Diabetes in America is in the public domain of the United States. You may use the work without restriction in the United States.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lawrence JM, Casagrande SS, Herman WH, et al., editors. Diabetes in America [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2023-.
Summary
Type 1 diabetes results from autoimmune destruction of insulin-producing beta cells. There is a genetic predisposition to the disease, with the greatest contribution conferred by alleles present within the major histocompatibility complex (MHC; known as the human leukocyte antigen [HLA] region) on the short arm of chromosome 6. Whether the initiation of this immune response results from environmental triggers remains to be determined. Over years, immune infiltration into pancreatic islets leads to beta cell damage, impairment of cell function, and destruction of beta cells. This notion has led to clinical trials to arrest the progression of disease and potentially prevent or reverse the clinical syndrome.
Clinical trials using various immunologic agents have been conducted at multiple stages of the disease process. Primary prevention trials have been conducted in individuals with genetic predisposition who have not yet developed immunologic markers. Secondary prevention trials have been conducted in individuals with two or more type 1 diabetes-related autoantibodies, either during Stage 1 (normal metabolic function) or Stage 2 (abnormal metabolic function). Intervention trials, also referred to as tertiary prevention trials, have been conducted after diagnosis of hyperglycemia (Stage 3), mostly shortly after clinical onset of disease.
This article provides brief summaries of randomized controlled clinical trials that have been performed and mentions some nonrandomized pilot studies. Success has been limited in primary and secondary prevention trials, with one recent and notable exception (teplizumab). Some tertiary intervention trials have demonstrated improved beta cell function, but these studies have not permanently prevented the decline in beta cell function (eventually declining in parallel to controls). Future interventions with combinations of agents that can target multiple immunologic mechanisms may be needed, including strategies to improve regulatory immunity, as well as to replace and restore beta cell function.
Introduction
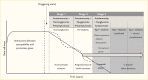
Type 1 diabetes is a progressive disease with a genetic predisposition, where a putative environmental trigger(s) initiates an immune response that results in pancreatic islet beta cell damage, impairment of beta cell function, and destruction of beta cells (1,2,3). Although initial characterization suggested a slow, linear progression of disease (1), more recent thought is that progression of type 1 diabetes is more variable, with waxing and waning of beta cell mass (Figure 1, solid line versus dotted line in Stages 1 and 2) (3,4). Moreover, the disease may be a consequence of imbalance between the immune system and the ability of the pancreatic beta cell to withstand attack (5).
A genetic basis for type 1 diabetes in association with human leukocyte antigens (HLA) was first described in the early 1970s (6), and subsequently, that relationship has been extensively characterized (7,8,9,10), with both HLA class II (10,11) and class I (12) contributing to genetic susceptibility and protection (13). Although multiple other genes have been identified (14,15), the HLA region remains the major contributor to genetic predisposition (16). In a study of the general population of Denver, Colorado, children born with the high-risk genotype HLA-DR3/4-DQ8 account for almost 50% of children who develop islet autoimmunity by age 5 years (17). Non-major histocompatibility complex (MHC) susceptibility genes may play a role in determining the rate of disease progression (17,18). More detail on genetic factors is described in the Diabetes in America article Genetics of Type 1 Diabetes (19).
An environmental or other acquired factor(s) that activates immunologically mediated destruction of beta cells has been suggested, but this (these) factor(s) has not been definitively identified (20,21). The role that the beta cell may play in its own demise, via endoplasmic reticulum (ER) stress and more, is a critical area of ongoing research (22). Moreover, the association between environmental factors and the course of the disease is complicated by observations that not only initiation of the disease process but also the rate of progression to clinical onset may be affected by environmental determinants. Further, metabolic decompensation at disease onset may be a consequence of unrelated or nonspecific environmental events that may, for example, induce inflammation in both the endocrine and exocrine pancreas. Observational cohort studies, such as The Environmental Determinants of Diabetes in the Young (TEDDY) study (23,24), are designed to ascertain environmental determinants that may trigger islet autoimmunity and affect the rate of progression to clinical onset in subjects with persistent islet autoimmunity. Please see the Diabetes in America article Risk Factors for Type 1 Diabetes for more discussion of putative environmental triggers of type 1 diabetes (25).
The immune response is initiated by antigen presentation in the context of HLA class II molecules with beta cell destruction mediated by T lymphocytes (26,27), resulting in a lymphocytic inflammatory response in pancreatic islets, called insulitis (28). Both effector CD4 (29) and cytotoxic CD8 (30) T lymphocytes are involved. These immune cells have the capacity to mediate damage both via cytokine effects, involving such cytokines as interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), or direct cytotoxic T lymphocyte-mediated lysis. This initial immune response, with continued lysis, creates the potential of a vicious cycle of inflammation, which also may engender secondary and tertiary immune responses that contribute to the impairment and eventual destruction of beta cells (3,5,26). This insidious process evolves over a variable amount of time—even many years in some individuals, particularly if diagnosed in adulthood. The eventual overt manifestation of clinical symptoms becomes apparent only when most beta cells have lost function and many have been destroyed.
The initial laboratory manifestation of this immune response to beta cell injury is seroconversion, i.e., the appearance of type 1 diabetes-related autoantibodies. These antibodies are not thought to mediate beta cell injury but to be markers of such injury. Type 1 diabetes-related autoantibodies were first described in the early 1970s, when islet cell antibodies (ICA) were identified by immunofluorescence (31). Subsequently, additional antibodies were identified with specific autoantigen targets, including insulin autoantibodies (IAA) and autoantibodies to glutamic acid decarboxylase (GAD), an aborted tyrosine phosphatase (islet antibody-2 or IA2), and the zinc transporter (ZnT8) (32), all components of beta cells. Seroconversion is an important marker of the type 1 diabetes disease process. Indeed, in longitudinal studies of birth cohorts identified by genetic screening, such as DAISY (Diabetes AutoImmunity Study in the Young) (33), BABYDIAB (34), and DIPP (DIabetes Prediction and Prevention study) (35), as well as the TEDDY study, if two or more autoantibodies appear, 61.6%–79.1% of children followed will progress to type 1 diabetes over the next 15 years (36,37,38). This finding has led to a new classification of type 1 diabetes (Figure 1), in which the presence of two or more autoantibodies defines Stage 1 type 1 diabetes (39).
During further evolution of the disease, progressive metabolic changes are observable (40). Lack of beta cell sensitivity to glucose—that is, failure of the beta cell to recognize glucose and appropriately secrete insulin—is an early defect (41), like that seen in type 2 diabetes (42). This defect may be manifested by loss of first-phase insulin response to intravenous glucose (43) and dysglycemia (abnormal glucose levels not reaching the threshold for clinical diagnosis), which defines Stage 2 type 1 diabetes. Dysglycemia typically refers to fasting glucose of 100–125 mg/dL (5.6–6.9 mmol/L), 2-hour oral glucose tolerance test (OGTT) glucose of 140–199 mg/dL (7.8–11.0 mmol/L), or 30, 60, or 90-minute OGTT glucose ≥200 mg/dL (≥11.1 mmol/L) (39). There is progression to symptomatic hyperglycemia (44), or Stage 3 type 1 diabetes (45) (defined as fasting glucose ≥126 mg/dL [≥7.0 mmol/L] or 2-hour OGTT glucose ≥200 mg/dL). Risk scores that consider several of these metabolic changes (e.g., glucose and C-peptide from OGTTs) have been developed (46,47) and validated (48,49,50), and these scores further help to distinguish progressors from non-progressors. After the clinical onset of type 1 diabetes, further progressive decline of beta cell function occurs (51).
If type 1 diabetes is an immunologically mediated disease, then immune intervention should alter the natural history of the disease and potentially abrogate the clinical syndrome. This concept has certainly been the case in animal models of type 1 diabetes (52,53,54). The first reported attempt at immune intervention in type 1 diabetes was in the late 1970s in a handful of subjects (55). In the 1980s, a number of small trials were conducted with a variety of immunologic agents (56). Then, stimulated by a provocative pilot study with cyclosporine (57), many studies have been conducted, mostly in recent-onset type 1 diabetes, in an attempt to interdict the disease process and preserve beta cell function (58,59). More and more studies have been conducted during the period prior to any evidence of autoimmunity (primary prevention) or after the development of type 1 diabetes-related autoantibodies (secondary prevention) (60). The goal of such primary and secondary interventions is to arrest the immune process and, thus, prevent or delay clinical disease.
This article describes significant progress made toward prevention of type 1 diabetes since the publication of the Diabetes in America, 3rd edition chapter Prevention of Type 1 Diabetes in 2018 (61). Table 1 lists both completed and ongoing primary and secondary prevention trials. Table 2 lists many contemporary intervention trials in subjects at clinical Stage 3 type 1 diabetes (tertiary prevention), mostly in recent-onset subjects, but some with established disease. Most studies listed in the tables are randomized controlled clinical trials, although a few pilot studies of significance are included.
TABLE 1.
Summary of the Results of Primary and Secondary Prevention Trials for Type 1 Diabetes Published Through April 2024
TABLE 2.
Summary of the Results of Intervention Studies in Recent-Onset Type 1 Diabetes Published Through April 2024
Primary Prevention Trials
Primary prevention trials (Table 1) have been conducted in birth cohorts identified by genetic screening for high-risk HLA haplotypes (e.g., DR3, DR4), with the interventions initiated at a time when there were neither signs of autoimmunity nor overt metabolic impairment. Since there is uncertainty as to whether those infants identified by genetic screening will progress to type 1 diabetes, any interventions evaluated must be extremely safe. Consequently, most primary prevention trials to date have involved dietary interventions directed at putative environmental triggers (62,63,64,65,66,67,68), although more recently, oral insulin antigen-specific therapy aimed at inducing early tolerance has been tested (69,70).
A 1994 meta-analysis demonstrated a correlation between onset of type 1 diabetes and either early introduction of cow’s milk formula or a brief period of breastfeeding (71). Consequently, two studies evaluated whether at the time of weaning, replacement of breast milk with a formula based on casein hydrolysate rather than conventional cow’s milk-based formula could abrogate autoimmunity (62,63). Eligible infants had HLA-conferred susceptibility to type 1 diabetes and at least one family member with type 1 diabetes. A pilot study in Finland enrolled 230 infants (62). The investigators reported that the group assigned to casein hydrolysate formula had a reduced risk of development of beta cell autoimmunity (appearance of one or more of the type 1 diabetes-related autoantibodies). A hazard ratio (HR) of 0.54 (95% confidence interval [CI] 0.29–0.95) was reported; similarly, the hazard ratio adjusted for observed difference in duration of exposure to study formula was 0.51 (95% CI 0.28–0.91) (62). The larger Trial to Reduce IDDM in the Genetically at Risk (TRIGR), a multinational trial involving 77 centers in 15 countries, registered more than 5,000 newborns and randomized 2,159 with risk genotypes (approximately 45% of those screened) (63). After 7 years, the TRIGR Study Group found no difference in the rate of appearance of type 1 diabetes-related autoantibodies (63). In the group assigned to casein hydrolysate formula, 13.4% had two or more islet autoantibodies versus 11.4% among those randomized to the conventional formula (unadjusted HR 1.21, 95% CI 0.94–1.54). When adjusted for HLA risk, duration of breastfeeding, vitamin D use, study formula duration and consumption, and region of the world, the hazard ratio was 1.23 (95% CI 0.96–1.58). Nonetheless, TRIGR continued follow-up for >10 years because it was designed with a primary outcome of the development of type 1 diabetes. The frequency of type 1 diabetes development was similar between the casein hydrolysate formula group (8.4%) and the conventional formula group (7.6%, p=0.47) with an adjusted hazard ratio of 1.1 (95% CI 0.8–1.5, p=0.46) (68). Casein hydrolysate had no effect on progression through the stages of type 1 diabetes in the TRIGR study.
In earlier studies, to evaluate whether bovine insulin might be the component of cow’s milk that serves as a trigger for type 1 diabetes, the Finnish Dietary Intervention Trial for the Prevention of Type 1 Diabetes (FINDIA) compared three formulas: cow’s milk-based formula (control), whey-based hydrolyzed formula, or whey-based FINDIA formula essentially free of bovine insulin, whenever breast milk was not available during the first 6 months of life (64). Of 5,003 infants screened, 1,113 were found eligible, 1,104 were randomized, and 908 provided at least one follow-up sample. By age 3 years, the group assigned to the FINDIA formula had a reduced risk of development of beta cell autoimmunity, defined as the appearance of one or more islet autoantibodies, in both the intention-to-treat analysis (odds ratio [OR] 0.39, 95% CI 0.17–0.91, p=0.03) and the actual treatment-received analysis (OR 0.23, 95% CI 0.08–0.69, p<0.01) for those in the FINDIA group when compared with the cow’s milk formula group (64).
The BABYDIET study, another randomized controlled trial, evaluated whether delayed exposure to gluten reduces the risk of type 1 diabetes autoimmunity (65). The rationale for the study was based on the investigators’ earlier prospective observational cohort demonstrating increased risk of islet autoimmunity in children who are exposed to gluten early in life (72). The BABYDIET trial randomized 150 infants with a first-degree relative with type 1 diabetes and an HLA risk genotype. They were assigned either to first gluten exposure at age 6 months (control group) or at age 12 months (late-exposure group) and were followed every 3 months until age 3 years and yearly thereafter. BABYDIET found that delaying gluten exposure until the age of 12 months was safe but did not reduce the risk for islet autoimmunity (3-year risk: 12% vs. 13%, p=0.6) (65), although the open-label nature and difficulty adhering to the dietary recommendations may have limited the outcome.
The Type 1 Diabetes TrialNet (TrialNet) Nutritional Intervention to Prevent (NIP) Type 1 Diabetes Pilot Trial assessed the feasibility of implementing a study to determine the effect of nutritional supplements with the omega-3 fatty acid docosahexaenoic acid (DHA), which has anti-inflammatory effects, during the last trimester of pregnancy and the first few years of life (66). NIP found that supplementation of infant diets with DHA was safe and resulted in an increased level of DHA in infant erythrocytes but did not find consistent reduction in inflammatory cytokines (66).
Based on putative observations that vitamin D administration in pregnancy may be protective of the subsequent development of diabetes in offspring of mothers with type 1 diabetes and that supplementation with vitamin D in infancy has been associated with decreased risk of type 1 diabetes (73,74), a group of Canadian investigators conducted a small pilot study. The group showed that it was possible to recruit infants from the general population for identification of HLA-associated risk status followed by enrollment before 1 month of age to a randomized controlled prevention trial of vitamin D supplementation (67). Therefore, they proposed a nationwide study (in Canada) to evaluate the hypothesis that vitamin D supplementation can decrease the risk of islet autoimmunity and type 1 diabetes. As of 2023, such a study has not been initiated.
The Primary Oral Insulin Therapy (Pre-POInT) study was a pilot safety study across four countries of the use of oral insulin in children age 2–7 years at risk of developing type 1 diabetes (69). The study found that daily oral administration of 67.5 mg insulin is safe and can actively engage the immune system with features of immune regulation in children who are genetically at risk of developing type 1 diabetes. Pre-POInT was followed by the Pre-POInT-EARLY study in Germany, assessing the use of daily oral insulin (67.5 mg) in children age 6 months to 3 years (70). In this small study, the authors did not find a statistically significant difference in immune responses to insulin in the children who received oral insulin compared to the control group (70).
Ongoing Primary Prevention Trials
A large, quadruple-blinded randomized controlled trial, POInT, has been initiated, powered to assess the clinically relevant outcomes of development of islet autoimmunity and/or type 1 diabetes (75). Other primary prevention trials underway include use of intranasal insulin (76) and Bifidobacterium probiotic supplementation (77). Philosophically, these types of therapies can be thought of as Generally Recognized As Safe (GRAS) agents, which are appealing in the setting of Stage 1 type 1 diabetes (78).
Building on these efforts, it would seem desirable to conduct more studies in those with genetic predisposition, particularly if a safe vaccine-type approach can be studied. This might involve an antigen-based vaccine (such as that used in the Pre-POInT study) or a vaccine directed against potential viral triggers of disease. To conduct such trials, it will be necessary to screen various populations at or shortly after birth, looking for high-risk genetic predisposition as is being done in the Global Platform for the Prevention of Autoimmune Diabetes (GPPAD) and their GPPAD-02 screening program (79). Theoretically, if such trials resulted in prevention of type 1 diabetes, this outcome would change public health practice, leading to routine screening at birth for high genetic risk. Further, if the intervention were safe, it could be included in routine neonatal or infant vaccination programs.
Secondary Prevention Trials
Secondary prevention trials (Table 1) are those conducted in individuals with Stage 1 (two or more islet autoantibodies with normoglycemia) or Stage 2 (islet autoantibodies and dysglycemia) type 1 diabetes (45). Most of these studies have been conducted in individuals who are first- or second-degree relatives of people with type 1 diabetes and who had been identified initially by screening for type 1 diabetes-associated autoantibodies. Because not all those with autoantibodies will progress to symptomatic disease (Stage 3), interventions to be evaluated have been selected cautiously to balance risk of therapy with risk of developing the disease. Initial studies tested nicotinamide (a vitamin B3 derivative) and insulin used in various routes of administration for its potential antigenic effects (to induce immune tolerance). This strategy was followed by GAD antigen therapy. With limited success, stronger therapeutics, such as anti-inflammatory and T lymphocyte-directed therapies, are now being studied.
Nicotinamide Trials
Nicotinamide, a water-soluble form of vitamin B3, had been shown to prevent diabetes in animal models and was asserted to have beneficial effect in school children. Consequently, two studies evaluated the effects of nicotinamide in at-risk relatives of individuals with type 1 diabetes: the German (Deutsch) Nicotinamide Diabetes Intervention Study (DENIS) (80) and the European Nicotinamide Diabetes Intervention Trial (ENDIT) (81); both were randomized placebo-controlled trials. DENIS randomized 55 relatives age 3–12 years and did not demonstrate a meaningful reduction in cumulative diabetes incidence at 3 years, resulting in early trial termination (80). ENDIT screened more than 35,000 relatives age 5–40 years and randomized 552 individuals to nicotinamide or placebo (81). The relatives who screened positive were projected to have a 5-year risk of type 1 diabetes of 40% (inclusion criteria of ICA-positivity, first-degree relative, and the relative having type 1 diabetes onset age <20 years). During 4 years of follow-up, the rate of development of type 1 diabetes was identical in both the nicotinamide and placebo groups (81). Thus, in these two studies, nicotinamide failed to delay the development of type 1 diabetes.
Insulin Trials
The Diabetes Prevention Trial-Type 1 (DPT-1) Study Group conducted two studies concomitantly: (1.) the DPT-1 Parenteral Insulin Trial (82) evaluated injected (parenteral) insulin in individuals with a projected 5-year risk of type 1 diabetes of at least 50% (who had Stage 2 type 1 diabetes) and (2.) the DPT-1 Oral Insulin Trial (83) evaluated oral insulin in individuals with a projected 5-year risk of type 1 diabetes of 25%–50% (who had Stage 1 type 1 diabetes). DPT-1 screened more than 100,000 relatives of individuals with type 1 diabetes for ICA by immunofluorescence and randomized 339 and 372 subjects, respectively, in the two trials.
Eligibility for the DPT-1 Parenteral Insulin Trial required, in addition to autoantibodies, evidence of reduced first-phase insulin response to intravenous glucose or glucose intolerance during an OGTT, thus meeting the criteria for Stage 2 type 1 diabetes. The experimental intervention was two daily injections of long-acting ultralente insulin plus a 96-hour continuous intravenous insulin infusion at baseline and annually thereafter. The randomized control group was closely observed but did not receive placebo. The rate of development of diabetes was the same in both the treated and control groups (HR 0.96, 95% CI 0.69–1.34, p=0.80) (82).
Eligibility for the DPT-1 Oral Insulin Trial required, in addition to autoantibodies, intact first-phase insulin response and normal glucose tolerance, thus meeting the criteria for Stage 1 type 1 diabetes. Randomized subjects received either oral insulin (7.5 mg) or matched placebo taken daily. The rate of development of diabetes was the same in both groups (HR 0.76, 95% CI 0.51–1.14, p=0.189) (83). In a post hoc analysis, a subgroup (individuals with higher IAA titers at baseline) was identified in which oral insulin appeared to have a beneficial effect. This subgroup had a projected delay of 4.5–5 years in onset of type 1 diabetes if baseline IAA titer was >80 nU/mL (83) and a projected delay of 10 years if baseline IAA titer was >300 nU/mL (84). Further follow-up of the DPT-1 oral insulin cohort showed that effects were maintained after administration of oral insulin ceased (85). Because the subgroup with a potential beneficial effect was identified in a post hoc analysis, another trial was conducted by TrialNet to examine oral insulin in subjects like those in the DPT-1 subgroup with higher titer IAA (86).
The TrialNet Oral Insulin Study was designed to test the beneficial post hoc effect seen in DPT-1, whereby those with high IAA titers (via radioimmunoassay) were noted to progress faster but also demonstrated benefit from oral insulin (86). However, improvements in the microinsulin autoantibody assay necessitated a deviation in IAA measurement from DPT-1 procedures. Eligibility criteria also included at least one additional autoantibody (GAD or IA2 autoantibodies) followed by ICA testing (of note, ICA testing was done first in DPT-1) and normal glucose tolerance. Participants were randomized to oral insulin (7.5 mg/day) or placebo. The primary analysis stratum (n=389) did not demonstrate a difference in the rate of diabetes (annualized rate of 8.8% in the oral insulin group and 10.2% in the placebo group) with a hazard ratio of 0.87 (95% CI 0–1.2, p=0.21). However, a prespecified secondary stratum of subjects (n=55) with lower first-phase insulin release in response to intravenous glucose were noted to have a high rate of progression to type 1 diabetes but a beneficial effect from oral insulin (annualized rate of 18.1% in the oral insulin group and 34.1% in the placebo group) with a hazard ratio of 0.45 (95% CI 0–0.82, p=0.006) (86). Overall, this therapy has been ineffective in the DPT-1 and TrialNet primary stratum trial populations.
The Belgian Diabetes Registry also evaluated whether parenteral insulin might delay the development of type 1 diabetes (87). In this study, the experimental group (n=25) received regular insulin twice daily before the most carbohydrate-rich meals, and the randomized control group (n=25) was closely observed but did not receive placebo. Eligible subjects were age 5–40 years, with IA2 antibodies and normal oral glucose tolerance. Diabetes-free survival did not differ between the two groups (p=0.97), with 5-year progression of 44% in the treated group and 49% in the control group.
The DIPP study was conducted in Finland among newborns from the general population (i.e., without relatives with type 1 diabetes) with high-risk HLA-DQB1 susceptibility alleles for type 1 diabetes (88). Cord blood samples from 116,720 consecutively born infants were screened, which identified 17,397 with high or moderate genetic risk, of whom 10,577 participated in a prospective study. The intervention study required at least two islet autoantibodies (Stage 1 type 1 diabetes); 224 infants were randomized to receive either intranasal insulin or placebo. DIPP also screened siblings of those infants and followed those who also had increased genetic risk, with 40 siblings randomized to receive intranasal insulin or placebo. During follow-up, within each of the cohorts (infants and siblings), the rate of progression to type 1 diabetes was the same in the intranasal insulin and placebo groups (88).
The Intranasal Insulin Trial (INIT-I), conducted in Australia, used a double-blind crossover design to evaluate the safety of intranasal insulin (89). The study included 38 individuals at risk for type 1 diabetes, who were treated with either intranasal insulin or placebo daily for 10 days and then 2 days per week for 6 months, after which they were crossed over to the other treatment. No acceleration of onset of type 1 diabetes was observed nor were there other adverse outcomes. Intranasal insulin was associated with an increase in antibodies and a decrease in T cell responses to insulin. Since there were no safety issues, another study, Intranasal Insulin Trial-II (INIT-II), under the auspices of the Diabetes Vaccine Development Centre (DVDC) in Australia, evaluated whether intranasal insulin in escalated doses could delay or prevent the onset of type 1 diabetes (90). No results have been formally reported, but an abstract indicated that rate of progression was similar in the nasal insulin and placebo groups.
GAD Trials
The Diabetes Prevention–Immune Tolerance study (DIAPREV-IT) conducted sequential trials with a GAD vaccine in (1.) a pilot study for efficacy and safety (91) and (2.) a larger trial with the addition of vitamin D supplementation (92). Children with GAD autoantibodies plus one other biochemical autoantibody were randomized to two injections of GAD or placebo. GAD autoantibody titers increased following therapy, and no adverse effects were found. The time to Stage 3 type 1 diabetes was similar between the GAD vaccine and placebo groups in the DIAPREV-IT study (91). In the DIAPREV-IT2 study, where both randomized groups also received vitamin D supplementation, the trial was terminated early for futility (92).
Innate and Adaptive Immune Modulation Trials
TrialNet conducted three studies in relatives of individuals with type 1 diabetes using hydroxychloroquine (93), abatacept (94,95), and teplizumab (96,97) with the goal of preventing or delaying disease progression. The use of hydroxychloroquine, an antiparasitic and immunomodulatory agent, was proposed due to evidence of its suppression of inflammatory biomarkers and potential positive effect on insulin signaling. The TrialNet hydroxychloroquine study included first- or second-degree relatives with at least two type 1 diabetes-related autoantibodies and normal glucose tolerance (Stage 1 type 1 diabetes). The trial was stopped early, after enrollment of 275 participants, due to lack of beneficial effect on delay of glucose intolerance (Stage 2 type 1 diabetes) during interim analysis (93).
Eligibility for the TrialNet abatacept study (94) required at least two autoantibodies, one of which was not IAA (due to competing study enrollment), and normal glucose tolerance (Stage 1 type 1 diabetes). Abatacept, a therapeutic used in other autoimmune diseases, acts through attenuation of T cell activation via co-stimulation blockade. In this trial, the drug was given for 1 year. Overall, the hazard rate was not statistically significant (HR 0.702, 95% CI 0.452–1.09, p=0.11) (95).
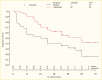
Eligibility for the TrialNet teplizumab study (96,97) required at least two autoantibodies and dysglycemia during an OGTT (Stage 2 type 1 diabetes). Teplizumab is an anti-CD3 monoclonal antibody posited to modulate effector T cell phenotypes (96). In this landmark trial, the time to development of diabetes (Stage 3 type 1 diabetes) was delayed in the group randomized to teplizumab (median 48.4 months) compared to those who received placebo (24.4 months) (Figure 2). The hazard ratio for type 1 diabetes was 0.41 (95% CI 0.22–0.78, p=0.006) (96). However, the entire cohort was a very high-risk population—within the teplizumab arm, 43% of participants developed diabetes, and 72% in the placebo arm progressed. Extended follow-up further supported the delay of progression in this Stage 2 group, with median time to Stage 3 type 1 diabetes of 59.6 months in those who received teplizumab and 27.1 months for those who received placebo (rate of type 1 diabetes diagnosis HR 0.457, p=0.01) (97). Consequently, teplizumab has been approved by the U.S. Food and Drug Administration (FDA) for use in Stage 2 type 1 diabetes to delay progression to Stage 3 (98). Post hoc studies identified responder phenotypes that suggest that those without ZnT8 autoantibodies or without HLA-DR3 may be more likely to respond to teplizumab, although validation studies are needed to confirm this finding.
Ongoing Secondary Prevention Trials
As noted in Table 1, other ongoing secondary prevention trials include the Fr1da Insulin Intervention study using oral insulin with a dose escalating to 67.5 mg daily in children age 2–12 years (99,100) and a TrialNet comparator trial of 67.5 mg daily versus 500 mg every other week of oral insulin in the mechanistic Immune Effects of Oral Insulin in Relatives at Risk for Type 1 Diabetes Mellitus study (101). Another immunomodulatory therapy with transient success in recently diagnosed individuals is anti-thymocyte globulin (ATG), which is now being studied in the TrialNet low-dose ATG (STOP-T1D) trial (102). Cell therapy, using autologous umbilical cord blood rich in regulatory T cells, is being studied in the CoRD pilot trial for safety and feasibility (103).
Challenges of Screening and Enrollment for Secondary Prevention Trials
Secondary prevention trials involve screening of relatives of people with type 1 diabetes and enrollment of those with early markers of disease, either autoantibodies alone (Stage 1) or autoantibodies and metabolic dysfunction (Stage 2). In cross-sectional screening of relatives for autoantibodies in DPT-1 and TrialNet, ~5% of relatives are found to have autoantibodies. Although this rate is much higher than the 0.3% in the general population as studied in the United States and Europe, it still means that to enroll secondary prevention trials, large numbers of subjects must be screened. For example, DPT-1 screened more than 100,000 relatives to enroll 711 subjects in the two arms of that study (parenteral and oral insulin). Now that a therapy to delay progression from Stage 2 to Stage 3 type 1 diabetes has been identified and approved by the FDA, the impetus for general population screening has increased. However, general population screening is not without challenges and requires the buy-in of many stakeholders. At this time, the main goal of early identification of those at risk for type 1 diabetes is to provide psychological support, metabolic monitoring, and education for prevention of diabetic ketoacidosis (DKA). In addition, as more therapies are found to preserve beta cell function in individuals with recent-onset (Stage 3 type 1 diabetes), therapies will continue to be moved from the tertiary prevention space to secondary prevention.
Tertiary Prevention Trials
Tertiary prevention trials (Table 2) have been conducted in subjects with Stage 3 type 1 diabetes—this is typically classic symptomatic type 1 diabetes requiring insulin therapy (Figure 1), mostly recent-onset, but some with established disease. As noted, many early pilot trials with a variety of immune interventions (56,104) will not be discussed here. Rather, this discussion is confined to randomized controlled trials and studies that have either evaluated contemporary immunologic approaches or ones that offer special insights.
Primary outcomes assessed in tertiary prevention trials, in the present day, focus on stimulated C-peptide production at the endpoint of the trial. Mean differences between treated and placebo groups at this timepoint are then compared for statistical significance. For the majority of trials, endogenous insulin (and C-peptide production) was stimulated by a standard liquid meal containing carbohydrate, protein, and fat (i.e., mixed meal tolerance test [MMTT]). Glucagon-stimulated C-peptide values have also been compared between treated and placebo groups. Several trials have used a measure of “remission” or “partial remission” to compare between treated and placebo groups. Remission has typically been the proportion who do not require exogenous insulin at a prespecified time point. The definition of partial remission, however, varies by trial; in general, this term refers to the proportion of individuals who require low doses of exogenous insulin and have a lower glycated hemoglobin (A1C) level. As trialists and participants weigh the potential benefits of such intervention trials, the risks are not inconsequential for some immunomodulatory therapies and are discussed below.
Early Intervention Studies
Cyclosporine
In 1984, Stiller et al. reported a pilot study using cyclosporine, an immunosuppressive agent targeting T lymphocytes (57), which stimulated several cyclosporine intervention studies (105,106,107,108,109,110). Two large randomized controlled trials compared “remission” rates with cyclosporine versus placebo in subjects with recent-onset Stage 3 type 1 diabetes (105,106). In the French study (105), “complete remission” was defined as metabolic control (aiming at fasting blood glucose <140 mg/dL, postprandial blood glucose <200 mg/dL, and A1C <7.5% [<58 mmol/mol]) in the absence of insulin treatment. “Partial remission” was defined by the same metabolic criteria obtained with <0.25 units/kg/day of insulin. In the Canadian-European study (106), the same metabolic targets were used, but “remission” also required a stimulated C-peptide level >0.6 nmol/L (>1.8 ng/mL) or a non-insulin-requiring (NIR) state. Doses of cyclosporine were progressively lowered and stopped after a period if remission was not achieved. Both studies showed more subjects in remission compared to placebo, but the rate of remission progressively declined in both groups during the 1-year course of the study.
Two smaller studies, in Miami (107) and Denver (108), were also conducted. The Miami study showed a slower rate of decline of stimulated C-peptide with cyclosporine compared to placebo. The Denver study showed a slightly greater, but not statistically significant, difference in the rate of remission in the cyclosporine group than the placebo group.
Buoyed by two randomized controlled trials showing the beneficial effects of cyclosporine, a French team initiated a study of cyclosporine in which all eligible subjects received the drug (109). In that study, 27 of 40 subjects (67.5%), all of whom were children, achieved remission. Enrollment was expanded, and subjects were followed for a protracted period of time, during which subjects lost their remission in spite of continued cyclosporine therapy (110). This lack of long-term benefit, coupled with the then-emerging recognition of cyclosporine side effects (particularly renal disease), led to virtual abandonment of this therapy in type 1 diabetes.
Azathioprine
In the same era, the mid-1980s, several studies were conducted with azathioprine, an immunomodulatory agent that accelerates lymphocyte apoptosis via inhibition of nucleic acid synthesis, in recent-onset Stage 3 type 1 diabetes (111,112,113). One study initiated therapy with a 10-week course of corticosteroids followed by 1 year of treatment with azathioprine and found better beta cell function at 1 year, as measured by peak C-peptide/glucose ratio, than in the randomized but untreated control group (111). Another, nonrandomized study gave alternate patients azathioprine and found that most azathioprine subjects achieved “remission,” whereas only one comparison subject did (112). A third azathioprine study was a double-masked, placebo-controlled study that enrolled 49 people age 2–20 years with newly diagnosed type 1 diabetes (113). This study found equal rates of remission in both groups. Given the nonrandomized nature of the other studies and the side effects of azathioprine, further studies with azathioprine were not pursued.
Linomide
The immunomodulatory agent linomide (quinoline-3-carboxamide), thought to activate or modulate regulatory T lymphocytes, was evaluated in a randomized placebo-controlled trial in 63 subjects age 10–20 years with recent-onset Stage 3 type 1 diabetes (114). Subjects were treated for 1 year, and beta cell function was evaluated by glucagon-stimulated C-peptide. Although the initial analysis suggested no difference between groups, when the analysis was confined to those with residual C-peptide at baseline (40 of 63 subjects), a beneficial effect was observed. Although side effects were minimal, the manufacturer did not continue development of linomide; thus, this agent was not further pursued.
Bacille Calmette-Guérin Vaccine
The Bacille Calmette-Guérin (BCG) vaccine, used for the prevention of tuberculosis, had previously shown benefit in animal models, and epidemiologic evidence suggested that BCG may play a role in the prevention of type 1 diabetes (115). Two double-masked, placebo-controlled trials in the 1990s evaluated the effects of BCG in subjects with recent-onset Stage 3 type 1 diabetes (116,117). One trial, conducted in Alberta, Canada (116), enrolled 26 subjects with mean age 13 years, while the other, conducted in Colorado and Massachusetts (117), enrolled 47 subjects age 5–18 years. Similar outcomes were seen in both studies: namely, there was no effect of BCG on preservation of beta cell function. Indeed, both studies showed a trend to greater decline of beta cell function in the BCG group than in the control group.
Oral Insulin
Three studies used oral insulin (in various doses) in recent-onset Stage 3 type 1 diabetes (118,119,120). In the French (118) and Italian (119) studies, no effect was seen in beta cell function. In the study conducted in the United States (120), retention of endogenous beta cell function was said to be dependent upon initial stimulated C-peptide response, age at diabetes onset, and numbers of specific islet autoantibodies found (120). The complex analysis did not permit a clear conclusion to be drawn.
Anti-CD5 Monoclonal Antibodies
Using an anti-CD5 monoclonal antibody, which targets T lymphocytes, linked to ricin A-chain, a toxin, a small open-label, dose-escalation pilot study was conducted in 15 subjects with recent-onset Stage 3 type 1 diabetes (121). With only 5 days of treatment, beta cell function (evaluated by an MMTT) appeared to decline more slowly than anticipated over 1 year, but in the absence of a control group, it was not possible to infer a beneficial effect. Nonetheless, the use of a monoclonal antibody directed at T lymphocytes served to stimulate other investigations of monoclonal antibodies in type 1 diabetes.
Non-antigen Specific Therapies
Therapeutics targeting the adaptive immune system in a non-antigen specific manner aim to transiently reduce inflammation and effector T cells, thus shifting the balance of immune cells toward a regulatory phenotype. In contrast, antigen-specific therapies in type 1 diabetes elicit a specific autoantigen-directed regulatory response, as described in the next section.
Mycophenolate Mofetil
TrialNet has conducted several studies with immunologic interventions in subjects with recently diagnosed type 1 diabetes. All studies enrolled subjects within 100 days of diagnosis (recent-onset Stage 3 type 1 diabetes) and measured beta cell function by C-peptide in response to serial MMTTs. One study evaluated the immunosuppressive agent mycophenolate mofetil, which impairs nucleic acid synthesis needed for lymphocyte proliferation, either alone or in combination with the anti-CD25 monoclonal antibody daclizumab, which targets the alpha chain of the interleukin-2 (IL-2) receptor expressed on T lymphocytes (122). The study enrolled 126 subjects age 8–45 years; it was stopped early by the Data and Safety Monitoring Board due to futility of the potential of seeing a beneficial treatment effect (122).
Anti-CD3 Intervention Studies
Extensive studies have been conducted with two anti-CD3 monoclonal antibodies targeting T lymphocytes—teplizumab and otelixizumab—which are humanized Fc-mutated (Fc receptor [FcR] nonbinding) monoclonal antibodies. The first study reported was a small study involving only 12 treated subjects and 12 untreated comparison subjects (123). Subjects received a single 14-day course of treatment with teplizumab within 6 weeks of diagnosis of Stage 3 type 1 diabetes and were found to have slower decline of beta cell function (by MMTT) at 1 year (123). In these and an expanded group of subjects (total of 21 treated, 21 untreated), sustained improvement of beta cell function was seen at 2 years (124). Meanwhile, the first randomized placebo-controlled trial with an anti-CD3 monoclonal antibody (125) included 80 subjects age 12–39 years. Within 4 weeks from diagnosis of Stage 3 type 1 diabetes, subjects were randomized to a 6-day course of either otelixizumab or placebo and followed for 18 months. Beta cell function measured using a hyperglycemic clamp followed by glucagon stimulation was found to be better in the otelixizumab than the placebo group, particularly in subjects with higher baseline insulin secretory response (125). After 4 years of follow-up, although beta cell function was not measured, the otelixizumab group had lower insulin requirements despite similar glycemic control as measured by A1C (126). Thus, the effects of a 6-day treatment course were evident 4 years later.
The results from these early Phase 2 studies with anti-CD3 treatment led to the initiation of Phase 3 clinical trials with both agents. However, the Phase 3 studies did not meet their primary outcome criteria. For teplizumab, the primary outcome was the combination of A1C <6.5% (<48 mmol/mol) and insulin dose <0.5 units/kg/day (127,128,129). This outcome measure was arbitrarily selected and highly criticized for several reasons. Moreover, by using a composite outcome that requires a subject to meet two criteria, the outcome became a dichotomous measure that dilutes the effect of two continuous variables—A1C and insulin dose. More importantly, when the conventional outcome measure of C-peptide was assessed, evidence of efficacy was observed both at 1 year (127) and 2 years (128) following two 14-day courses of teplizumab (at entry and at 26 weeks into the study). This effect was especially evident in subjects enrolled in the United States (who had lower A1C at entry and during the study), in younger subjects (age 8–17 years), in subjects enrolled within 6 weeks of diagnosis, and in subjects with higher levels of C-peptide at entry (127). For otelixizumab, the Phase 3 studies used a dose that was one-sixteenth (total of 3.1 mg over 8 days) that used in the positive Phase 2 study described in the previous paragraph (total of 48 mg) to avoid any side effects (130,131). Not only were side effects completely obviated, but beneficial effects were also obviated. This outcome highlights the challenge with significant dose reduction—all effective therapies are likely to have some side effects, and eliminating the side effects of a drug may also eliminate its potential benefits.
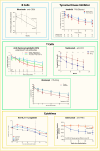
Two other studies with teplizumab are worth noting. In the Autoimmunity-Blocking Antibody for Tolerance in Recently Diagnosed Type 1 Diabetes (AbATE) Trial, conducted by the Immune Tolerance Network (ITN), efficacy was demonstrated (132), although this study was not blinded as the control group did not receive a placebo. The trial met the primary endpoint based on area under the curve (AUC) C-peptide from an MMTT, and the results from this trial and other successful new-onset type 1 diabetes randomized controlled trials are displayed in Figure 3. More importantly, however, subjects could be divided into two groups: “responders” and “non-responders” to treatment. Responders were those who maintained C-peptide better than the randomized but untreated comparison group at 24 months. This subgroup, which constituted 45% of subjects treated with teplizumab, maintained beta cell function for 2 years, whereas the non-responders lost beta cell function at a rate like the control group (132). In another teplizumab study, the Delay trial, subjects diagnosed with Stage 3 type 1 diabetes at least 4 but not more than 12 months before enrollment (thus “Delayed” compared to recent onset) were randomized to receive infusions of teplizumab or placebo (133). The decline of beta cell function slowed in the treated group, driven by beneficial effect in those treated within 4–8 months of diagnosis, as the effects were not significant in the subgroup treated 9–12 months after diagnosis. Another Phase 3 trial with teplizumab, the PROTECT study, which randomized 328 subjects, met its primary endpoint (134,135). At week 78, those treated with teplizumab not only had greater C-peptide AUC than placebo-treated (least-squares mean difference of 0.13 nmol/L, 95% CI 0.09–0.17, p<0.001), but also a greater frequency maintained “clinically meaningful peak C-peptide” ≥0.2 nmol/L (94.9% versus 79.2%, respectively).
Anti-thymocyte Globulin Intervention Studies
A small pilot study has evaluated the combination of low-dose ATG and pegylated granulocyte colony-stimulating factor (GCSF) (136). This study enrolled 25 subjects age 12–45 years with type 1 diabetes of 4–24 months duration, who were randomized 2:1 to active treatment or placebo. Subjects received intravenous ATG (or placebo) over 2 days, followed by subcutaneous GCSF every 2 weeks for six doses. At the end of 1 year, beta cell function was preserved, as measured by an MMTT. At the end of 2 years, the difference between groups was no longer statistically significant (137).
Most recently, TrialNet evaluated the efficacy of a polyclonal T cell-directed immunosuppressant ATG (Thymoglobulin) at low doses (2.5 mg/kg/course) to obviate the T cell-mediated immune destruction of the beta cell (138,139). In this trial, 89 individuals age 12–45 years were randomized in a 1:1:1 study design to receive ATG alone (two doses over 2 days), ATG and pegylated GCSF (six doses over 12 weeks), or placebo. In this study, C-peptide production (via an MMTT) was preserved in those who received low-dose ATG compared to placebo but not in the combination ATG+GCSF group compared to placebo at 1 year (138). Effector CD4 T lymphocytes were reduced, but regulatory T cells were preserved. This effect, along with lower A1C, was also seen in those treated with low-dose ATG 2 years after the initial therapy (139).
The ITN conducted an earlier study evaluating ATG (Thymoglobulin) at 6.5 mg/kg/course in recent-onset type 1 diabetes, the Study of Thymoglobulin to Arrest Type 1 diabetes (START) (140). In START, 58 subjects age 12–35 years with recent-onset Stage 3 type 1 diabetes were randomized in a 2:1 design to receive ATG or placebo over 4 days. There was no between-group difference in beta cell function at 1 year; however, ATG resulted in generalized depletion of T lymphocytes rather than the hoped-for specific depletion of effector memory T lymphocytes with preservation of regulatory T cells. Of note, post hoc analysis demonstrated a beneficial effect of this ATG dose at 2 years for those who were age >21 years (141).
Abatacept Intervention Study
TrialNet evaluated effects of the fusion protein abatacept (soluble CTLA4-Ig), which binds to CD80 and CD86, the ligands for CD28, a co-stimulatory molecule on T lymphocytes (142). In this study, 112 subjects age 6–45 years were randomized in a 2:1 design to receive either monthly infusions of abatacept or placebo for 2 years. After those 2 years, beta cell function was better maintained in the abatacept group than in the placebo group, although a progressive decline was seen in the abatacept group as well (142). After therapy was stopped, subjects were followed for an additional year, with the abatacept group maintaining a difference from the placebo group; a progressive parallel rate of decline was observed in both groups but shifted by 9.5 months in abatacept-treated subjects (143). Thus, the beneficial effect was sustained for at least 1 year after cessation of abatacept infusions or 3 years from the diagnosis of type 1 diabetes.
Alefacept Intervention Study
In another ITN study, the Inducing Remission in New-Onset Type 1 Diabetes with Alefacept (T1DAL) trial, alefacept was used to target memory T lymphocytes (144). In this trial, 49 subjects age 12–35 years with recent-onset Stage 3 type 1 diabetes were randomized in a 2:1 design to receive either alefacept or placebo, given as two 12-week courses of monthly intramuscular injections separated by a 12-week hiatus. Because the manufacturer withdrew alefacept from production during the trial, enrollment was smaller than planned. Beta cell function was preserved in the alefacept group, i.e., it did not decline over 12 months, but the results of the primary outcome—C-peptide during the first 2 hours of the MMTT—just missed statistical significance (p=0.065). In contrast, the secondary outcome—C-peptide during the full 4 hours of the MMTT—indicated a significant difference in beta cell function (p=0.019) (144). At 24 months, both the 4-hour and the 2-hour C-peptide levels were greater in the alefacept group than the placebo group (145). Thus, had the study been fully enrolled, the primary outcome may have been met. Moreover, alefacept appeared to have a greater impact on central memory and effector memory T lymphocytes, with sparing of naïve and regulatory T lymphocyte populations. Taken together, these findings suggest that targeting memory T lymphocytes may be an attractive immunomodulatory approach. A study of a different CD2-targeting therapeutic is underway as of January 2024 (146).
Rituximab Intervention Study
A TrialNet study evaluated the anti-CD20 monoclonal antibody rituximab, which depletes B lymphocytes (147). In this study, 87 subjects age 8–40 years were randomized in a 2:1 design to receive either four weekly doses of rituximab or placebo. After 1 year, beta cell function was better maintained in the rituximab group than in the placebo group, although a progressive decline was observed in the rituximab group as well (147). Over 2 years, the rate of decline of C-peptide was parallel between groups but shifted by 8.2 months in rituximab-treated subjects (148). Thus, the effect of rituximab was transient, with no fundamental alteration of the disease process.
Antigen-Specific Therapies
GAD Intervention Studies
A vaccine, consisting of GAD with the adjuvant aluminum hydroxide (GAD-Alum), created much excitement based on an initial report of a Phase 2 trial, which claimed benefit at least in those subjects enrolled early after diagnosis (149). However, this result was not confirmed in a TrialNet study (150) nor in two Phase 3 trials conducted by the manufacturer (151,152).
More recently, a single-center randomized controlled trial utilized gamma aminobutyric acid (GABA) with or without GAD. GABA is an inhibitory neurotransmitter that is synthesized by the decarboxylation of glutamate by GAD. While this 1:1:1 randomized trial found that neither GABA nor GABA+GAD preserved C-peptide over placebo, secondary outcomes confirmed safety and tolerability in addition to identifying a reduction in glucagon levels post-treatment in the combination arm (153).
DiaPep277 Intervention Studies
Several Phase 2 clinical trials were conducted using DiaPep277, a 24-amino acid peptide derived from heat shock protein 60 (an antigen for beta cells). The first of the Phase 2 trials appeared to have promising results (154,155), but results from the other Phase 2 trials (156,157,158) were conflicting. A Phase 3 trial was reported and had inherently confusing results with improved C-peptide versus placebo during a glucagon-stimulated test but no difference between groups with an MMTT (159,160). Subsequently, the papers describing this trial were retracted.
Insulin Antigen Vaccine Intervention Studies
The ITN conducted two small pilot studies of insulin vaccines. One trial evaluated the safety of a vaccine using human insulin B-chain in incomplete Freund’s adjuvant, administered as a single intramuscular injection (161). In this pilot safety study, 12 subjects age 18–35 years were randomized to receive either the vaccine or placebo. There were no safety issues. No difference in beta cell function was found, but there was suggestive evidence that antigen-specific regulatory T lymphocytes were generated.
A study using an insulin B-chain altered peptide ligand enrolled 188 subjects age 10–35 years (162). Subjects were randomized into one of four groups—three doses of the drug or placebo. After 2 years, no difference in beta cell function was observed among the four groups.
Another study evaluated a plasmid-encoded proinsulin (163). Subjects age 18–40 years were randomized into one of five groups—four doses of the drug or placebo. Although beta cell function improved at one time point for one of the four doses, the overall intervention failed to show benefit.
Cytokine-Directed Therapies
Anti-interleukin-1 (IL-1)
Another treatment strategy evaluated by TrialNet was antagonism of the cytokine IL-1, which is thought to be a key mediator of innate immunity, as it is a proinflammatory cytokine that recruits effector T lymphocytes in inflamed tissues and has direct toxic effects on beta cells. In a TrialNet study of the anti-IL-1beta monoclonal antibody canakinumab, 71 subjects age 6–45 years were randomized in a 2:1 design to receive either monthly subcutaneous injections of canakinumab or placebo for 1 year (164). No difference in beta cell function was observed between groups.
The canakinumab study was reported together with another study examining antagonism of IL-1, using the human IL-1 receptor antagonist anakinra (164). For the anakinra trial, 69 subjects age 18–35 years were randomized to receive either daily subcutaneous injections of anakinra or placebo for 9 months. With anakinra as well, no difference in beta cell function was found between groups. Thus, by itself, antagonism of IL-1 failed to show benefit.
Anti-TNF-Alpha
A small pilot study evaluated etanercept, a blocker of the proinflammatory cytokine TNF (165). Although only 18 subjects were enrolled, the etanercept group had increased beta cell function at 6 months, whereas the placebo group had decreased beta cell function at that time, thus achieving statistical significance between groups. This study was followed by another anti-TNF therapy, golimumab, in a trial that randomized 84 subjects in a 2:1 design to active therapy or placebo (166). At 1 year, the golimumab group experienced higher C-peptide (during a 4-hour MMTT) than the placebo group. Additionally, more golimumab-treated subjects achieved partial remission (as defined by an insulin dose-adjusted A1C level score ≤9, calculated as the A1C level [in %] plus four times the insulin dose [in units/kg/day]). The drug was discontinued after 1 year, and during the following year, C-peptide declined parallel to the placebo group. Additional studies have not been pursued despite anti-TNF therapy being used continuously over many years in other autoimmune diseases.
Interleukin-2 (IL-2) Agonism
Another ITN pilot study was an open-label Phase 1 study using the combination of IL-2 and rapamycin (167). Nine subjects were enrolled, age 20–36 years, between 4 and 48 months from diagnosis of type 1 diabetes if they had a peak C-peptide value of at least 0.4 nmol/L (1.2 ng/mL) during an MMTT. The study was halted due to the reported acute decline in C-peptide during the first 3 months; without a comparison group and without much literature data on the rate of C-peptide decline in this period after diagnosis, it is not clear whether this observation was unusual. There appeared to be a subsequent recovery of C-peptide in four of the subjects. Although regulatory T lymphocytes increased, natural killer cells and eosinophils also increased, with no difference in effector T lymphocytes.
Two small safety studies, one with adult participants (168) and one with pediatric participants (169), involving 24 subjects each, evaluated three dosing regimens of a low dose of IL-2. Regulatory T lymphocytes increased, and no safety issues emerged, including no decline in C-peptide that was reported with higher doses of IL-2 in combination with rapamycin (167).
Metabolic and Anti-inflammatory-Based Intervention Studies
A pilot Phase 1 safety study used alpha-1-antitrypsin (AAT), an anti-inflammatory agent that had beneficial effects in animal models (170). No safety issues were identified. The study showed that AAT was associated with down-modulation of IL-1beta, which may indicate potential benefit for type 1 diabetes.
The Diabetes and Atorvastatin (DIATOR) Trial randomized 89 subjects age 18–39 years to atorvastatin or placebo, on the basis that atorvastatin has immunomodulatory properties (171). This provocative, but small, study did not meet its primary outcome (difference in C-peptide between groups at 18 months). However, when the authors examined the decline in C-peptide within the atorvastatin group, there was a nonsignificant decline, whereas the decline in C-peptide within the placebo group was significant (171). A further analysis suggested that individuals with markers of inflammation may be the ones that benefit (172). Because atorvastatin is a common, orally administered generic drug, further evaluation of atorvastatin may be warranted.
Another study examined the combination of sitagliptin and lansoprazole in patients with recent-onset Stage 3 type 1 diabetes (173). The rationale of this study was that a dipeptidyl-peptidase 4 (DPP-4) inhibitor (sitagliptin) would increase serum levels of glucagon-like peptide-1 (GLP-1), while a proton pump inhibitor (lansoprazole) would increase serum levels of gastrin. In experimental animals, the combination of GLP-1 and gastrin increases beta cell mass and function. The human study—REPAIR T1D—randomized 68 subjects age 11–36 years in a 2:1 design to receive either the combination of sitagliptin and lansoprazole or placebo for both drugs. At 1 year, there was no difference in the rate of decline of beta cell function comparing treated and control subjects (173).
The liraglutide (GLP-1 receptor agonist) and anti-interleukin-21 (IL-21) randomized controlled trial of 308 individuals age 18-45 years tested the hypothesis that the combination of IL-21 (transient immunomodulation) plus liraglutide (increased proliferation and decreased apoptosis of beta cells) could enable beta cell survival in those who were newly diagnosed (174). While neither agent alone slowed the decline in metabolic function (C-peptide via an MMTT), individuals who received liraglutide plus anti-IL-21 had a 10% decline from baseline (compared to 39% C-peptide decline in the placebo group) and a resultant treatment ratio of 1.48 (95% CI 1.16–1.89, p=0.0017) (174). The drugs were then discontinued, and after a further half-year of follow-up, the beneficial effects had dissipated (174).
Imatinib, a tyrosine kinase inhibitor, was effective at preserving C-peptide above placebo-treatment individuals at 1 year (p=0.048), but not 2 years; gastrointestinal issues were seen at a higher rate in the imatinib group than the placebo group (175).
Verapamil, a calcium channel blocker introduced more than 4 decades ago for treatment of angina and arrhythmias, is also used as an adjunctive antihypertensive. Verapamil was found to reduce elevated levels of thioredoxin-interacting protein (TXNIP), which inhibit insulin secretion and cause beta cell apoptosis. A small trial in adults (176) and a larger trial in children (177) with recently diagnosed type 1 diabetes demonstrated C-peptide preservation at 1 year compared to placebo (difference 0.28 nmol/L [0.84 ng/mL], 95% CI 0.05–0.51, p=0.0186, and 0.14 nmol/L [0.42 ng/mL], 95% CI 0.01–0.27, p=0.04, respectively). Future trials using verapamil (likely in combination) are planned, due to its putative mechanism and safety profile.
Cell Therapy Intervention Studies
Cell therapy has been investigated for the tertiary prevention of type 1 diabetes. The transfer of ex vivo-expanded autologous CD4+CD127-CD25+ polyclonal regulatory T cells, due to their role in dampening autoimmune-mediated beta cell destruction, is under study. No safety issues have been identified, but preservation of C-peptide has been limited (178,179) apart from one study of children age 8–16 years who received two doses of regulatory T cells and four doses of rituximab and demonstrated preserved C-peptide at 2 years compared to placebo (180).
Other cell-based therapies have explored the use of mesenchymal stem cells (MSCs) to suppress aberrant T cell proliferation and upregulate regulatory T cells. These therapies have not demonstrated any major safety issues, and one study of 20 adults demonstrated an increase in C-peptide production at 1 year compared to a decline of 13% in the placebo group (181,182).
In 2007, a group of investigators from Brazil reported an open-label trial of 15 patients with Stage 3 type 1 diabetes age 13–31 years diagnosed within the previous 6 weeks, who were treated with the combination of high-dose immunotherapy, with cyclophosphamide and ATG, together with nonmyeloablative autologous hematopoietic stem cell therapy (AHSCT) using CD34+ cells isolated from bone marrow (183). The investigators reported that during 7–36 months of follow-up, 14 of 15 subjects became insulin free. Subsequently, they updated their findings in a total of 23 subjects, asserting that 20 had achieved freedom from insulin therapy, with 12 subjects maintaining that outcome for a mean of 31 months (184,185). Additional studies were subsequently conducted in Poland and China, and the data from those studies have been summarized (186). The findings confirmed that many subjects achieved insulin independence. However, substantial side effects occurred, including a death from Pseudomonas sepsis (186), and the death rate in other disease states with AHSCT can be as high as 25%. In addition, these were all nonrandomized, open-label studies, with incomplete characterization of the subjects, so it is not clear that all participants had autoimmune type 1 diabetes.
Intensive Insulin Therapy Intervention Studies
Following studies in rodent models of diabetes, early recovery of glucose toxicity and glucose values maintained in tight ranges were presupposed to preserve beta cells. A small pilot study in 1989 suggested this to be true in recently diagnosed adolescents who underwent 2-week hospitalization with a tightly controlled continuous insulin infusion compared to adolescents who received NPH insulin (187). A significant difference in stimulated C-peptide was found at 1 year (p<0.01). However, trials conducted mostly in adolescents since that time with more sophisticated automated insulin delivery systems have not demonstrated a difference in C-peptide preservation for those randomized to intensive insulin therapy (188,189,190).
Ongoing Intervention Studies
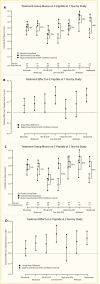
Generally Recognized as Safe (GRAS) therapies are being studied in those with newly diagnosed type 1 diabetes, including probiotic supplementation (191,192) and omega-3 fatty acids plus vitamin D supplementation (193). Several provocative studies demonstrating at least transient preservation of beta cell function have been conducted, but no controlled studies have demonstrated sufficient sustained beta cell function, such that insulin therapy is not required. As new therapies are explored, immunotherapies that have demonstrated transient beta cell preservation continue to be studied; however, direct comparison of therapeutics is unlikely as head-to-head comparison trials are unfeasible. Statistical modeling of six trials in recent-onset type 1 diabetes, adjusted for age and baseline C-peptide production, identified ATG and teplizumab as drugs that produced the greatest improvement in C-peptide over their respective placebo groups at 1 and 2 years (Figure 4) (194).
Heterogeneity in Inclusion Criteria and Response to Therapy
While the results of these intervention trials appear modest (e.g., 0.24 nmol/L difference in C-peptide at 1 year), we suspect that the wide inclusion criteria for trials, used to aid enrollment, may dilute the effect of certain therapeutics. The pathophysiology and clinical course of type 1 diabetes are variable according to age at diagnosis, for example, and yet all ages (e.g., 12–45 years) are treated with the same therapeutic. In adults, the course of type 1 diabetes is more indolent and marked by less autoimmune infiltrate in the pancreas (195). Short courses of therapy may not be successful in altering the disease trajectory in these cases. The field has come to assess for this post hoc in responder analyses, where subpopulations of participants have been identified who respond better to a therapeutic than others (196). This information will aid in the design of future clinical trials; however, the limiting factor of participant numbers remains.
Conclusions
Additional studies aimed at intervening in early type 1 diabetes are underway with a variety of approaches, some of which include: (1.) further study of teplizumab and ustekinumab in children and young adults; (2.) a probiotic in combination with teplizumab; (3.) T and B cell combination therapy with rituximab and abatacept; (4.) anti-CD40 antibody; (5.) low-dose IL-2; (6.) JAK (Janus kinase) pathway inhibitor; (7.) methyldopa to inhibit the binding pocket for DQ8 antigen presentation; (8.) intralymphatic delivery of GAD vaccine in HLA-DR3-DQ2-restricted individuals; and (9.) dendritic cell-based vaccines (134,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211). A combination approach may be needed—one that combines an anti-inflammatory agent targeting innate immunity, with an immunomodulatory agent targeting adaptive immunity, with agents that stimulate regulatory immunity, and an agent that helps preserve beta cell health (212). Additionally, lifelong or repeated courses of therapy during the time of detectable C-peptide production may be required, like the model of treatment for other autoimmune diseases.
To promote drug development and timely advancement along the regulatory approval process, if agents tested in Stage 3 type 1 diabetes studies are found to be efficacious and safe, they can be brought to earlier disease (Stage 1 and Stage 2 type 1 diabetes). In addition, initiatives to screen and detect individuals at risk for type 1 diabetes should facilitate the ability to conduct larger Stage 1 and Stage 2 prevention studies. If only successful Stage 3 therapeutics are used in the at-risk population, there is the potential risk of missing an effective treatment. The key is understanding precise mechanisms leading to type 1 diabetes. Without that knowledge, therapeutic measures will be broad and nonspecific.
Next, it is essential to understand whether type 1 diabetes heterogeneity is related solely to age or other phenotypes. The development and validation of biomarkers to identify subtypes (e.g., latent autoimmune diabetes in adults [LADA]), or “endotypes,” of type 1 diabetes could allow for therapeutic selection based on an individual’s specific mechanism of disease, while also increasing efficacy (213,214). Given that the heterogeneity of type 1 diabetes has been well documented (3,214,215), more homogeneous study populations will be needed to achieve robust and durable outcomes in clinical trials. In addition, the field has not achieved equal representation of racial and ethnic minority populations in clinical trials or equitable health outcomes for these individuals (216). The risk of progressing to clinical type 1 diabetes can present with different risk factors (e.g., HLA, autoantibodies) in African American populations compared to Caucasian/Northern European populations (217). This concern is vital, as one disease-modifying therapy is unlikely to be effective for everyone at risk of or diagnosed with type 1 diabetes.
List of Abbreviations
- A1C
glycated hemoglobin
- AAT
alpha-1-antitrypsin
- AHSCT
autologous hematopoietic stem cell therapy
- ATG
anti-thymocyte globulin
- BCG
Bacille Calmette-Guérin
- CI
confidence interval
- DAISY
Diabetes AutoImmunity Study in the Young
- DENIS
German (Deutsch) Nicotinamide Diabetes Intervention Study
- DHA
docosahexaenoic acid
- DIAPREV-IT
Diabetes Prevention–Immune Tolerance
- DIPP
DIabetes Prediction and Prevention Study
- DPT-1
Diabetes Prevention Trial-Type 1
- ENDIT
European Nicotinamide Diabetes Intervention Trial
- FDA
U.S. Food and Drug Administration
- FINDIA
Finnish Dietary Intervention Trial for the Prevention of Type 1 Diabetes
- GABA
gamma aminobutyric acid
- GAD
glutamic acid decarboxylase
- GCSF
granulocyte colony-stimulating factor
- GLP-1
glucagon-like peptide-1
- GRAS
Generally Recognized As Safe
- HLA
human leukocyte antigen
- HR
hazard ratio
- IA2
islet antibody-2
- IAA
insulin autoantibodies
- ICA
islet cell antibodies
- IDDM
insulin-dependent diabetes mellitus
- IL
interleukin
- INIT
Intranasal Insulin Trial
- ITN
Immune Tolerance Network
- MMTT
mixed meal tolerance test
- NIP
Nutritional Intervention to Prevent Type 1 Diabetes
- OGTT
oral glucose tolerance test
- OR
odds ratio
- POInT
Primary Oral Insulin Therapy study
- TEDDY
The Environmental Determinants of Diabetes in the Young
- TNF
tumor necrosis factor
- TrialNet
Type 1 Diabetes TrialNet
- TRIGR
Trial to Reduce IDDM in the Genetically at Risk
- ZNT8
zinc transporter 8
Conversions
A1C: (% x 10.93) - 23.50 = mmol/mol
C-peptide: ng/mL x 0.333 = nmol/L
Glucose: mg/dL x 0.0555 = mmol/L
Funding
Dr. Jacobsen is supported by funding from the NIH. Dr. Schatz is supported by funding from the NIH and an NIH/NCATS Clinical and Translational Science Award to the University of Florida. Dr. Herold is supported by funding from the NIH and JDRF. Dr. Skyler is supported by the Diabetes Research Institute Foundation.
Acknowledgment
This is an update of: Skyler JS, Krischer JP, Becker DJ, Rewers M: Prevention of Type 1 Diabetes. Chapter 37 in Diabetes in America, 3rd ed. Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, Eds. Bethesda, MD, National Institutes of Health, NIH Pub No. 17-1468, 2018, p. 37.1–37.21
Article History
Received in final form on May 25, 2024.
References
- 1.
- Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314(21):1360-1368. doi:10.1056/NEJM198605223142106 [PubMed: 3517648] [CrossRef]
- 2.
- Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293-1300. doi:10.1038/nature08933 [PMC free article: PMC4959889] [PubMed: 20432533] [CrossRef]
- 3.
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69-82. doi:10.1016/S0140-6736(13)60591-7 [PMC free article: PMC4380133] [PubMed: 23890997] [CrossRef]
- 4.
- Della Manna T, Setian N, Savoldelli RD, et al. Diabetes mellitus in childhood: an emerging condition in the 21st century. Rev Assoc Med Bras. 2016;62(6):594-601. doi:10.1590/1806-9282.62.06.594 [PubMed: 27849238] [CrossRef]
- 5.
- Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop? The notion of homicide or β-cell suicide revisited. Diabetes. 2011;60(5):1370-1379. doi:10.2337/db10-1797 [PMC free article: PMC3292309] [PubMed: 21525508] [CrossRef]
- 6.
- Nerup J, Platz P, Andersen OO, et al. HL-A antigens and diabetes mellitus. Lancet. 1974;2(7885):864-866. doi:10.1016/s0140-6736(74)91201-x [PubMed: 4137711] [CrossRef]
- 7.
- Schenker M, Hummel M, Ferber K, et al. Early expression and high prevalence of islet autoantibodies for DR3/4 heterozygous and DR4/4 homozygous offspring of parents with type I diabetes: the German BABYDIAB study. Diabetologia. 1999;42(6):671-677. doi:10.1007/s001250051214 [PubMed: 10382586] [CrossRef]
- 8.
- Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res. 2001;56:69-89. doi:10.1210/rp.56.1.69 [PubMed: 11237226] [CrossRef]
- 9.
- Lambert AP, Gillespie KM, Thomson G, et al. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J Clin Endocrinol Metab. 2004;89(8):4037-4043. doi:10.1210/jc.2003-032084 [PubMed: 15292346] [CrossRef]
- 10.
- Thomson G, Valdes AM, Noble JA, et al. Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: a meta-analysis. Tissue Antigens. 2007;70(2):110-127. doi:10.1111/j.1399-0039.2007.00867.x [PubMed: 17610416] [CrossRef]
- 11.
- Erlich H, Valdes AM, Noble J, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the Type 1 Diabetes Genetics Consortium families. Diabetes. 2008;57(4):1084-1092. doi:10.2337/db07-1331 [PMC free article: PMC4103420] [PubMed: 18252895] [CrossRef]
- 12.
- Noble JA, Valdes AM, Varney MD, et al. HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes. 2010;59(11):2972-2979. doi:10.2337/db10-0699 [PMC free article: PMC2963558] [PubMed: 20798335] [CrossRef]
- 13.
- Pugliese A, Gianani R, Moromisato R, et al. HLA-DQB1*0602 is associated with dominant protection from diabetes even among islet cell antibody-positive first-degree relatives of patients with IDDM. Diabetes. 1995;44(6):608-613. doi:10.2337/diab.44.6.608 [PubMed: 7789622] [CrossRef]
- 14.
- Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360(16):1646-1654. doi:10.1056/NEJMra0808284 [PubMed: 19369670] [CrossRef]
- 15.
- Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–707. doi:10.1038/ng.381 [PMC free article: PMC2889014] [PubMed: 19430480] [CrossRef]
- 16.
- Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(1):a007732. doi:10.1101/cshperspect.a007732 [PMC free article: PMC3253030] [PubMed: 22315720] [CrossRef]
- 17.
- Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57(2):176-185. doi:10.1373/clinchem.2010.148221 [PMC free article: PMC4874193] [PubMed: 21205883] [CrossRef]
- 18.
- Shapiro MR, Thirawatananond P, Peters L, et al. De-coding genetic risk variants in type 1 diabetes. Immunol Cell Biol. 2021;99(5):496-508. doi:10.1111/imcb.12438 [PMC free article: PMC8119379] [PubMed: 33483996] [CrossRef]
- 19.
- Redondo MJ, Onengut-Gumuscu S, Gaulton KJ. Genetics of Type 1 Diabetes. In: Lawrence JM, Casagrande SS, Herman WH, Wexler DJ, Cefalu WT, eds. Diabetes in America. eBook. National Institutes of Health; 2023. https://www
.ncbi.nlm .nih.gov/books/NBK597411/ - 20.
- Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(7):a007690. doi:10.1101/cshperspect.a007690 [PMC free article: PMC3385937] [PubMed: 22762021] [CrossRef]
- 21.
- Eringsmark Regnéll S, Lernmark Å. The environment and the origins of islet autoimmunity and type 1 diabetes. Diabet Med. 2013;30(2):155-160. doi:10.1111/dme.12099 [PMC free article: PMC3552102] [PubMed: 23252770] [CrossRef]
- 22.
- Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021;17(3):150-161. doi:10.1038/s41574-020-00443-4 [PMC free article: PMC7722981] [PubMed: 33293704] [CrossRef]
- 23.
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286-298. doi:10.1111/j.1399-5448.2007.00269.x [PubMed: 17850472] [CrossRef]
- 24.
- Hagopian WA, Erlich H, Lernmark Å, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12(8):733-743. doi:10.1111/j.1399-5448.2011.00774.x [PMC free article: PMC3315186] [PubMed: 21564455] [CrossRef]
- 25.
- Stene LC, Norris JM, Rewers MJ. Risk Factors for Type 1 Diabetes. In: Lawrence JM, Casagrande SS, Herman WH, Wexler DJ, Cefalu WT, eds. Diabetes in America. eBook. National Institutes of Health; 2023. https://www
.ncbi.nlm .nih.gov/books/NBK597412/ - 26.
- Peakman M. Immunological pathways to β-cell damage in type 1 diabetes. Diabet Med. 2013;30(2):147-154. doi:10.1111/dme.12085 [PubMed: 23199020] [CrossRef]
- 27.
- Roep BO, Tree TI. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol. 2014;10(4):229-242. doi:10.1038/nrendo.2014.2 [PubMed: 24468651] [CrossRef]
- 28.
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14(10):619-633. doi:10.2337/diab.14.10.619 [PubMed: 5318831] [CrossRef]
- 29.
- Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113(3):451-463. doi:10.1172/JCI19585 [PMC free article: PMC324541] [PubMed: 14755342] [CrossRef]
- 30.
- Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209(1):51-60. doi:10.1084/jem.20111187 [PMC free article: PMC3260877] [PubMed: 22213807] [CrossRef]
- 31.
- Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279-1283. doi:10.1016/s0140-6736(74)90140-8 [PubMed: 4139522] [CrossRef]
- 32.
- Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32(4):468-478. doi:10.1016/j.immuni.2010.03.018 [PMC free article: PMC2861716] [PubMed: 20412757] [CrossRef]
- 33.
- Barker JM, Barriga KJ, Yu L, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab. 2004;89(8):3896-3902. doi:10.1210/jc.2003-031887 [PubMed: 15292324] [CrossRef]
- 34.
- Hummel S, Ziegler AG. Early determinants of type 1 diabetes: experience from the BABYDIAB and BABYDIET studies. Am J Clin Nutr. 2011;94(6 Suppl):1821S-1823S. doi:10.3945/ajcn.110.000646 [PubMed: 21633073] [CrossRef]
- 35.
- Kupila A, Muona P, Simell T, et al. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia. 2001;44(3):290-297. doi:10.1007/s001250051616 [PubMed: 11317658] [CrossRef]
- 36.
- Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473-2479. doi:10.1001/jama.2013.6285 [PMC free article: PMC4878912] [PubMed: 23780460] [CrossRef]
- 37.
- Krischer JP, Liu X, Lernmark Å, et al. Predictors of the initiation of islet autoimmunity and progression to multiple autoantibodies and clinical diabetes: the TEDDY study. Diabetes Care. 2022;45(10):2271-2281. doi:10.2337/dc21-2612 [PMC free article: PMC9643148] [PubMed: 36150053] [CrossRef]
- 38.
- Ghalwash M, Anand V, Lou O, et al. Islet autoantibody screening in at-risk adolescents to predict type 1 diabetes until young adulthood: a prospective cohort study. Lancet Child Adolesc Health. 2023;7(4):261-268. doi:10.1016/S2352-4642(22)00350-9 [PMC free article: PMC10038928] [PubMed: 36681087] [CrossRef]
- 39.
- Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. doi:10.2337/dc15-1419 [PMC free article: PMC5321245] [PubMed: 26404926] [CrossRef]
- 40.
- Sosenko JM, Skyler JS, Herold KC, Palmer JP, Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups. The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-Type 1. Diabetes. 2012;61(6):1331-1337. doi:10.2337/db11-1660 [PMC free article: PMC3357303] [PubMed: 22618768] [CrossRef]
- 41.
- Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS, DPT-1 Study Group. Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes. 2010;59(3):679-685. doi:10.2337/db09-1378 [PMC free article: PMC2828663] [PubMed: 20028949] [CrossRef]
- 42.
- Ferrannini E, Mari A. β-cell function in type 2 diabetes. Metabolism. 2014;63(10):1217-1227. doi:10.1016/j.metabol.2014.05.012 [PubMed: 25070616] [CrossRef]
- 43.
- Sosenko JM, Skyler JS, Beam CA, et al. Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in Diabetes Prevention Trial-Type 1 participants. Diabetes. 2013;62(12):4179-4183. doi:10.2337/db13-0656 [PMC free article: PMC3837047] [PubMed: 23863814] [CrossRef]
- 44.
- Sosenko JM, Palmer JP, Rafkin-Mervis L, et al. Incident dysglycemia and progression to type 1 diabetes among participants in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32(9):1603-1607. doi:10.2337/dc08-2140 [PMC free article: PMC2732147] [PubMed: 19487644] [CrossRef]
- 45.
- Insel RA, Dunne JL, Ziegler AG. General population screening for type 1 diabetes: Has its time come? Curr Opin Endocrinol Diabetes Obes. 2015;22(4):270-276. doi:10.1097/MED.0000000000000173 [PubMed: 26087338] [CrossRef]
- 46.
- Sosenko JM, Krischer JP, Palmer JP, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2008;31(3):528-533. doi:10.2337/dc07-1459 [PubMed: 18000175] [CrossRef]
- 47.
- Sosenko JM, Skyler JS, DiMeglio LA, et al. A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care. 2015;38(2):271-276. doi:10.2337/dc14-1813 [PMC free article: PMC4302258] [PubMed: 25519451] [CrossRef]
- 48.
- Sosenko JM, Skyler JS, Mahon J, et al. Validation of the Diabetes Prevention Trial-Type 1 Risk Score in the TrialNet Natural History Study. Diabetes Care. 2011;34(8):1785-1787. doi:10.2337/dc11-0641 [PMC free article: PMC3142063] [PubMed: 21680724] [CrossRef]
- 49.
- Nathan BM, Redondo MJ, Ismail H, et al. Index60 identifies individuals at appreciable risk for stage 3 among an autoantibody-positive population with normal 2-hour glucose levels: implications for current staging criteria of type 1 diabetes. Diabetes Care. 2022;45(2):311-318. doi:10.2337/dc21-0944 [PMC free article: PMC8914436] [PubMed: 34853027] [CrossRef]
- 50.
- Jacobsen LM, Bundy BN, Ismail HM, et al. Index60 is superior to HbA1c for identifying individuals at high risk for type 1 diabetes. J Clin Endocrinol Metab. 2022;107(10):2784-2792. doi:10.1210/clinem/dgac440 [PMC free article: PMC9516117] [PubMed: 35880956] [CrossRef]
- 51.
- Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61(8):2066-2073. doi:10.2337/db11-1538 [PMC free article: PMC3402330] [PubMed: 22688329] [CrossRef]
- 52.
- Mordes JP, Desemone J, Rossini AA. The BB rat. Diabetes Metab Rev. 1987;3(3):725-750. doi:10.1002/dmr.5610030307 [PubMed: 3301238] [CrossRef]
- 53.
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447-485. doi:10.1146/annurev.immunol.23.021704.115643 [PubMed: 15771578] [CrossRef]
- 54.
- Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23(2):115-126. doi:10.1016/j.immuni.2005.08.002 [PubMed: 16111631] [CrossRef]
- 55.
- Leslie R, Pyke D. Immunosuppression of acute insulin-dependent diabetes. In: Irvine WJ, ed. Immunology of Diabetes. Teviot Scientific Publications Ltd; 1980.
- 56.
- Skyler JS. Immune intervention studies in insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1987;3(4):1017-1035. doi:10.1002/dmr.5610030410 [PubMed: 3315520] [CrossRef]
- 57.
- Stiller CR, Dupré J, Gent M, et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984;223(4643):1362-1367. doi:10.1126/science.6367043 [PubMed: 6367043] [CrossRef]
- 58.
- Skyler JS, Pugliese A. Immunotherapy trials for type 1 diabetes: the contribution of George Eisenbarth. Diabetes Technol Ther. 2013;15(Suppl 2):S2-13 – S2-20. doi:10.1089/dia.2013.010710.1089/dia.2013.0107 [PMC free article: PMC3676656] [PubMed: 23786294] [CrossRef] [CrossRef]
- 59.
- von Herrath M, Peakman M, Roep B. Progress in immune-based therapies for type 1 diabetes. Clin Exp Immunol. 2013;172(2):186-202. doi:10.1111/cei.12085 [PMC free article: PMC3628322] [PubMed: 23574316] [CrossRef]
- 60.
- Skyler JS. Primary and secondary prevention of type 1 diabetes. Diabet Med. 2013;30(2):161-169. doi:10.1111/dme.12100 [PMC free article: PMC3580116] [PubMed: 23231526] [CrossRef]
- 61.
- Skyler JS, Krischer JP, Becker DJ, Rewers M. Prevention of Type 1 Diabetes. In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America, 3rd ed. National Institutes of Health; 2018:37.1-37.21:chap 37. Accessed January 25, 2024. https://www
.ncbi.nlm .nih.gov/books/NBK567991/ - 62.
- Knip M, Virtanen SM, Seppä K, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363(20):1900-1908. doi:10.1056/NEJMoa1004809 [PMC free article: PMC4242902] [PubMed: 21067382] [CrossRef]
- 63.
- Knip M, Åkerblom HK, Becker D, et al. Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA. 2014;311(22):2279-2287. doi:10.1001/jama.2014.5610 [PMC free article: PMC4225544] [PubMed: 24915259] [CrossRef]
- 64.
- Vaarala O, Ilonen J, Ruohtula T, et al. Removal of bovine insulin from cow’s milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch Pediatr Adolesc Med. 2012;166(7):608-614. doi:10.1001/archpediatrics.2011.1559 [PubMed: 22393174] [CrossRef]
- 65.
- Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34(6):1301-1305. doi:10.2337/dc10-2456 [PMC free article: PMC3114350] [PubMed: 21515839] [CrossRef]
- 66.
- Chase HP, Boulware D, Rodriguez H, et al. Effect of docosahexaenoic acid supplementation on inflammatory cytokine levels in infants at high genetic risk for type 1 diabetes. Pediatr Diabetes. 2015;16(4):271-279. doi:10.1111/pedi.12170 [PMC free article: PMC4291300] [PubMed: 25039804] [CrossRef]
- 67.
- Wicklow BA, Taback SP. Feasibility of a type 1 diabetes primary prevention trial using 2000 IU vitamin D3 in infants from the general population with increased HLA-associated risk. Ann N Y Acad Sci. 2006;1079:310-312. doi:10.1196/annals.1375.047 [PubMed: 17130571] [CrossRef]
- 68.
- Knip M, Åkerblom HK, Al Taji E, et al. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: the TRIGR randomized clinical trial. JAMA. 2018;319(1):38-48. doi:10.1001/jama.2017.19826 [PMC free article: PMC5833549] [PubMed: 29297078] [CrossRef]
- 69.
- Bonifacio E, Ziegler AG, Klingensmith G, et al. Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA. 2015;313(15):1541-1549. doi:10.1001/jama.2015.2928 [PubMed: 25898052] [CrossRef]
- 70.
- Assfalg R, Knoop J, Hoffman KL, et al. Oral insulin immunotherapy in children at risk for type 1 diabetes in a randomised controlled trial. Diabetologia. 2021;64(5):1079-1092. doi:10.1007/s00125-020-05376-1 [PMC free article: PMC8012335] [PubMed: 33515070] [CrossRef]
- 71.
- Gerstein HC. Cow’s milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care. 1994;17(1):13-19. doi:10.2337/diacare.17.1.13 [PubMed: 8112184] [CrossRef]
- 72.
- Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290(13):1721-1728. doi:10.1001/jama.290.13.1721 [PubMed: 14519706] [CrossRef]
- 73.
- The EURODIAB Substudy 2 Study Group. Vitamin D supplement in early childhood and risk for type I (insulin-dependent) diabetes mellitus. Diabetologia. 1999;42(1):51-54. doi:10.1007/s001250051112 [PubMed: 10027578] [CrossRef]
- 74.
- Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500-1503. doi:10.1016/S0140-6736(01)06580-1 [PubMed: 11705562] [CrossRef]
- 75.
- Ziegler AG, Achenbach P, Berner R, et al. Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (Global Platform for the Prevention of Autoimmune Diabetes Primary Oral Insulin Trial) study protocol. BMJ Open. 2019;9(6):e028578. doi:10.1136/bmjopen-2018-028578 [PMC free article: PMC6609035] [PubMed: 31256036] [CrossRef]
- 76.
- U.S. National Library of Medicine: ClinicalTrials.gov. PINIT Study: Primary Intranasal Insulin Trial. Updated June 22, 2021. Accessed January 24, 2023. https:
//clinicaltrials .gov/ct2/show/NCT03182322 - 77.
- Ziegler AG, Arnolds S, Kolln A, et al. Supplementation with Bifidobacterium longum subspecies infantis EVC001 for mitigation of type 1 diabetes autoimmunity: the GPPAD-SINT1A randomised controlled trial protocol. BMJ Open. 2021;11(11):e052449. doi:10.1136/bmjopen-2021-052449 [PMC free article: PMC8578987] [PubMed: 34753762] [CrossRef]
- 78.
- Schatz DA, Levine SR, Atkinson MA. It’s time to mow the GRAS in type 1 diabetes. Diabetes. 2011;60(11):2669-2671. doi:10.2337/db11-1158 [PMC free article: PMC3198084] [PubMed: 22025772] [CrossRef]
- 79.
- Winkler C, Haupt F, Heigermoser M, et al. Identification of infants with increased type 1 diabetes genetic risk for enrollment into primary prevention trials—GPPAD-02 study design and first results. Pediatr Diabetes. 2019;20(6):720-727. doi:10.1111/pedi.12870 [PMC free article: PMC6851563] [PubMed: 31192505] [CrossRef]
- 80.
- Lampeter EF, Klinghammer A, Scherbaum WA, et al. The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS Group. Diabetes. 1998;47(6):980-984. doi:10.2337/diabetes.47.6.980 [PubMed: 9604880] [CrossRef]
- 81.
- Gale EA, Bingley PJ, Emmett CL, Collier T, European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925-931. doi:10.1016/S0140-6736(04)15786-3 [PubMed: 15043959] [CrossRef]
- 82.
- Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685-1691. doi:10.1056/NEJMoa012350 [PubMed: 12037147] [CrossRef]
- 83.
- Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care. 2005;28(5):1068-1076. doi:10.2337/diacare.28.5.1068 [PubMed: 15855569] [CrossRef]
- 84.
- Skyler JS, Type 1 Diabetes TrialNet Study Group. Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci. 2008;1150:190-196. doi:10.1196/annals.1447.055 [PMC free article: PMC2928677] [PubMed: 19120293] [CrossRef]
- 85.
- Vehik K, Cuthbertson D, Ruhlig H, et al. Long-term outcome of individuals treated with oral insulin: Diabetes Prevention Trial-Type 1 (DPT-1) oral insulin trial. Diabetes Care. 2011;34(7):1585-1590. doi:10.2337/dc11-0523 [PMC free article: PMC3120180] [PubMed: 21610124] [CrossRef]
- 86.
- Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ, Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA. 2017;318(19):1891-1902. doi:10.1001/jama.2017.17070 [PMC free article: PMC5798455] [PubMed: 29164254] [CrossRef]
- 87.
- Vandemeulebroucke E, Gorus FK, Decochez K, et al. Insulin treatment in IA-2A-positive relatives of type 1 diabetic patients. Diabetes Metab. 2009;35(4):319-327. doi:10.1016/j.diabet.2009.02.005 [PubMed: 19647467] [CrossRef]
- 88.
- Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372(9651):1746-1755. doi:10.1016/S0140-6736(08)61309-4 [PubMed: 18814906] [CrossRef]
- 89.
- Harrison LC, Honeyman MC, Steele CE, et al. Pancreatic β-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27(10):2348-2355. doi:10.2337/diacare.27.10.2348 [PubMed: 15451899] [CrossRef]
- 90.
- U.S. National Library of Medicine: ClinicalTrials.gov. Trial of Intranasal Insulin in Children and Young Adults at Risk of Type 1 Diabetes (INITII). Updated October 8, 2020. Accessed February 3, 2023. https:
//clinicaltrials .gov/ct2/show/NCT00336674 - 91.
- Elding Larsson H, Lundgren M, Jonsdottir B, Cuthbertson D, Krischer J, DiAPREV-IT Study Group. Safety and efficacy of autoantigen-specific therapy with 2 doses of alum-formulated glutamate decarboxylase in children with multiple islet autoantibodies and risk for type 1 diabetes: a randomized clinical trial. Pediatr Diabetes. 2018;19(3):410-419. doi:10.1111/pedi.12611 [PubMed: 29171140] [CrossRef]
- 92.
- U.S. National Library of Medicine: ClinicalTrials.gov. Prevention Trial: Immune-tolerance With Alum-GAD (Diamyd) and Vitamin D3 to Children With Multiple Islet Autoantibodies (DiAPREV-IT2). Updated November 17, 2020. Accessed February 3, 2023. https:
//clinicaltrials .gov/ct2/show/NCT02387164 - 93.
- U.S. National Library of Medicine: ClinicalTrials.gov. Hydroxychloroquine in Individuals At-risk for Type 1 Diabetes Mellitus (TN-22). Accessed January 24, 2023. https://www
.clinicaltrials .gov/ct2/show/NCT03428945 - 94.
- U.S. National Library of Medicine: ClinicalTrials.gov. CTLA4-Ig (Abatacept) for Prevention of Abnormal Glucose Tolerance and Diabetes in Relatives At-Risk for Type 1. Accessed January 24, 2023. https://www
.clinicaltrials .gov/ct2/show/NCT01773707 - 95.
- Russell WE, Bundy BN, Anderson MS, et al. Abatacept for delay of type 1 diabetes progression in stage 1 relatives at risk: a randomized, double-masked, controlled trial. Diabetes Care. 2023;46(5):1005-1013. doi:10.2337/dc22-2200 [PMC free article: PMC10154649] [PubMed: 36920087] [CrossRef]
- 96.
- Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613. doi:10.1056/NEJMoa1902226 [PMC free article: PMC6776880] [PubMed: 31180194] [CrossRef]
- 97.
- Sims EK, Bundy BN, Stier K, et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med. 2021;13(583):eabc8980. doi:10.1126/scitranslmed.abc8980 [PMC free article: PMC8610022] [PubMed: 33658358] [CrossRef]
- 98.
- Evans-Molina C, Oram RA. Teplizumab approval for type 1 diabetes in the USA. Lancet Diabetes Endocrinol. 2023;11(2):76-77. doi:10.1016/S2213-8587(22)00390-4 [PubMed: 36623518] [CrossRef]
- 99.
- Raab J, Haupt F, Scholz M, et al. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open. 2016;6(5):e011144. doi:10.1136/bmjopen-2016-011144 [PMC free article: PMC4874167] [PubMed: 27194320] [CrossRef]
- 100.
- U.S. National Library of Medicine: ClinicalTrials.gov. Fr1da Insulin Intervention. Accessed January 24, 2023. https:
//clinicaltrials .gov/ct2/show/NCT02620072 - 101.
- U.S. National Library of Medicine: ClinicalTrials.gov. Immune Effects of Oral Insulin in Relatives at Risk for Type 1 Diabetes Mellitus (TN20). Updated July 27, 2020. Accessed February 3, 2023. https://www
.clinicaltrials .gov/ct2/show/NCT02580877 - 102.
- U.S. National Library of Medicine: ClinicalTrials.gov. STOP-T1D Low-Dose (ATG) (TN28). Accessed January 24, 2023. https:
//clinicaltrials .gov/ct2/show/NCT04291703 - 103.
- Australian New Zealand Clinical Trials Registry. Cord Reinfusion in Diabetes Pilot Study. Accessed January 24, 2023. https://www
.anzctr.org .au/Trial/Registration/TrialReview .aspx?id=363694 - 104.
- Skyler JS. Immune intervention for type 1 diabetes mellitus. Int J Clin Pract Suppl. 2011;65(170):61-70. doi:10.1111/j.1742-1241.2010.02580.x [PubMed: 21323814] [CrossRef]
- 105.
- Feutren G, Papoz L, Assan R, et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2(8499):119-124. doi:10.1016/s0140-6736(86)91943-4 [PubMed: 2873396] [CrossRef]
- 106.
- The Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. . Diabetes. 1988;37(11):1574-1582. doi:10.2337/diab.37.11.1574 [PubMed: 2903105] [CrossRef]
- 107.
- Skyler JS, Rabinovitch A, Miami Cyclosporine Diabetes Study Group. Cyclosporine in recent onset type I diabetes mellitus. Effects on islet β cell function. J Diabetes Complications. 1992;6(2):77-88. doi:10.1016/1056-8727(92)90016-e [PubMed: 1611143] [CrossRef]
- 108.
- Chase HP, Butler-Simon N, Garg SK, et al. Cyclosporine A for the treatment of new-onset insulin-dependent diabetes mellitus. Pediatrics. 1990;85(3):241-245. doi:10.1542/peds.85.3.241 [PubMed: 2304776] [CrossRef]
- 109.
- Bougneres PF, Carel JC, Castano L, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318(11):663-670. doi:10.1056/NEJM198803173181103 [PubMed: 3125434] [CrossRef]
- 110.
- Bougnères PF, Landais P, Boisson C, et al. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39(10):1264-1272. doi:10.2337/diab.39.10.1264 [PubMed: 2210078] [CrossRef]
- 111.
- Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319(10):599-604. doi:10.1056/NEJM198809083191002 [PubMed: 3045545] [CrossRef]
- 112.
- Harrison LC, Colman PG, Dean B, Baxter R, Martin FI. Increase in remission rate in newly diagnosed type I diabetic subjects treated with azathioprine. Diabetes. 1985;34(12):1306-1308. doi:10.2337/diab.34.12.1306 [PubMed: 3905463] [CrossRef]
- 113.
- Cook JJ, Hudson I, Harrison LC, et al. Double-blind controlled trial of azathioprine in children with newly diagnosed type I diabetes. Diabetes. 1989;38(6):779-783. doi:10.2337/diab.38.6.779 [PubMed: 2656346] [CrossRef]
- 114.
- Coutant R, Landais P, Rosilio M, et al. Low dose linomide in type I juvenile diabetes of recent onset: a randomised placebo-controlled double blind trial. Diabetologia. 1998;41(9):1040-1046. doi:10.1007/s001250051028 [PubMed: 9754822] [CrossRef]
- 115.
- Dias HF, Mochizuki Y, Kühtreiber WM, Takahashi H, Zheng H, Faustman DL. Bacille Calmette Guerin (BCG) and prevention of types 1 and 2 diabetes: results of two observational studies. PLoS One. 2023;18(1):e0276423. doi:10.1371/journal.pone.0276423 [PMC free article: PMC9858877] [PubMed: 36662841] [CrossRef]
- 116.
- Elliott JF, Marlin KL, Couch RM. Effect of bacille Calmette-Guérin vaccination on C-peptide secretion in children newly diagnosed with IDDM. Diabetes Care. 1998;21(10):1691-1693. doi:10.2337/diacare.21.10.1691 [PubMed: 9773732] [CrossRef]
- 117.
- Allen HF, Klingensmith GJ, Jensen P, Simoes E, Hayward A, Chase HP. Effect of Bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care. 1999;22(10):1703-1707. doi:10.2337/diacare.22.10.1703 [PubMed: 10526739] [CrossRef]
- 118.
- Chaillous L, Lefèvre H, Thivolet C, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabète Insuline Orale group. Lancet. 2000;356(9229):545-549. doi:10.1016/s0140-6736(00)02579-4 [PubMed: 10950231] [CrossRef]
- 119.
- Pozzilli P, Pitocco D, Visalli N, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000;43(8):1000–1004. doi:10.1007/s001250051482 [PubMed: 10990077] [CrossRef]
- 120.
- Ergun-Longmire B, Marker J, Zeidler A, et al. Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann N Y Acad Sci. 2004;1029(1):260-277. doi:10.1196/annals.1309.057 [PubMed: 15681764] [CrossRef]
- 121.
- Skyler JS, Lorenz TJ, Schwartz S, et al. Effects of an anti-CD5 immunoconjugate (CD5-plus) in recent onset type I diabetes mellitus: a preliminary investigation. J Diabetes Complications. 1993;7(4):224-232. doi:10.1016/S0002-9610(05)80249-1 [PubMed: 7693056] [CrossRef]
- 122.
- Gottlieb PA, Quinlan S, Krause-Steinrauf H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care. 2010;33(4):826-832. doi:10.2337/dc09-1349 [PMC free article: PMC2845036] [PubMed: 20067954] [CrossRef]
- 123.
- Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692-1698. doi:10.1056/NEJMoa012864 [PubMed: 12037148] [CrossRef]
- 124.
- Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54(6):1763-1769. doi:10.2337/diabetes.54.6.1763 [PMC free article: PMC5315015] [PubMed: 15919798] [CrossRef]
- 125.
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598-2608. doi:10.1056/NEJMoa043980 [PubMed: 15972866] [CrossRef]
- 126.
- Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53(4):614-623. doi:10.1007/s00125-009-1644-9 [PubMed: 20225393] [CrossRef]
- 127.
- Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487-497. doi:10.1016/S0140-6736(11)60931-8 [PMC free article: PMC3191495] [PubMed: 21719095] [CrossRef]
- 128.
- Hagopian W, Ferry RJ, Sherry N, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62(11):3901-3908. doi:10.2337/db13-0236 [PMC free article: PMC3806608] [PubMed: 23801579] [CrossRef]
- 129.
- U.S. National Library of Medicine: ClinicalTrials.gov. Protege Encore Study-Clinical Trial of Teplizumab (MGA031) in Children and Adults With Recent-Onset Type 1 Diabetes Mellitus. Accessed February 4, 2023. https:
//clinicaltrials .gov/ct2/show/NCT00920582 - 130.
- Aronson R, Gottlieb PA, Christiansen JS, et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37(10):2746-2754. doi:10.2337/dc13-0327 [PMC free article: PMC4392937] [PubMed: 25011949] [CrossRef]
- 131.
- Ambery P, Donner TW, Biswas N, Donaldson J, Parkin J, Dayan CM. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med. 2014;31(4):399-402. doi:10.1111/dme.12361 [PubMed: 24236828] [CrossRef]
- 132.
- Herold KC, Gitelman SE, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62(11):3766-3774. doi:10.2337/db13-0345 [PMC free article: PMC3806618] [PubMed: 23835333] [CrossRef]
- 133.
- Herold KC, Gitelman SE, Willi SM, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56(2):391-400. doi:10.1007/s00125-012-2753-4 [PMC free article: PMC3537871] [PubMed: 23086558] [CrossRef]
- 134.
- U.S. National Library of Medicine: ClinicalTrials.gov. Recent-Onset Type 1 Diabetes Trial Evaluating Efficacy and Safety of Teplizumab (PROTECT). Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT03875729 - 135.
- Ramos EL, Dayan CM, Chatenoud L et al. Teplizumab and β-cell function in newly diagnosed type 1 diabetes. N Engl J Med 2023;389(23):2151-2161. doi:10.1056/NEJMoa2308743 [PubMed: 37861217] [CrossRef]
- 136.
- Haller MJ, Gitelman SE, Gottlieb PA, et al. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125(1):448-455. doi:10.1172/JCI78492 [PMC free article: PMC4382237] [PubMed: 25500887] [CrossRef]
- 137.
- Haller MJ, Gitelman SE, Gottlieb PA, et al. Antithymocyte globulin plus G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes. Diabetes. 2016;65(12):3765-3775. doi:10.2337/db16-0823 [PMC free article: PMC5127248] [PubMed: 27669730] [CrossRef]
- 138.
- Haller MJ, Schatz DA, Skyler JS, et al. Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care. 2018;41(9):1917-1925. doi:10.2337/dc18-0494 [PMC free article: PMC6105329] [PubMed: 30012675] [CrossRef]
- 139.
- Haller MJ, Long SA, Blanchfield JL, et al. Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes. 2019;68(6):1267-1276. doi:10.2337/db19-0057 [PMC free article: PMC6610026] [PubMed: 30967424] [CrossRef]
- 140.
- Gitelman SE, Gottlieb PA, Rigby MR, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):306-316. doi:10.1016/S2213-8587(13)70065-2 [PMC free article: PMC6489466] [PubMed: 24622416] [CrossRef]
- 141.
- Gitelman SE, Gottlieb PA, Felner EI, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59(6):1153-1161. doi:10.1007/s00125-016-3917-4 [PMC free article: PMC4869699] [PubMed: 27053235] [CrossRef]
- 142.
- Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412-419. doi:10.1016/S0140-6736(11)60886-6 [PMC free article: PMC3462593] [PubMed: 21719096] [CrossRef]
- 143.
- Orban T, Bundy B, Becker DJ, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37(4):1069-1075. doi:10.2337/dc13-0604 [PMC free article: PMC3964491] [PubMed: 24296850] [CrossRef]
- 144.
- Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):284-294. doi:10.1016/S2213-8587(13)70111-6 [PMC free article: PMC3957186] [PubMed: 24622414] [CrossRef]
- 145.
- Rigby MR, Harris KM, Pinckney A, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125(8):3285-3296. doi:10.1172/JCI81722 [PMC free article: PMC4623571] [PubMed: 26193635] [CrossRef]
- 146.
- U.S. National Library of Medicine: ClinicalTrials.gov. A Study of TCD601 (Siplizumab) in New Onset Type 1 Diabetes Patients (STRIDE). Updated October 30, 2023. Accessed January 31, 2024. https://classic
.clinicaltrials .gov/ct2/show/NCT06025110 - 147.
- Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143-2152. doi:10.1056/NEJMoa0904452 [PMC free article: PMC6410357] [PubMed: 19940299] [CrossRef]
- 148.
- Pescovitz MD, Greenbaum CJ, Bundy B, et al. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37(2):453-459. doi:10.2337/dc13-0626 [PMC free article: PMC3898764] [PubMed: 24026563] [CrossRef]
- 149.
- Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359(18):1909-1920. doi:10.1056/NEJMoa0804328 [PubMed: 18843118] [CrossRef]
- 150.
- Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378(9788):319-327. doi:10.1016/S0140-6736(11)60895-7 [PMC free article: PMC3580128] [PubMed: 21714999] [CrossRef]
- 151.
- Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366(5):433-442. doi:10.1056/NEJMoa1107096 [PubMed: 22296077] [CrossRef]
- 152.
- U.S. National Library of Medicine: ClinicalTrials.gov. A Phase III Study to Investigate the Impact of Diamyd in Patients Newly Diagnosed With Type 1 Diabetes (USA)-DIAPREVENT. Updated October 10, 2012. Accessed February 4, 2023. https:
//clinicaltrials .gov/ct2/show/NCT00751842 - 153.
- Martin A, Mick GJ, Choat HM, et al. A randomized trial of oral gamma aminobutyric acid (GABA) or the combination of GABA with glutamic acid decarboxylase (GAD) on pancreatic islet endocrine function in children with newly diagnosed type 1 diabetes. Nat Commun. 2022;13(1):7928. doi:10.1038/s41467-022-35544-3 [PMC free article: PMC9790014] [PubMed: 36566274] [CrossRef]
- 154.
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. β-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358(9295):1749-1753. doi:10.1016/S0140-6736(01)06801-5 [PubMed: 11734230] [CrossRef]
- 155.
- Raz I, Avron A, Tamir M, et al. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev. 2007;23(4):292-298. doi:10.1002/dmrr.712 [PubMed: 17124720] [CrossRef]
- 156.
- Huurman VA, Decochez K, Mathieu C, Cohen IR, Roep BO. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab Res Rev. 2007;23(4):269-275. doi:10.1002/dmrr.691 [PubMed: 17024692] [CrossRef]
- 157.
- Lazar L, Ofan R, Weintrob N, et al. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab Res Rev. 2007;23(4):286-291. doi:10.1002/dmrr.711 [PubMed: 17124721] [CrossRef]
- 158.
- Schloot NC, Meierhoff G, Lengyel C, et al. Effect of heat shock protein peptide DiaPep277 on β-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev. 2007;23(4):276-285. doi:10.1002/dmrr.707 [PubMed: 17103487] [CrossRef]
- 159.
- Pozzilli P, Raz I, Peled D, et al. Evaluation of long-term treatment effect in a type 1 diabetes intervention trial: differences after stimulation with glucagon or a mixed meal. Diabetes Care. 2014;37(5):1384-1391. doi:10.2337/dc13-1392 [PubMed: 24408401] [CrossRef]
- 160.
- Raz I, Ziegler AG, Linn T, et al. Treatment of recent-onset type 1 diabetic patients with DiaPep277: results of a double-blind, placebo-controlled, randomized phase 3 trial. Diabetes Care. 2014;37(5):1392-1400. doi:10.2337/dc13-1391 [PubMed: 24757230] [CrossRef]
- 161.
- Orban T, Farkas K, Jalahej H, et al. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J Autoimmun. 2010;34(4):408-415. doi:10.1016/j.jaut.2009.10.005 [PMC free article: PMC2860016] [PubMed: 19931408] [CrossRef]
- 162.
- Walter M, Philotheou A, Bonnici F, Ziegler AG, Jimenez R, NBI-6024 Study Group. No effect of the altered peptide ligand NBI-6024 on β-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care. 2009;32(11):2036-2040. doi:10.2337/dc09-0449 [PMC free article: PMC2768201] [PubMed: 19690081] [CrossRef]
- 163.
- Roep BO, Solvason N, Gottlieb PA, et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci Transl Med. 2013;5(191):191ra82. doi:10.1126/scitranslmed.3006103 [PMC free article: PMC4516024] [PubMed: 23803704] [CrossRef]
- 164.
- Moran A, Bundy B, Becker DJ, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905-1915. doi:10.1016/S0140-6736(13)60023-9 [PMC free article: PMC3827771] [PubMed: 23562090] [CrossRef]
- 165.
- Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32(7):1244-1249. doi:10.2337/dc09-0054 [PMC free article: PMC2699714] [PubMed: 19366957] [CrossRef]
- 166.
- Quattrin T, Haller MJ, Steck AK, et al. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. 2020;383(21):2007-2017. doi:10.1056/NEJMoa2006136 [PubMed: 33207093] [CrossRef]
- 167.
- Long SA, Rieck M, Sanda S, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61(9):2340-2348. doi:10.2337/db12-0049 [PMC free article: PMC3425404] [PubMed: 22721971] [CrossRef]
- 168.
- Hartemann A, Bensimon G, Payan CA, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1(4):295-305. doi:10.1016/S2213-8587(13)70113-X [PubMed: 24622415] [CrossRef]
- 169.
- Rosenzwajg M, Salet R, Lorenzon R, et al. Low-dose IL-2 in children with recently diagnosed type 1 diabetes: a phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia. 2020;63(9):1808-1821. doi:10.1007/s00125-020-05200-w [PubMed: 32607749] [CrossRef]
- 170.
- Gottlieb PA, Alkanani AK, Michels AW, et al. α1-antitrypsin therapy downregulates toll-like receptor-induced IL-1β responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. J Clin Endocrinol Metab. 2014;99(8):E1418-E1426. doi:10.1210/jc.2013-3864 [PMC free article: PMC4121034] [PubMed: 24527714] [CrossRef]
- 171.
- Martin S, Herder C, Schloot NC, et al. Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the randomized DIATOR Trial. PLoS One. 2011;6(3):e17554. doi:10.1371/journal.pone.0017554 [PMC free article: PMC3055882] [PubMed: 21412424] [CrossRef]
- 172.
- Strom A, Kolb H, Martin S, et al. Improved preservation of residual beta cell function by atorvastatin in patients with recent onset type 1 diabetes and high CRP levels (DIATOR trial). PLoS One. 2012;7(3):e33108. doi:10.1371/journal.pone.0033108 [PMC free article: PMC3308960] [PubMed: 22448235] [CrossRef]
- 173.
- Griffin KJ, Thompson PA, Gottschalk M, Kyllo JH, Rabinovitch A. Combination therapy with sitagliptin and lansoprazole in patients with recent-onset type 1 diabetes (REPAIR-T1D): 12-month results of a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2014;2(9):710-718. doi:10.1016/S2213-8587(14)70115-9 [PMC free article: PMC4283272] [PubMed: 24997559] [CrossRef]
- 174.
- von Herrath M, Bain SC, Bode B, et al. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9(4):212-224. doi:10.1016/S2213-8587(21)00019-X [PubMed: 33662334] [CrossRef]
- 175.
- Gitelman SE, Bundy BN, Ferrannini E, et al. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9(8):502-514. doi:10.1016/S2213-8587(21)00139-X [PMC free article: PMC8494464] [PubMed: 34214479] [CrossRef]
- 176.
- Ovalle F, Grimes T, Xu G, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med. 2018;24(8):1108-1112. doi:10.1038/s41591-018-0089-4 [PMC free article: PMC6092963] [PubMed: 29988125] [CrossRef]
- 177.
- Forlenza GP, McVean J, Beck RW, et al. Effect of verapamil on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA. 2023;329(12):990-999. doi:10.1001/jama.2023.2064 [PMC free article: PMC9960020] [PubMed: 36826844] [CrossRef]
- 178.
- Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189. doi:10.1126/scitranslmed.aad4134 [PMC free article: PMC4729454] [PubMed: 26606968] [CrossRef]
- 179.
- Dong S, Hiam-Galvez KJ, Mowery CT, et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight. 2021;6(18):e147474. doi:10.1172/jci.insight.147474 [PMC free article: PMC8492314] [PubMed: 34324441] [CrossRef]
- 180.
- Zieliński M, Żalińska M, Iwaszkiewicz-Grześ D, et al. Combined therapy with CD4(+) CD25highCD127(-) T regulatory cells and anti-CD20 antibody in recent-onset type 1 diabetes is superior to monotherapy: randomized phase I/II trial. Diabetes Obes Metab. 2022;24(8):1534-1543. doi:10.1111/dom.14723 [PubMed: 35441440] [CrossRef]
- 181.
- Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587-592. doi:10.2337/db14-0656 [PubMed: 25204974] [CrossRef]
- 182.
- Izadi M, Sadr Hashemi Nejad A, Moazenchi M, et al. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. 2022;13(1):264. doi:10.1186/s13287-022-02941-w [PMC free article: PMC9208234] [PubMed: 35725652] [CrossRef]
- 183.
- Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297(14):1568-1576. doi:10.1001/jama.297.14.1568 [PubMed: 17426276] [CrossRef]
- 184.
- Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301(15):1573-1579. doi:10.1001/jama.2009.470 [PubMed: 19366777] [CrossRef]
- 185.
- Voltarelli JC, Martinez EZ, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus—reply. JAMA. 2009;302(6):624-625. doi:10.1001/jama.2009.1100 [PubMed: 19671901] [CrossRef]
- 186.
- D’Addio F, Valderrama Vasquez A, Ben Nasr M, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes. 2014;63(9):3041-3046. doi:10.2337/db14-0295 [PubMed: 24947362] [CrossRef]
- 187.
- Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320(9):550-554. doi:10.1056/NEJM198903023200902 [PubMed: 2644534] [CrossRef]
- 188.
- Buckingham B, Beck RW, Ruedy KJ, et al. Effectiveness of early intensive therapy on β-cell preservation in type 1 diabetes. Diabetes Care. 2013;36(12):4030-4035. doi:10.2337/dc13-1074 [PMC free article: PMC3836135] [PubMed: 24130350] [CrossRef]
- 189.
- Boughton CK, Allen JM, Ware J, et al. Closed-loop therapy and preservation of C-peptide secretion in type 1 diabetes. N Engl J Med. 2022;387(10):882-893. doi:10.1056/NEJMoa2203496 [PubMed: 36069870] [CrossRef]
- 190.
- McVean J, Forlenza GP, Beck RW, et al. Effect of tight glycemic control on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA. 2023;329(12):980-989. doi:10.1001/jama.2023.2063 [PMC free article: PMC9960023] [PubMed: 36826834] [CrossRef]
- 191.
- U.S. National Library of Medicine: ClinicalTrials.gov. Probiotics in Newly Diagnosed T1D. Accessed July 30, 2023. https:
//clinicaltrials .gov/study/NCT04141761 - 192.
- U.S. National Library of Medicine: ClinicalTrials.gov. Reducing Innate Inflammation in New Onset Type 1 Diabetes. Accessed July 30, 2023. https:
//clinicaltrials .gov/study/NCT04335656 - 193.
- U.S. National Library of Medicine: ClinicalTrials.gov. Pilot Study of OMEGA-3 and Vitamin D in High-Dose in Type I Diabetic Patients (POSEIDON). Accessed July 30, 2023. https:
//clinicaltrials .gov/study/NCT03406897 - 194.
- Jacobsen LM, Bundy BN, Greco MN, et al. Comparing beta cell preservation across clinical trials in recent-onset type 1 diabetes. Diabetes Technol Ther. 2020;22(12):948-953. doi:10.1089/dia.2020.0305 [PMC free article: PMC7757538] [PubMed: 32833543] [CrossRef]
- 195.
- Leete P, Mallone R, Richardson SJ, Sosenko JM, Redondo MJ, Evans-Molina C. The effect of age on the progression and severity of type 1 diabetes: potential effects on disease mechanisms. Curr Diab Rep. 2018;18(11):115. doi:10.1007/s11892-018-1083-4 [PMC free article: PMC10043737] [PubMed: 30259209] [CrossRef]
- 196.
- Jacobsen LM, Diggins K, Blanchfield L, et al. Responders to low-dose ATG induce CD4+ T cell exhaustion in type 1 diabetes. JCI Insight. 2023;8(16):e161812. doi:10.1172/jci.insight.161812 [PMC free article: PMC10543726] [PubMed: 37432736] [CrossRef]
- 197.
- U.S. National Library of Medicine: ClinicalTrials.gov. Clinical Phase II/III Trial of Ustekinumab to Treat Type 1 Diabetes (UST1D2). Updated June 8, 2022. Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT03941132 - 198.
- Gregory JW, Carter K, Cheung WY, et al. Phase II multicentre, double-blind, randomised trial of ustekinumab in adolescents with new-onset type 1 diabetes (USTEK1D): trial protocol. BMJ Open. 2021;11(10):e049595. doi:10.1136/bmjopen-2021-049595 [PMC free article: PMC8524290] [PubMed: 34663658] [CrossRef]
- 199.
- U.S. National Library of Medicine: ClinicalTrials.gov. A Study to Assess the Safety and Tolerability of Different Doses of AG019 Administered Alone or in Combination With Teplizumab in Participants With Recent-onset Diagnosed Type 1 Diabetes (T1D). Accessed January 30, 2023, 2023. https:
//clinicaltrials .gov/ct2/show/NCT03751007 - 200.
- U.S. National Library of Medicine: ClinicalTrials.gov. Rituximab and Abatacept for Prevention or Reversal of Type 1 Diabetes (TN25). Accessed January 24, 2023. https:
//clinicaltrials .gov/ct2/show/NCT03929601 - 201.
- U.S. National Library of Medicine: ClinicalTrials.gov. Study of Safety and Efficacy of CFZ533 in Type 1 Diabetes Pediatric and Young Adult Subjects (CCFZ533X2207). Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT04129528 - 202.
- CORDIS. Ultra-Low Dose of IL-2 for the Treatment of Recently-Diagnosed Type 1 Diabetes. Accessed January 30, 2023. https://cordis
.europa .eu/project/id/305380/reporting - 203.
- U.S. National Library of Medicine: ClinicalTrials.gov. Baricitinib in New-Onset Type 1 Diabetes (BANDIT). Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT04774224 - 204.
- Waibel M, Thomas HE, Wentworth JM, et al. Investigating the efficacy of baricitinib in new onset type 1 diabetes mellitus (BANDIT)—study protocol for a phase 2, randomized, placebo controlled trial. Trials. 2022;23(1):433. doi:10.1186/s13063-022-06356-z [PMC free article: PMC9125350] [PubMed: 35606820] [CrossRef]
- 205.
- Waibel M, Wentworth JM, So M, et al. Baricitinib and β-cell function in patients with new-onset type 1 diabetes. N Engl J Med 2023;389(23):2140-2150. doi:10.1056/NEJMoa2306691 [PubMed: 38055252] [CrossRef]
- 206.
- U.S. National Library of Medicine: ClinicalTrials.gov. Effect of Methyldopa on MHC Class II Antigen Presentation in Type 1 Diabetes. Updated January 18, 2022. Accessed January 30, 2023. https://www
.clinicaltrials .gov/ct2/show/NCT01883804 - 207.
- Ludvigsson J, Eriksson L, Nowak C, et al. Phase III, randomised, double-blind, placebo-controlled, multicentre trial to evaluate the efficacy and safety of rhGAD65 to preserve endogenous beta cell function in adolescents and adults with recently diagnosed type 1 diabetes, carrying the genetic HLA DR3-DQ2 haplotype: the DIAGNODE-3 study protocol. BMJ Open. 2022;12(10):e061776. doi:10.1136/bmjopen-2022-061776 [PMC free article: PMC9628549] [PubMed: 36316084] [CrossRef]
- 208.
- U.S. National Library of Medicine: ClinicalTrials.gov. Safety, Tolerability and Potential Efficacy of AVT001 in Patients With Type 1 Diabetes. Updated December 12, 2022. Accessed February 4, 2023. https://www
.clinicaltrials .gov/ct2/show/NCT03895996 - 209.
- Gaglia JL, Mackey M, Daley HL, et al. 825-P: Novel autologous dendritic cell therapy AVT001 for type 1 diabetes. Diabetes 2023;72(Suppl 1):825–P. doi:10.2337/db23-825-P10.2337/db23-825-P [PubMed: 38916421] [CrossRef] [CrossRef]
- 210.
- U.S. National Library of Medicine: ClinicalTrials.gov. IMCY-0098 Proof of ACtion in Type 1 Diabetes (IMPACT Study). Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT04524949 - 211.
- U.S. National Library of Medicine: ClinicalTrials.gov. A Multiple Ascending Dose Trial Investigating Safety, Tolerability and Pharmacokinetics of NNC0361-0041 (TOPPLE T1D). Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT04279613 - 212.
- Skyler JS. Prevention and reversal of type 1 diabetes—past challenges and future opportunities. Diabetes Care. 2015;38(6):997-1007. doi:10.2337/dc15-0349 [PubMed: 25998292] [CrossRef]
- 213.
- Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5-12. doi:10.2337/dc19-0880 [PMC free article: PMC6925574] [PubMed: 31753960] [CrossRef]
- 214.
- Woittiez NJ, Roep BO. Impact of disease heterogeneity on treatment efficacy of immunotherapy in type 1 diabetes: different shades of gray. Immunotherapy. 2015;7(2):163-174. doi:10.2217/imt.14.104 [PubMed: 25713991] [CrossRef]
- 215.
- Jacobsen LM, Bocchino L, Evans-Molina C, et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia. 2020;63(3):588-596. doi:10.1007/s00125-019-05047-w [PMC free article: PMC7229995] [PubMed: 31768570] [CrossRef]
- 216.
- Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161-165. doi:10.15585/mmwr.mm6906a3 [PMC free article: PMC7017961] [PubMed: 32053581] [CrossRef]
- 217.
- Perry DJ, Wasserfall CH, Oram RA, et al. Application of a genetic risk score to racially diverse type 1 diabetes populations demonstrates the need for diversity in risk-modeling. Sci Rep. 2018;8(1):4529. doi:10.1038/s41598-018-22574-5 [PMC free article: PMC5852207] [PubMed: 29540798] [CrossRef]
- 218.
- Carel J-C, Landais P, Bougnères P. Therapy to prevent type 1 diabetes mellitus. N Engl J Med. 2002;347(14):1115-1116. doi:10.1056/NEJM200210033471415 [PubMed: 12362017] [CrossRef]
- 219.
- Keymeulen B, van Maurik A, Inman D, et al. A randomised, single-blind, placebo-controlled, dose-finding safety and tolerability study of the anti-CD3 monoclonal antibody otelixizumab in new-onset type 1 diabetes. Diabetologia. 2021;64(2):313-324. doi:10.1007/s00125-020-05317-y [PMC free article: PMC7801303] [PubMed: 33145642] [CrossRef]
- 220.
- Ludvigsson J, Routray I, Elluru S, et al. Combined vitamin D, ibuprofen and glutamic acid decarboxylase-alum treatment in recent onset type I diabetes: lessons from the DIABGAD randomized pilot trial. Future Sci OA. 2020;6(7):FSO604. doi:10.2144/fsoa-2020-0078 [PMC free article: PMC7421935] [PubMed: 32802401] [CrossRef]
- 221.
- Casas R, Dietrich F, Barcenilla H, et al. Glutamic acid decarboxylase injection into lymph nodes: beta cell function and immune responses in recent onset type 1 diabetes patients. Front Immunol. 2020;11:564921. doi:10.3389/fimmu.2020.564921 [PMC free article: PMC7583358] [PubMed: 33162978] [CrossRef]
- 222.
- Ludvigsson J, Sumnik Z, Pelikanova T, et al. Intralymphatic glutamic acid decarboxylase with vitamin D supplementation in recent-onset type 1 diabetes: a double-blind, randomized, placebo-controlled phase IIb trial. Diabetes Care. 2021;44(7):1604-1612. doi:10.2337/dc21-0318 [PMC free article: PMC8323180] [PubMed: 34021020] [CrossRef]
- 223.
- Greenbaum CJ, Serti E, Lambert K, et al. IL-6 receptor blockade does not slow β cell loss in new-onset type 1 diabetes. JCI Insight. 2021;6(21):e150074. doi:10.1172/jci.insight.150074 [PMC free article: PMC8663550] [PubMed: 34747368] [CrossRef]
- 224.
- Marwaha AK, Chow S, Pesenacker AM, et al. A phase 1b open-label dose-finding study of ustekinumab in young adults with type 1 diabetes. Immunother Adv. 2022;2(1):ltab022. doi:10.1093/immadv/ltab022 [PMC free article: PMC8769169] [PubMed: 35072168] [CrossRef]
- 225.
- Thrower SL, James L, Hall W, et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man phase I safety study. Clin Exp Immunol. 2009;155(2):156-165. doi:10.1111/j.1365-2249.2008.03814.x [PMC free article: PMC2675245] [PubMed: 19040615] [CrossRef]
- 226.
- U.S. National Library of Medicine: ClinicalTrials.gov. Safety Study to Assess Whether Proinsulin Peptide Injections Can Slow or Stop the Body Damaging Its Own Insulin-Making Cells in the Pancreas in Patients Newly Diagnosed With Type 1 Diabetes (MonoPepT1De). Updated July 28, 2015. Accessed March 21, 2023. https:
//clinicaltrials .gov/ct2/show/NCT01536431 - 227.
- Lebenthal Y, Brener A, Hershkovitz E, et al. A phase II, double-blind, randomized, placebo-controlled, multicenter study evaluating the efficacy and safety of alpha-1 antitrypsin (AAT) (Glassia®) in the treatment of recent-onset type 1 diabetes. Int J Mol Sci. 2019;20(23):6032. doi:10.3390/ijms20236032 [PMC free article: PMC6928874] [PubMed: 31795482] [CrossRef]
- 228.
- U.S. National Library of Medicine: ClinicalTrials.gov. Study of Human Plasma-Derived Alpha1-Proteinase Inhibitor in Subjects With New-Onset Type 1 Diabetes Mellitus. Updated September 5, 2018. Accessed January 30, 2023. https://www
.clinicaltrials .gov/ct2/show/NCT02093221 - 229.
- Piemonti L, Keymeulen B, Gillard P, et al. Ladarixin, an inhibitor of the interleukin-8 receptors CXCR1 and CXCR2, in new-onset type 1 diabetes: a multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2022;24(9):1840-1849. doi:10.1111/dom.14770 [PMC free article: PMC9540558] [PubMed: 35589610] [CrossRef]
- 230.
- Cabrera-Rode E, Cubas-Dueñas I, Rodríguez-Acosta J, et al. An exploratory study of itolizumab on the preservation of beta cell function in adults with recent-onset type 1 diabetes. J Clin Med. 2022;11(7):1789. doi:10.3390/jcm11071789 [PMC free article: PMC8999981] [PubMed: 35407396] [CrossRef]
- 231.
- U.S. National Library of Medicine: ClinicalTrials.gov. MELD-ATG: Phase II, Dose Ranging, Efficacy Study of Anti-thymocyte Globulin (ATG) Within 6 Weeks of Diagnosis of Type 1 Diabetes (T1D) (Meld-ATG). Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT04509791 - 232.
- U.S. National Library of Medicine: ClinicalTrials.gov. Tauroursodeoxycholic Acid (TUDCA) in New-Onset Type 1 Diabetes. Updated December 4, 2018. Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT02218619 - 233.
- U.S. National Library of Medicine: ClinicalTrials.gov. Verapamil SR in Adults With Type 1 Diabetes (Ver-A-T1D). Accessed January 30, 2023. https:
//clinicaltrials .gov/ct2/show/NCT04545151 - 234.
- U.S. National Library of Medicine: ClinicalTrials.gov. The Diabetes Virus Detection and Intervention Trial (DiViDInt). Updated April 8, 2021. Accessed September 28, 2023. https:
//clinicaltrials .gov/study/NCT04838145 - 235.
- Krogvold L, Mynarek IM, Ponzi E et al. Pleconaril and ribavirin in new-onset type 1 diabetes: a phase 2 randomized trial. Nat Med 2023;29(11):2902-2908. doi:10.1038/s41591-023-02576-1 [PMC free article: PMC10667091] [PubMed: 37789144] [CrossRef]
- 236.
- U.S. National Library of Medicine: ClinicalTrials.gov. Safety and Efficacy of CLBS03 in Adolescents With Recent Onset Type 1 Diabetes (The Sanford Project T-Rex Study). Updated January 8, 2021. Accessed January 30, 2023. https://beta
.clinicaltrials .gov/study/NCT02691247
Drs. Jacobsen and Schatz reported no conflicts of interest. Dr. Herold reported that he has consulted for Sanofi, Sonoma Biotherapeutics, and Vertex and is on the Scientific Advisory Board for NexImmune. He is listed as a co-inventor on a patent for use of teplizumab for delay/prevention of type 1 diabetes but receives no royalties. Dr. Skyler reported that he: is the Chair of the Strategic Advisory Committee for INNODIA, INNODIA-HARVEST, INNODIA-iVZW, and EDENT1FI; is currently on the Data and Safety Monitoring Boards for three studies of disease-modifying therapies for type 1 diabetes; and has consulted (in the last 12 months) with several companies developing disease-modifying or other therapies for type 1 diabetes, including 4Immune, Abvance, ActoBiotics, Adocia, Avotres, COUR, Imcyse, ImmunoMolecular Therapeutics, Kriya, Novo-Nordisk, Provention Bio, Sanofi, Signos, Vertex, and Viacyte.
- Review Prevention of Type 1 Diabetes.[Diabetes in America. 2018]Review Prevention of Type 1 Diabetes.Skyler JS, Krischer JP, Becker DJ, Rewers M. Diabetes in America. 2018 Aug
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Prediction and prevention of type 1 diabetes: update on success of prediction and struggles at prevention.[Pediatr Diabetes. 2015]Review Prediction and prevention of type 1 diabetes: update on success of prediction and struggles at prevention.Michels A, Zhang L, Khadra A, Kushner JA, Redondo MJ, Pietropaolo M. Pediatr Diabetes. 2015 Nov; 16(7):465-84. Epub 2015 Jul 23.
- A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto's Thyroiditis.[J Assoc Physicians India. 2023]A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto's Thyroiditis.Bhakat B, Pal J, Das S, Charaborty SK, SircarMedical NR, Kolkata, RGKar, NorthBengal, Siliguri. J Assoc Physicians India. 2023 Jan; 71(1):1.
- Type 1 diabetes pathogenesis - Prevention???[Indian J Endocrinol Metab. 2015]Type 1 diabetes pathogenesis - Prevention???Krishna CS, Srikanta S. Indian J Endocrinol Metab. 2015 Apr; 19(Suppl 1):S58-63.
- Prevention of Type 1 Diabetes - Diabetes in AmericaPrevention of Type 1 Diabetes - Diabetes in America
Your browsing activity is empty.
Activity recording is turned off.
See more...

 1
1