NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.

TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades.
Show detailsChapter 11TRPA1 : A Sensory Channel of Many Talents
Marilia Z. P. Guimaraes and Sven-Eric Jordt.
Author Information and AffiliationsAuthors
Marilia Z. P. Guimaraes1 and Sven-Eric Jordt2.Affiliations
Abstract
In mammals, TRPA1 is the sole member of the TRPA gene subfamily. Recent reports identified TRPA1 as a target for the noxious and inflammatory irritant mustard oil in peripheral sensory neurons, implicating a functional role in pain and neurogenic inflammation. Other studies suggest that TRPA1 participates in additional sensory processes, such as cold sensation and hearing. In this chapter, we summarize and discuss these recent findings and speculate about the potential physiological role of TRPA1 in chemosensation and pain transduction.
INTRODUCTION
TRPA1 is a member of the TRPA branch of the TRP ion channel gene family. On the structural level, TRPA channels are characterized by multiple N-terminal ankyrin repeats (~14 in the N-terminus of human TRPA1). Superficially, TRPA channels resemble TRPN channels that were implicated in mechanotransduction and hearing in Drosophila and zebrafish. However, the ion channel domain of TRPA channels is evolutionarily distant from TRPN channels. While other animals express two or more TRPA genes, mammals have only a sole TRPA gene, TRPA1. Far from having only a rudimentary presence, TRPA1 has attracted significant attention from different areas of sensory research. In mammals, TRPA1 is expressed in a subset of peripheral sensory neurons, implicating a specialized role in sensory transduction. Recent studies found evidence that TRPA1 is involved in sensory neural responses to mustard oil, allicin, and other chemical irritants. Moreover, TRPA1 may serve as a sensor for noxious cold temperature. Other studies identified TRPA1 as a candidate for the auditory hair cell transduction channel. The potential role of TRPA1 in hearing will be discussed in a different chapter in this volume. Here, we focus on TRPA1 as a target for chemical sensory irritants and discuss its physiological role in acute and inflammatory pain.
TRPA1 IN THE SENSORY NEURAL RESPONSE TO MUSTARD OIL
Mustard Oil and Capsaicin as Chemical Probes for the Pain Pathway
Mustard and pungent roots such as wasabi or radish have been staples in the human diet for millennia. The pungent ingredient in these and other plants of the genus Brassica (the cabbages) is mustard oil (allyl isothiocyanate). Mustard oil is produced from a chemical precursor, sinigrin, by the enzyme myrosinase that is activated when mustard seeds are crushed in the presence of water. Besides inducing acute pain and irritation, mustard oil is also a potent inflammatory agent. Applying it to the skin induces reddening, swelling, edema, and plasma extravasation, accompanied by thermal and mechanical hyperalgesia, the painful hypersensitivity to otherwise innocuous thermal and mechanical stimuli. Since it was discovered that mustard oil activated inflammation through a neurogenic mechanism, mustard oil has became an invaluable tool in the study of the pain pathway [1]. Mustard oil induces neurogenic inflammation by triggering the release of neuropeptides such as CGRP (calcitonin gene-related peptide) and Substance P from sensory nerve endings [2]. These peptides activate local dilation and permeabilization of the vasculature and promote infiltration of the affected tissue by neutrophils and other immune cells [3]. Mustard oil–induced neurogenic inflammation closely resembles neurogenic inflammation caused by capsaicin, the pungent ingredient in chili peppers [4]. Capsaicin is a vanilloid compound and is structurally distinct from isothiocyanates. Both capsaicin and mustard oil were crucial for the discovery and characterization of a specific subset of nociceptive inflammatory sensory neurons, the C-fibers. Pretreatment of the skin with capsaicin reversibly abolishes the induction of pain and inflammation by mustard oil, indicating that both compounds target the same populations of neurons. Psychophysical tests in human subjects also demonstrate a close relationship between the actions of capsaicin and mustard oil. When the human tongue is pretreated with capsaicin, placement of a mustard oil–soaked filter disk on the tongue failed to induce painful sensations [5]. When newborn rats were injected with a large dose of capsaicin, they became insensitive to both capsaicin and mustard oil throughout their life spans [6]. Injection of capsaicin causes permanent ablation of a subpopulation of sensory neurons with small soma diameters in all sensory ganglia [7,8]. These neurons give rise to unmyelinated sensory fibers, the C-fibers, which are characterized by their sensitivity to capsaicin.
Although mustard oil and capsaicin have similar effects, the pharmacology of capsaicin is much better defined. The discovery of the high-affinity capsaicin receptor ligand resiniferatoxin enabled the localization of capsaicin receptor sites on sensory neurons. Synthetic agonists and antagonists such as olvanil and capsazepine facilitated further detailed pharmacological and electrophysiological studies of vanilloid action. The cDNA encoding for the capsaicin receptor, TRPV1, was cloned in 1997, allowing receptor studies on the molecular level [9]. Deleting the TRPV1 gene in mice revealed that TRPV1 is essential for inflammatory thermal hyperalgesia and that it contributes to heat- and acid-evoked pain [10,11]. These and many other studies affirmed the crucial role of TRPV1 in pain transduction, and TRPV1 has become one of the major drug discovery targets for the development of new analgesics. In contrast, very little was known about potential targets for mustard oil on sensory neurons. In most studies, mustard oil was topically applied or injected in pure form or as an oil emulsion. Specific antagonists or analogues with higher potencies were unknown, and for some time it was thought that mustard oil might either exert its effects through nonspecific chemical damage of sensory neurons or through activation of the capsaicin receptor. However, TRPV1-deficient mice retained normal sensitivity to mustard oil [10], and TRPV1 is not activated by mustard oil in vitro [12]. Indicating a more specific action, a few select studies showed that isothiocyanates induce neurogenic effects at much lower concentrations. In one study, mustard oil induced contraction of the rat bladder at micromolar concentrations through a neurogenic mechanism [13]. Interestingly, this activity was blocked by ruthenium red, a cation channel blocker that also blocks TRP channels such as TRPV1. In a different study, benzyl isothiocyanate, a pungent structural congener of mustard oil, caused the neurogenic relaxation of precontracted arteries at micromolar concentrations [14]. Similar to the discovery of the capsaicin receptor, the detection and analysis of the molecular targets for mustard oil in sensory neurons may devise new strategies for the development of analgesics and anti-inflammatory agents.
TRPA1 Mediates Mustard Oil Effects In Sensory Neurons
To investigate the cellular and molecular basis of mustard oil action, we studied responses of cultured rat sensory neurons to mustard oil by ratiometric calcium imaging. We found that mustard oil activated influx of Ca2+ into ~35 percent of sensory neurons [12]. All mustard oil–responsive neurons were also sensitive to capsaicin. Mustard oil–induced Ca2+ influx could be blocked by ruthenium red. Thus, we hypothesized that mustard oil activates a ruthenium red–sensitive Ca2+-permeable ion channel in TRPV1-expressing neurons. Intriguingly, these characteristics were shared by the ion channel TRPA1, previously discovered as a potential mediator of responses to noxious cold stimuli in sensory neurons [15]. TRPA1 is expressed in a subset of peptidergic TRPV1-positive neurons. Moreover, TRPA1 channel currents are sensitive to ruthenium red [15]. These coincidences encouraged us to test responses of human and rat TRPA1 channels to mustard oil. We found that mustard oil and other pungent isothiocyanates induced robust ruthenium red–sensitive currents in Xenopus oocytes and in cultured mammalian cells expressing human and rat TRPA1. The potencies of mustard oil for TRPA1 activation and for sensory neural responses were comparable [12]. Similarly, extracts from mustard seeds and wasabi activated TRPA1. Mouse TRPA1 was also found to be sensitive to mustard oil [16,17]. Our recent analysis of mice with a targeted deletion in the TRPA1 gene indicates that TRPA1 may represent the sole site for the pungent and inflammatory action of mustard oil. TRPA1-deficient mice do not display acute pain-related behavior after application of mustard oil to paws. Mustard oil–induced mechanical and thermal hyperalgesia were absent. Moreover, mustard oil failed to activate influx of Ca2+ into TRPA1-deficient cultured sensory neurons, indicating that TRPA1 may represent the sole site for the pungent and inflammatory action of these agents.
Activation of TRPA1 by Garlic Derivatives and Hazardous Unsaturated Aldehydes
In addition to mustard oil, TRPA1 is activated by other pungent plant products such as cinnamaldehyde, eugenol, gingerol, and methyl salicylate [16]. However, very high concentrations of these compounds are needed to activate TRPA1. Two recent studies showed that extracts from garlic activate TRPA1 [18,19]. Garlic contains a variety of pungent organosulfur compounds, including allicin, a thiosulfinate compound, and diallyl disulfide. Thiosulfinates and pungent disulfides are also present in onions and other plants of the genus allium. Thiosulfinates and allyl disulfide are structurally related to mustard oil, sharing allyl groups and labile carbon-sulfur bonds. Both allicin and allyl disulfide were found to be potent activators of TRPA1 [18,19]. Allicin and diallyl disulfide activate Ca2+ influx into a subset of capsaicin-sensitive neurons that are also sensitive to mustard oil [18,19]. Cultured neurons from TRPA1-deficient mice failed to respond to allicin with Ca2+ uptake, confirming that TRPA1 is the sole site of action of pungent organosulfur compounds.
Garlic extracts and derivatives have hypotensive properties in vitro and in vivo in animal models. Adventitial sensory nerve fibers in arteries contribute to vasodilation by activity-dependent release of the vasodilatory peptide CGRP. In mesenteric arterial preparations, garlic extracts induced vasodilation of precontracted arterial segments through a neurogenic pathway [18]. Allicin, diallyl disulfide, and mustard oil had the same effects [18]. Their activities were abolished by pretreatment of the preparation with capsaicin and by the TRP channel blocker ruthenium red. Capsaicin depletes CGRP from capsaicin-sensitive neurons, leaving subsequent neural activation ineffective. Taken together, these results suggest that allicin, diallyl disulfide, and mustard oil induce vasodilation in vitro by activating TRPA channels on capsaicin-sensitive perivascular sensory nerve endings. Clearly, future pharmacological and genetic experiments are required to clarify whether this mechanism contributes to the systemic hypotensive effects of garlic in vivo. It remains controversial whether dietary garlic has beneficial cardiovascular effects in humans.
In addition to pungent organosulfur compounds, TRPA1 is activated by noxious unsaturated aldehydes [20]. One of these aldehydes is acrolein (2-propenal), a potent lachrymator and pulmonary agent that was used in chemical warfare in the First World War. Acrolein is an environmental hazard produced during combustion, and it can be found in cigarette smoke, smoke from fires, and smog. Moreover, acrolein is a toxic by-product of cyclophosphamide chemotherapy, causing hemorrhagic cystitis through neurogenic effects. While acrolein triggers Ca2+ influx into cultured neurons from wild-type mice, neurons of TRPA1-deficient mice fail to respond to this noxious compound [20].
ACTIVATION OF TRPA1 BY CANNABINOIDS
The Cannabis plant has been cultivated for centuries both for the production of hemp fiber and for its presumed medicinal and psychoactive properties. The best-known effects of cannabinoids are changes in mood and motivation, as well as in appetite. However, cannabinoids also induce a complex mixture of dose-dependent cardiovascular effects that can cause postural hypertension or hypotension in humans [21]. These effects revealed important roles for cannabinoids in the cardiovascular system. The major active ingredient in cannabis is Δ-9-tetrahydrocannabinol (THC), which produces most of the characteristic pharmacological effects. The identification of this and other phytocannabinoids and their corresponding receptors suggested that endogenous substances, endocannabinoids, might exist that are capable of eliciting similar actions. Anandamide, a polyunsaturated fatty acid amide, was discovered as a potent endocannabinoid that activates cannabinoid receptors in the brain. It is thought that most cannabinoid actions happen through the activation of two cannabinoid receptors, CB1 and CB2, both G-protein-coupled receptors. CB1 was first identified in the brain, where it is expressed abundantly. CB1 usually couples to Gi, leading to a reduction in cAMP and to Ca2+ channel inhibition and K+ channel stimulation. The second receptor, CB2, is expressed in immune system cells. Both receptors have been implicated in the cardiovascular actions of cannabinoids [22].
In anesthetized rats, a bolus injection of anandamide induces a triphasic cardiovascular response, consisting of an initial transient fall in blood pressure and heart rate (phase I), followed by a pressor response (phase II), and culminating with prolonged hypotension (phase III) [22]. The third phase can be blocked by a synthetic cannabinoid antagonist, SR141716A (rimonabant), indicating that CB1 is involved. However, phase I could not be blocked by the same antagonist. In addition, in studies with isolated mesenteric arteries, it has been demonstrated that anandamide has powerful vasodilatory effects, whereas other synthetic cannabinoids known to activate CB1 do not. Furthermore, the vasodilator activity of anandamide in the perfused mesenteric vascular bed remains intact in CB1 knockout mice [23]. The discovery that this effect was endothelium independent suggested that anandamide might act through a neurogenic mechanism. Indeed, anandamide was found to induce vasodilation by releasing CGRP from perivascular sensory neurons [23]. Because this effect was blocked by ruthenium red and capsazepine, it was proposed that anandamide might stimulate TRPV1 receptors present in sensory nerves, which was shown to be the case [24]. This report showed that endocannabinoids can act through receptors other than CB1 and CB2, and it identified a TRP channel as an ionotropic cannabinoid receptor.
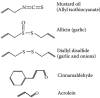
FIGURE 11.1
Diverse chemical activators of TRPA1: TRPA1 channels were initially identified as the target of mustard oil (allyl isothiocyanate), the pungent ingredient in mustard. Subsequently other pungent organosulfur compounds, such as allicin and diallyl disulfide, (more...)
THC is known to cause hypotension in laboratory animals, even though in humans it might have the opposite effect [21]. These differences might be due to the dose and/or experimental setting, such as anesthetized versus nonanesthetized subjects. In rat isolated mesenteric and hepatic arteries, THC induces extensive vessel relaxation [25]. Similar to anandamide, THC was shown to act via a neurogenic, CB1-and CB2-independent pathway. This effect is mediated by capsaicin-sensitive neurons, as depletion of CGRP by capsaicin abolished the vasodilatory actions of THC. Similar to the effect of anandamide, THC-induced vasodilation was sensitive to ruthenium red. However, THC was still able to induce vasodilation in arteries prepared from TRPV1 knockout mice, indicating that a target different from TRPV1 mediates this activity. This result led to the pursuit of yet another cannabinoid receptor with characteristics of a TRP channel. In subsequent experiments, it was found that THC activates Ca2+ influx into a capsaicin- and mustard oil–sensitive population of sensory neurons. Because of TRPA1’s coexpression with TRPV1, its sensitivity to mustard oil, and its sensitivity to ruthenium red, it was tested as a potential receptor for THC. Indeed, THC activated cationic currents in oocytes and mammalian cells expressing TRPA1. This indicates that TRPA1 might be responsible for the vasodilatory actions of THC in isolated vessels and establishes TRPA1 as an additional ionotropic cannabinoid receptor. Although anandamide does not promote TRPA1 activity, these results suggest that other endogenous cannabinoid-like compounds exist that may modulate TRPA1 activity.
TRPA1 IN COLD SENSATION: QUESTIONS AND ANSWERS
The physiological role of TRPA1 in sensory transduction is currently a matter of spirited debate. Initially, TRPA1 was identified as a potential mediator of noxious cold stimuli in nociceptive sensory neurons [15]. This hypothesis was based on the observation that TRPA1 responds to cold stimuli in heterologous expression systems. However, other recent studies did not observe cold sensitivity of TRPA1 channels [12,17]. Thus, it remains to be determined whether TRPA1 has intrinsic cold sensitivity in the reported range.
Another important question is how TRPA1 responses compare to sensory neural responses to cold. Cold responses in dissociated sensory neurons are heterogeneous [26]. Cold-sensitive neurons in rats and mice are divided into at least two populations, based on their pharmacology and their temperature-response profiles. The major cold-sensitive population is activated by moderate cooling and is sensitive to the cooling agent menthol, indicating that the cold/menthol receptor TRPM8 contributes to the observed responses. A smaller percentage of sensory neurons respond to cold, but not to menthol, indicating that mechanisms independent from TRPM8 are involved. These could include the activation of other depolarizing conductances such as TRPA1 or the inhibition of hyperpolarizing potassium channels. Comparison of the cellular distribution of TRPA1 with the prevalence of nonmenthol cold-responsive cells has not helped to resolve whether or not TRPA1 is involved in sensing cold. While initial in situ hybridization experiments detected TRPA1 in only 3.6 percent of mouse DRG neurons [15], a number comparable to the prevalence of nonmenthol cold-responsive cells, consensus is building that TRPA1 expression is more widespread. Recent reports found that TRPA1 is expressed in 20–36.7 percent of trigeminal neurons, 20–56.5 percent of DRG neurons, and 28.4 percent of neurons in nodose ganglia [12,17,18,27,28]. While these numbers compare very well with the percentages of mustard oil–sensitive cells, they are much larger than the percentage of nonmenthol cold-responsive neurons. Because no significant correlation between mustard oil responses and cold sensitivity was found in cultured neurons, Babes et al. concluded that TRPA1 is not essential for the cold response [26]. These authors also observed significant kinetic differences in the cold responses of menthol-insensitive neurons and of TRPA1 in heterologous cells. Additional comparisons of cellular responses to TRPA1 activators such as allicin or cinnamaldehyde with cold responses again led to divergent conclusions about the role of TRPA1 in cold sensation [12,18,19].
Cellular and behavioral analyses of TRPA1-deficient mice showed that TRPA1 is not essential for acute responses to cold [20]. The prevalence of menthol-insensitive cold-responsive neurons remained unchanged when compared with wild-type mice, indicating that a TRPA1-independent mechanism is responsible for activation of these cells by cold. Behavioral responses to evaporative cooling and to different temperatures on the cold plate were completely normal in TRPA1-deficient mice [20]. Thus, TRPA1 is not required for cold-induced pain in vivo. As noxious cold sensitivity of sensory neurons does not depend on TRPA1 expression, we are left with the question of whether the cold-activated currents in TRPA1-expressing heterologous cells that have been observed by some investigators can be attributed to intrinsic cold sensitivity of the channel or whether such currents are the result of indirect mechanisms, channel overexpression, disturbances of intracellular Ca2+ levels, or other causes.
While TRPA1 is not essential for acute cold-induced pain, a recent report found that the suppression of TRPA1 by antisense nucleotides reduces hypersensitivity to cold temperature (cold allodynia) in rat models of inflammation and nerve injury [27]. These authors also found that the transcription of TRPA1 is increased during inflammation. These data suggest that TRPA1 expression and regulation may affect the excitability of temperature-sensitive neurons in vivo.
TRPA1 IS A RECEPTOR-OPERATED CHANNEL ACTING IN CONCERT WITH TRPV1
TRP ion channels are regulated through signaling pathways that are activated by G-protein-coupled receptors and other membrane receptors. In sensory neurons, TRPV1 activity is regulated by a number of phospholipase C (PLC)–coupled receptors, including the B2 bradykinin receptor, TrkA, the receptor for nerve growth factor (NGF), and ATP receptors. Bradykinin and NGF sensitize TRPV1 to heat and to noxious chemical stimuli, leading to thermal hyperalgesia. Heat sensitivity of TRPV1 is increased by PLC-mediated pathways that remove inhibition of TRPV1 by the membrane phospholipid PIP2 [29,30]. Heterologous cells coexpressing TRPA1 and PLC-coupled receptors respond with immediately activating cationic currents when receptor agonists are applied [12,16]. This indicates that TRPA1 may function as a receptor-operated channel in vivo. Bradykinin, in addition to causing thermal hyperalgesia through sensitization of TRPV1, elicits acute pain through immediate depolarization of sensory neurons. This effect, while diminished, is maintained in TRPV1-deficient mice, suggesting that additional conductances such as TRPA1 may be required for full neural excitation. Indeed, neurons isolated from TRPA1-deficient mice fail to display bradykinin-induced Ca2+ influx [20]. Even more strikingly, bradykinin-induced thermal hyperalgesia is absent in TRPA1-deficient mice. While thermal hyperalgesia was initially attributed solely to TRPV1, this result indicates that TRPA1 and TRPV1 are interdependently regulated downstream of BK2 receptors to establish hypersensitivity to heat. Apparently, TRP channels from different branches of the TRP channel gene family can act in concert to exert physiological effects. Whether this interdependence reflects direct physical interaction between channels and receptors or an indirect regulatory relationship remains to be investigated.
TRPA1 AS A MULTIPLE IRRITANT SENSOR
TRPA1 channels respond to a multitude of irritants with diverse origins and chemical structures. Subsets of sensory neurons in the lung, eyes, and mucous membranes have a broad sensitivity to diverse volatile chemical irritants and environmental toxicants. These compounds induce pain, coughing, apnea, and lachrymation and inspire an individual’s behavior to be protected from further exposure. Responses to most chemical irritants are retained in mice deficient in the capsaicin receptor TRPV1, implicating other mechanisms of neural activation. Is TRPA1 involved as a multiple irritant sensor in these neurons? How is such a broad range of sensitivity achieved? Currently, it is unknown whether or not mustard oil and other activators interact with TRPA1 directly. We can discuss different options for the mechanism of channel activation. First, chemical irritants may bind to TRPA1 through “classical” ligand-receptor interaction. While most receptor-ligand systems require high degrees of specificity, some receptors bind multiple ligands with diverse structures. For example, bitter-taste receptors are known to interact with a multitude of plant alkaloids and other bitter-tasting chemicals that don’t show much resemblance to each other in their chemical structures [31]. Similarly, TRPA1 may bind to a large variety of irritant molecules to induce sensory neural excitation. Second, chemically reactive irritants such as mustard oil could form permanent or transient covalent bonds with TRPA1, thereby activating the channel. Isothiocyanates, allicin, and unsaturated aldehydes are reactive compounds capable of forming covalent bonds with cysteine and other residues in proteins. Third, reactive irritants could interfere with signaling pathways that regulate TRPA1, leading to channel activation. These pathways could include phosphorylation cascades, or regulation of intracellular Ca2+ that is known to affect TRPA1 function [12].
CONCLUSION
The studies discussed in this chapter show that TRPA1 channels in mammalian sensory neurons contribute to acute and inflammatory pain. TRPA1 is activated downstream of inflammatory PLC-coupled receptors for bradykinin and other proalgesic agents in vivo and acts in concert with TRPV1 to cause thermal hyperalgesia. Whether endogenous ligands for TRPA1 exist remains to be established. The sensitivity of TRPA1 to phytocannabinoids indicates that endogenous cannabinoid-like ligands may modulate TRPA1 function. While it is not known whether mustard oil–like compounds are endogenously expressed in mammals, other endogenous organosulfur compounds could affect the TRPA1 activity. Future pharmacological, electrophysiological, and genetic studies are needed to clarify these and other aspects of TRPA1 function. In addition to TRPV1, TRPA1 represents an exciting new target for the development of potential new analgesics and anti-inflammatory agents.
REFERENCES
- 1.
- Bruce AN. Über die Beziehungen der sensiblen Nervenendigungen zum Entzünd-ungsvorgang. Arch Exp Pathol Pharmakol. 1910;63:423–33.
- 2.
- Louis SM, Johnstone D, Russell NJ, Jamieson A, Dockray GJ. Antibodies to calcitonin-gene related peptide reduce inflammation induced by topical mustard oil but not that due to carrageenin in the rat. Neurosci Lett. 1989;102(2-3):257–60. [PubMed: 2812504]
- 3.
- Smith CH, Barker JN, Morris RW, MacDonald DM, Lee TH. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151(6):3274–82. [PubMed: 7690800]
- 4.
- Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol. 1967;31(1):138–51. [PMC free article: PMC1557289] [PubMed: 6055248]
- 5.
- Simons CT, Carstens MI, Carstens E. Oral irritation by mustard oil: self-desensitization and cross-desensitization with capsaicin. Chem Senses. 2003;28(6):459–65. [PubMed: 12907583]
- 6.
- Gamse R, Holzer P, Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980;68(2):207–13. [PMC free article: PMC2043922] [PubMed: 6153545]
- 7.
- Cuello AC, Gamse R, Holzer P, Lembeck F. Substance P immunoreactive neurons following neonatal administration of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1981;315(3):185–94. [PubMed: 6163995]
- 8.
- Hori T, Tsuzuki S. Thermoregulation in adult rats which have been treated with capsaicin as neonates. Pflügers Arch. 1981;390(3):219–23. [PubMed: 7196020]
- 9.
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. [PubMed: 9349813]
- 10.
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13. [PubMed: 10764638]
- 11.
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–87. [PubMed: 10821274]
- 12.
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–65. [PubMed: 14712238]
- 13.
- Patacchini R, Maggi CA, Meli A. Capsaicin-like activity of some natural pungent substances on peripheral endings of visceral primary afferents. Naunyn Schmiedebergs Arch Pharmacol. 1990;342(1):72–77. [PubMed: 1698263]
- 14.
- Wilson RK, Kwan TK, Kwan CY, Sorger GJ. Effects of papaya seed extract and benzyl isothiocyanate on vascular contraction. Life Sci. 2002;71(5):497–507. [PubMed: 12052434]
- 15.
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–29. [PubMed: 12654248]
- 16.
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–57. [PubMed: 15046718]
- 17.
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25(16):4052–61. [PMC free article: PMC6724946] [PubMed: 15843607]
- 18.
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102(34):12248–52. [PMC free article: PMC1189336] [PubMed: 16103371]
- 19.
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15(10):929–34. [PubMed: 15916949]
- 20.
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–82. [PubMed: 16564016]
- 21.
- Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(11 Suppl):58S–63S. [PubMed: 12412837]
- 22.
- Pacher P, Batkai S, Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48(8):1130–38. [PMC free article: PMC2225528] [PubMed: 15910888]
- 23.
- Hogestatt ED, Zygmunt PM. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2-3):343–51. [PubMed: 12052048]
- 24.
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–57. [PubMed: 10440374]
- 25.
- Zygmunt PM, Andersson DA, Hogestatt ED. Delta 9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci. 2002;22(11):4720–27. [PMC free article: PMC6758782] [PubMed: 12040079]
- 26.
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20(9):2276–82. [PubMed: 15525269]
- 27.
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115(9):2393–401. [PMC free article: PMC1187934] [PubMed: 16110328]
- 28.
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493(4):596–606. [PubMed: 16304633]
- 29.
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–62. [PubMed: 11418861]
- 30.
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300(5623):1284–88. [PubMed: 12764195]
- 31.
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434(7030):225–29. [PubMed: 15759003]
- TRPA1 : A Sensory Channel of Many Talents - TRP Ion Channel Function in Sensory ...TRPA1 : A Sensory Channel of Many Talents - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
Your browsing activity is empty.
Activity recording is turned off.
See more...