toward different molecular transporters could also be helpful to elucidate monoamine transport mechanisms. However, this approach is accompanied with upregulation of transporters to take up NE, facilitated by the promiscuous nature of neurotransmitter transporters (17). This has been observed in the brains of NET knockout mice (63), which exhibit increased SERT and DAT expression. In addition, mice with reduced SERT expression overexpress OCT3 and exhibit increased serotonin clearance through OCT3 (6). These points have been discussed in a previous report as it pertains to SERT knockout rats (44). This upregulation of transporters in genetic models of deficient transport would thereby make the interpretations of experimental results arising from these techniques difficult and is why we did not use the OCT3 knockout rodent in this study.
The anticontractile effect of PVAT due to NE transport at least in the rat aorta was discovered by Soltis and Cassis in 1991 (64), and we present the first biochemical measures to investigate the mechanism by which PVAT can take up NE. This study suggests that NE transport may be involved in the anticontractile effect of PVAT. We fully recognize that other mechanisms exist that reduce NE-induced contraction, such as the release of adiponectin from PVAT adipocytes (26). The contribution of these mechanisms in a physiological system may dictate the pathology observed in different vascular disorders. A decline in NE transport capacity of PVAT may exacerbate loss of adiponectin followed by adipocyte hypertrophy and dysfunction in disease. In addition, in situations of dysfunctional adiponectin release, PVAT NE transport may become a more important mechanism of reducing vascular tone. These are questions we will pursue in the future.
The method in which we euthanized the animal before tissue collection could have a potential effect on adrenergic system activation; thus, these factors and how they would affect our end points were considered. Hirota et al. (31) reported inhibition of NE (noradrenaline) and DA release after exposure of rat brain striatal slices to barbiturates, including pentobarbital. Other groups have similarly reported an inhibition of NE release (34, 50, 62) or no effect (41, 56) by pentobarbital in their studies. Our laboratory has previously euthanized rats using isoflurane or pentobarbital, and we have not observed a difference in tissue catecholamine content with either of them, suggesting similar functions of uptake and release. In the present study, most of the experiments allow comparisons within each animal by reporting percent NE uptake (opposed to absolute NE values) and every animal was euthanized the same way (with pentobarbital). Thus, we hope that small changes in baseline NE would not affect our results.
Great care was taken to clean our adipocyte fraction from any contaminating cells. A possibility is that there was some contamination of nerve fibers and SVF cells in the adipocyte isolates, and this is why it was especially revealing to observe fluorescent labeling of PVAT adipocytes with ASP+. With confocal microscopy, it was possible to confirm that ASP+ was labeling adipocytes and could readily distinguish if blood vessels or other structures that were in the visual field were exhibiting fluorescence with ASP+.
Conclusions, novelty, and significance.
The present study identified NE transport in PVAT and found OCT3 to be the prime candidate transporter of NE within the PVAT adipocyte. This is the first report of NE transport in PVAT adipocytes. This is also the first time that ASP+ has been applied to the study of adipose tissue. Experiments using ASP+ may be extended to compare NE transport in disease models of obesity and hypertension to investigate the regulation and dysfunction of NE transport.
The present study identified NE uptake into PVAT as part of a larger project defining the physiology and pathophysiology of an endogenous adrenergic system in PVAT. Adipose tissue maintains high expression of the amine-metabolizing enzymes monoamine oxidase and semicarbazide-sensitive amine oxidase (1). Therefore, it is likely that one way PVAT interacts with NE is by breaking it down with amine oxidases. Relative to NE storage, tyramine (a sympathomimetic drug)-induced PVAT-dependent contraction of the rat thoracic aorta and rat superior mesenteric artery was reduced by tetrabenazine (a vesicular monoamine transporter inhibitor). This finding, published by our group, supports that local stores of NE in PVAT could contribute to vascular contraction (5). Collectively, these findings will define an adrenergic system in PVAT that we can then investigate in obesity.
This study sheds light on the interaction between PVAT and the blood vessel within a local adrenergic system. The role of PVAT reducing vascular contraction in health could be, in part, due to NE uptake into PVAT, and this mechanism of NE removal may be dysfunctional in diseases of altered vascular tone. The scarcity of information on transporters of NE and NE uptake in PVAT led us to study normal (nondisease model) rats to test whether uptake is an important physiological mechanism in PVAT. Understanding the normal functional characteristics of NE uptake in the nondiseased rodent allows us to know what to look for when investigating a disease model. In this study, we developed an assay (confocal microscopy of ASP+ staining of adipose tissue) that we and/or other investigators could use to investigate PVAT mechanisms in an obese and/or hypertensive model organism. The present study is a first step in this direction.
GRANTS
This work was supported by National Institutes of Health Grants P01-HL-70687 and 5-T32-GM 92715-4.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.A.-L., W.F.J., J.N.W., and S.W.W. conception and design of research; N.A.-L. and J.M.T. performed experiments; N.A.-L. and R.B. analyzed data; N.A.-L. interpreted results of experiments; N.A.-L. prepared figures; N.A.-L. drafted manuscript; N.A.-L., W.F.J., R.B., J.N.W., J.M.T., and S.W.W. edited and revised manuscript; N.A.-L., W.F.J., R.B., J.N.W., J.M.T., and S.W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Cassandra LaMarche for technical assistance.
REFERENCES
- 1.Abella A, Garcia-Vicente S, Viguerie N, Ros-Baro A, Camps M, Palacin M, Zorzano A, Marti L. Adipocytes release a soluble form of VAP-1/SSAO by a metalloprotease-dependent process and in a regulated manner. Diabetologia 47: 429–438, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Aghamohammadzadeh R, Heagerty AM. Obesity-related hypertension: epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann Med 44, Suppl 1: S74–S84, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW. Perivascular adipose tissue contains functional catecholamines. Pharmacol Res Perspect 2: e00041, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci USA 105: 18976–18981, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 335: 165–189, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 20: 225–231, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Brandes RP. The fatter the better? Perivascular adipose tissue attenuates vascular contraction through different mechanisms. Br J Pharmacol 151: 303–304, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 34: 1621–1630, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, Ryan JJ, Stewart JK, Fischer-Stenger K. Catecholamines in a macrophage cell line. J Neuroimmunol 135: 47–55, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Bulloch JM, Daly CJ. Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther 143: 61–73, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Vital signs: prevalence, treatment, and control of hypertension–United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep 60: 103–108, 2011. [PubMed] [Google Scholar]
- 14.Chaldakov GN, Tonchev AB, Stankulov IS, Ghenev PI, Fiore M, Aloe L, Rancic G, Panayotov P, Kostov DD. Periadventitial adipose tissue (tunica adiposa): enemy or friend around? Arch Pathol Lab Med 131: 1766; author reply 1766–1767, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Pawlikowski B, Schlessinger A, More SS, Stryke D, Johns SJ, Portman MA, Chen E, Ferrin TE, Sali A, Giacomini KM. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet Genomics 20: 687–699, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davids E, Zhang K, Kula NS, Tarazi FI, Baldessarini RJ. Effects of norepinephrine and serotonin transporter inhibitors on hyperactivity induced by neonatal 6-hydroxydopamine lesioning in rats. J Pharmacol Exp Ther 301: 1097–1102, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther 121: 89–99, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Felice LJ, Adams SV. Serotonin and norepinephrine transporters: possible relationship between oligomeric structure and channel modes of conduction. Mol Membr Biol 18: 45–51, 2001. [DOI] [PubMed] [Google Scholar]
- 19.DeMartinis FD, Ashkin KT, Lampe KT. Fluorescence detection of mitochondrial clusters in mammalian white fat cells in vivo. Am J Physiol Cell Physiol 253: C783–C791, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 286: H1107–H1113, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Alfonso MS, Gil-Ortega M, Garcia-Prieto CF, Aranguez I, Ruiz-Gayo M, Somoza B. Mechanisms of perivascular adipose tissue dysfunction in obesity. Int J Endocrinol 2013: 402053, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75: 719–727, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 26: 1297–1302, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol 151: 323–331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res 71: 363–373, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Hariya N, Mochizuki K, Inoue S, Morioka K, Shimada M, Okuda T, Goda T. Insulin resistance in SHR/NDmc-cp rats correlates with enlarged perivascular adipocytes and endothelial cell dysfunction in skeletal muscle. J Nutr Sci Vitaminol (Tokyo) 60: 52–59, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Haunso A, Buchanan D. Pharmacological characterization of a fluorescent uptake assay for the noradrenaline transporter. J Biomol Screen 12: 378–384, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Henry SL, Barzel B, Wood-Bradley RJ, Burke SL, Head GA, Armitage JA. Developmental origins of obesity-related hypertension. Clin Exp Pharmacol Physiol 39: 799–806, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Hensler JG, Artigas F, Bortolozzi A, Daws LC, De Deurwaerdere P, Milan L, Navailles S, Koek W. Catecholamine/serotonin interactions: systems thinking for brain function and disease. Adv Pharmacol 68: 167–197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirota K, Kudo M, Kudo T, Kitayama M, Kushikata T, Lambert DG, Matsuki A. Barbiturates inhibit K+-evoked noradrenaline and dopamine release from rat striatal slices–involvement of voltage sensitive Ca2+ channels. Neurosci Lett 291: 175–178, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Horschitz S, Hummerich R, Schloss P. Functional coupling of serotonin and noradrenaline transporters. J Neurochem 86: 958–965, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11: 11–18, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Ida Y, Tsuda A, Tsujimaru S, Satoh M, Tanaka M. Pentobarbital attenuates stress-induced increases in noradrenaline release in specific brain regions of rats. Pharmacol Biochem Behav 36: 953–956, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3). J Pharmacol Exp Ther 308: 2–9, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Kang YS. Obesity associated hypertension: new insights into mechanism. Electrolyte Blood Press 11: 46–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocabas AM, Rudnick G, Kilic F. Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters. J Neurochem 85: 1513–1520, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koepsell H. Polyspecific organic cation transporters and their biomedical relevance in kidney. Curr Opin Nephrol Hypertens 22: 533–538, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24: 1227–1251, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens 23: 1170–1178, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Kubota T, Anzawa N, Hirota K, Yoshida H, Kushikata T, Matsuki A. Effects of ketamine and pentobarbital on noradrenaline release from the medial prefrontal cortex in rats. Can J Anaesth 46: 388–392, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 371: 1513–1518, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Andersen I, Aleke J, Golubinskaya V, Gustafsson H, Nilsson H. Reduced anti-contractile effect of perivascular adipose tissue on mesenteric small arteries from spontaneously hypertensive rats: role of Kv7 channels. Eur J Pharmacol 698: 310–315, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Linder AE, Diaz J, Ni W, Szasz T, Burnett R, Watts SW. Vascular reactivity, 5-HT uptake, and blood pressure in the serotonin transporter knockout rat. Am J Physiol Heart Circ Physiol 294: H1745–H1752, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B, Richter EA, Smulders YM, van Hinsbergh VW, Serne EH, Eringa EC. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 62: 590–598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meijer RI, Serne EH, Korkmaz HI, van der Peet DL, de Boer MP, Niessen HW, van Hinsbergh VW, Yudkin JS, Smulders YM, Eringa EC. Insulin-induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia 58: 1907–1915, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendizabal Y, Llorens S, Nava E. Vasoactive effects of prostaglandins from the perivascular fat of mesenteric resistance arteries in WKY and SHROB rats. Life Sci 93: 1023–1032, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Mizuno T, Ito E, Kimura F. Pentobarbital sodium inhibits the release of noradrenaline in the medial preoptic area in the rat. Neurosci Lett 170: 111–113, 1994. [DOI] [PubMed] [Google Scholar]
- 51.Mooney JJ, Samson JA, Hennen J, Pappalardo K, McHale N, Alpert J, Koutsos M, Schildkraut JJ. Enhanced norepinephrine output during long-term desipramine treatment: a possible role for the extraneuronal monoamine transporter (SLC22A3). J Psychiatr Res 42: 605–611, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen NL, Randall J, Banfield BW, Bartness TJ. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am J Physiol Regul Integr Comp Physiol 306: R375–R386, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311: 806–814, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 283: 1305–1322, 1997. [PubMed] [Google Scholar]
- 55.Paczkowski FA, Bryan-Lluka LJ, Porzgen P, Bruss M, Bonisch H. Comparison of the pharmacological properties of cloned rat, human, and bovine norepinephrine transporters. J Pharmacol Exp Ther 290: 761–767, 1999. [PubMed] [Google Scholar]
- 56.Pashkov VN, Hemmings HC Jr. The effects of general anesthetics on norepinephrine release from isolated rat cortical nerve terminals. Anesth Analg 95: 1274–1281, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Pizzinat N, Marti L, Remaury A, Leger F, Langin D, Lafontan M, Carpene C, Parini A. High expression of monoamine oxidases in human white adipose tissue: evidence for their involvement in noradrenaline clearance. Biochem Pharmacol 58: 1735–1742, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Qiu YH, Peng YP, Jiang JM, Wang JJ. Expression of tyrosine hydroxylase in lymphocytes and effect of endogenous catecholamines on lymphocyte function. Neuroimmunomodulation 11: 75–83, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39: 32–41, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Sala-Rabanal M, Li DC, Dake GR, Kurata HT, Inyushin M, Skatchkov SN, Nichols CG. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol Pharm 10: 1450–1458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz JW, Blakely RD, DeFelice LJ. Binding and transport in norepinephrine transporters. Real-time, spatially resolved analysis in single cells using a fluorescent substrate. J Biol Chem 278: 9768–9777, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Shimokawa A, Jin QH, Ishizuka Y, Kunitake T, Takasaki M, Kannan H. Effects of anesthetics on norepinephrine release in the hypothalamic paraventricular nucleus region of awake rats. Neurosci Lett 244: 21–24, 1998. [DOI] [PubMed] [Google Scholar]
- 63.Solich J, Faron-Gorecka A, Kusmider M, Palach P, Gaska M, Dziedzicka-Wasylewska M. Norepinephrine transporter (NET) knock-out upregulates dopamine and serotonin transporters in the mouse brain. Neurochem Int 59: 185–191, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 13: 277–296, 1991. [DOI] [PubMed] [Google Scholar]
- 65.Stern N, Marcus Y. Perivascular fat: innocent bystander or active player in vascular disease? J Cardiometab Syndr 1: 115–120, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Stunes AK, Reseland JE, Hauso O, Kidd M, Tommeras K, Waldum HL, Syversen U, Gustafsson BI. Adipocytes express a functional system for serotonin synthesis, reuptake and receptor activation. Diabetes Obes Metab 13: 551–558, 2011. [DOI] [PubMed] [Google Scholar]
- 66a.Stuurman N, Edelstein AD, Amodaj N, Hoover KH, and Vale RD. Computer Control of Microscopes Using μManager (online). http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3065365/ [15 October 2015]. [DOI] [PMC free article] [PubMed]
- 67.Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 9: 105–116, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 122: 1–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R. Adipocytes as a new source of catecholamine production. FEBS Lett 585: 2279–2284, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Verhaagh S, Schweifer N, Barlow DP, Zwart R. Cloning of the mouse and human solute carrier 22a3 (Slc22a3/SLC22A3) identifies a conserved cluster of three organic cation transporters on mouse chromosome 17 and human 6q26-q27. Genomics 55: 209–218, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 44: 271–276, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol 33: 1320–1328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson JN, Brown AS, Babinchak WM, Ridge CD, Walls JD. Fluorescent stilbazolium dyes as probes of the norepinephrine transporter: structural insights into substrate binding. Org Biomol Chem 10: 8710–8719, 2012. [DOI] [PubMed] [Google Scholar]
- 73a.World Health Organization. Obesity and Overweight (Fact Sheet 311) (online). http://www.who.int/mediacentre/factsheets/fs311/en/ [15 October 2015].
- 74.Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Zhu HJ, Appel DI, Grundemann D, Richelson E, Markowitz JS. Evaluation of organic cation transporter 3 (SLC22A3) inhibition as a potential mechanism of antidepressant action. Pharmacol Res 65: 491–496, 2012. [DOI] [PubMed] [Google Scholar]