NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.

TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades.
Show detailsChapter 5TRPV1 Receptors and Signal Transduction
Tamara Rosenbaum and Sidney A. Simon.
Author Information and AffiliationsAuthors
Tamara Rosenbaum1 and Sidney A. Simon2.Affiliations
INTRODUCTION
The perception of pain throughout the body arises when neural signals originating from the terminals of nociceptors are propagated to second-order neurons in the spinal cord or brainstem, whereupon they are transmitted to specific higher order brain areas (Price, 2000). Recent studies have begun to elucidate some of the molecular mechanisms underlying the transduction of noxious stimuli. Many stimuli have been found to activate ion channels present on nociceptor terminals that act as molecular transducers to depolarize these neurons, thereby setting off nociceptive impulses along the pain pathways (Price, 2000; Costigan and Woolf, 2000). Among these ion channels are the members of the transient receptor potential (TRP) family. To date, the most studied member of the TRP family is the TRPV1 receptor. This is because it is the only one activated by capsaicin, the compound in chili pepper responsible for its “hot” taste; also, inhibiting TRPV1 has been shown to have therapeutic value (DiMarzo et al., 2002; Cortright and Szallasi, 2004). Although we will focus on the presence of these channels in nociceptors, we note that they have been identified in many other cell types and in various cortical and subcortical areas (Toth et al., 2005).
The transient receptor potential vanilloid 1 (TRPV1) channel is predicted to have six transmembrane domains and a short, pore-forming hydrophobic stretch between the fifth and sixth transmembrane domains (see Figure 5.1A). It is activated not only by the vanilloid capsaicin (Caterina et al., 1997), but also by noxious heat (>43°C) and low pH (Caterina et al., 1997; Tominaga et al., 1998), voltage (Gunthorpe et al., 2000; Piper et al., 1999), and various lipids (Julius and Basbaum, 2001; Caterina and Julius, 2001; Clapham, 2003; Cortright and Szallasi, 2004, Szallasi and Blumberg, 1999; Prescott and Julius, 2003; Jung et al., 2004; Bhave et al., 2003). In cells, TRPV1 is inactivated by its binding to PIP2 and is released from this block by PLC-mediated PIP2 hydrolysis (Prescott and Julius, 2003).
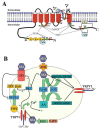
FIGURE 5.1
(A) A schematic diagram of a TRPV1 subunit in a bilayer. The subunit has six transmembrane domains (red) and a pore loop. The functional TRPV1 receptor is believed to form a tetramer. Residues involved in vanilloid binding are shown in orange. “A” (more...)
Since its cloning in 1997, many amino acid regions within the TPRV1 protein have been shown to be involved in specific functions, such as capsaicin, proton, and heat activation; voltage dependence; permeability and ion selectivity; antagonist regions; desensitization; phosphorylation; modulation by lipids; and multimerization. In regard to its subunit composition, functional TRPV1 channels likely exist as homomeric or heteromeric complexes composed of four subunits that assemble to form functional cation-(including calcium) permeable pores (Clapham, 2003; Kedei et al., 2001; Kuzhikanathil et al., 2001). Moreover, like other ion channels, these channels have been shown to be associated with regulatory proteins (see Figure 5.1B and Kim et al., 2006).
There are many signaling pathways that become activated (or inhibited) by the activation of TRPV1 (Farkas-Szallasi et al., 1995; Wood et al., 1988). Similar to many other channels, TRPV1 contains multiple phosphorylation sites in its amino acid sequence for protein kinase C (PKC) (Bhave et al., 2003; Dai et al., 2004; Premkumar et al., 2004), protein kinase A (PKA) (Bhave et al., 2002; De Petrocellis et al., 2001; Rathee et al., 2002) and Ca2+/calmodulin-dependent protein kinase II (CaMKII). The presence of multiple phosphorylation sites in TRPV1 implies possible regulatory actions by these kinases (Wood et al., 1988).
Discussed later in this chapter are several lines of evidence that show that two types of lipids—endocannabinoids and eicosanoids that are products of lipoxygenase (LOX)—activate TRPV1 channels (Zygmunt et al., 1999; Hwang et al., 2000).
Because TRPV1 functions as a molecular integrator for multiple types of sensory input, in this chapter we will explore the molecular mechanisms underlying the activation and modulation of this channel.
TRPV1 ACTIVATORS
Before proceeding with the identification of regions on TRPV1 associated with the binding of activators, care must be taken that these are actually the binding sites as opposed to gating sites that will also lead to changes in the allosteric properties of the protein. This is important because, for example, capsaicin, even at concentrations that fail to activate a current, can sensitize TRPV1 receptors to protons and heat. Similarly, protons can sensitize TRPV1 receptors to capsaicin and heat. As discussed below, this likely occurs as a consequence of decreasing the free energy of one of the many closed states for these stimuli to be closer to one of the open states (Hui et al., 2003; Ryu et al., 2003). That is, TRPV1 is a multistate ion channel whose rate constants between the rate limiting closed and open states may depend on voltage, temperature, and a variety of agonists.
Activation by Capsaicin and Other TRPV1 Agonists
TRPV1 receptors are activated by vanilloids like capsaicin (Spath and Darling, 1930; Thresh, 1846). At negative holding potentials, this activation results in the influx of calcium and sodium, thereby depolarizing the cell. TRPV1 can be activated by capsaicin in isolated membrane patches that are devoid of the intracellular signaling machinery. Judging from results obtained from binding assays (Szallasi et al., 1993) and from electrophysiological recordings (Hui et al., 2003), there is good agreement that the binding of at least two capsaicin molecules is required for complete activation of this channel.
There is good evidence that the capsaicin-binding site is intracellular on TRPV1 receptors. One form of evidence is that when added extracellularly, membrane-impermeant forms of capsaicin are inactive, but are active when applied intracellularly (Jung et al., 1999). In the rodent form of TRPV1, residues Arg114 and Glu761 in the intracellular N- and C-termini, respectively, have been identified as agonist recognition sites (Jung et al., 2002). By analyzing the avian form of TRPV1 that is not activated by capsaicin with murine forms that are, it was found that residues Tyr511 and Thr550 in the third and fifth transmembrane domains are responsible for binding capsaicin to TRPV1 (Jordt and Julius, 2002; Gavva et al., 2004). In addition, three residues located at the transition between the second intracellular loop and the third transmembrane domain (Tyr511 and Ser512) and at the bottom of the fifth transmembrane domain (Tyr550) have been proposed as sites where vanilloid agonists might interact with the TRPV1 channel (Jordt and Julius, 2002; Gavva et al., 2004).
One of the best characterized of the less-pungent capsaicin analogues is olvanil. Olvanil, like capsaicin, has nociceptive and inflammatory properties (Dray and Dickenson, 1991), and it is about as efficient as capsaicin in its ability to increase Ca2+ influx in cultured rat DRGs (Walpole et al., 1993; Koplas et al., 1997) and induce currents in nociceptive neurons (Liu and Simon, 1997). One reason that it is a less-pungent analogue is that its rate of activation of TRPV1 is slower than that of capsaicin; while TRPV1 is being activated and the receptor potential is slowly depolarizing, voltage-dependent sodium and calcium channels will become inactivated to a greater extent. This in turn will decrease the probability of this neuron firing action potentials (Liu et al., 1997). Another reason is that olvanil also activates CB1 receptors, which leads to anti-nociceptive responses.
An important advance was the identification of resiniferatoxin (RTX), a diterpene related to the phorbol esters, as a potent capsaicin analogue (Szallasi and Blumberg, 1989). This compound, which shares a vanillyl group with capsaicin, is a particularly strong irritant that was isolated from the latex of a Moroccan cactus-like plant Euphorbia resinifera (Hergenhahn et al., 1984; Appendino and Szallasi, 1997). In several assays, RTX is several thousandfold more potent than capsaicin (Szolcsanyi et al., 1990).
Rat and human TRPV1 have been pharmacologically characterized, proving that RTX is indeed an agonist of TRPV1 in DRG neurons. The use of radio-labeled RTX for TRPV1 has allowed the detection of TRPV1 in peripheral tissues such as the urinary bladder (Szallasi et al., 1993; Acs et al., 1994), urethra (Parlani et al., 1993), nasal mucosa (Rinder et al., 1996), airways (Szallasi et al., 1993), and colon (Goso et al., 1993), as well as in several brain nuclei (Mezey et al., 2000).
RTX binding requires the presence of a methionine in position 547 of the third transmembranal segment (Gavva et al., 2004); these residues are involved also in the binding of some endogenous agonists as well as of competitive antagonists (Jordt and Julius, 2002; Gavva et al., 2004; Phillips et al., 2004).
In addition to capsaicin and RTX, several other natural and synthetic hydrophobic TRPV1 agonists have been identified (Rami and Gunthorpe, 2004; Krause et al., 2005; Cortright and Szallasi, 2004). Among the natural agonists found in the body are the arachidonic acid metabolites anandamide (which also activates CB1 and TRPV4 receptors) and 12-hydroxyeicosatetranenoic acid (12-HETE) (see Figure 5.1B). In addition, N-arachidonoyldopamine (NADA) activates TRPV1, but its biological function remains unknown. Piperine and zingerone, two pungent tasting compounds found in black pepper and ginger, respectively, have also been shown to activate TRPV1 receptors (Liu and Simon, 1996; McNamara et al., 2005). In summary, TRPV1 can be activated by a variety of molecules, and many more are certain to be identified.
TRPV1 Activation by Protons
Pain sensation is augmented by the acidic extracellular pH during ischemia or inflammation. It has been shown that Aδ and C-fiber neurons transduce extracellular acid signals (hydrated protons) by means of at least two different classes of cation-selective channels, TRPV1 (Caterina et al., 1997; Gunthorpe et al., 2002) and the acid-sensing ion channels (ASICs) (Bianchi and Driscoll, 2002; Kellenberger et al., 2002; Waldmann et al., 1999).
For TRPV1 receptors, it has been found that protons only activate the channels when they are added from the extracellular solution, suggesting that the proton-active gate is on the extracellular part of the molecule (Jordt et al., 2000; Welch et al., 2000). Lowering the pH causes sigmoidal increases in the current. This current has a reversal potential close to zero millivolts (Bevan and Yeats, 1991; Liu and Simon, 2000), suggesting that, at neutral pH, protons do not carry much of the current in buffers containing physiological concentrations of sodium and calcium. Nevertheless, under specific experimental conditions, it has recently been suggested that TRPV1 is permeable to protons and that the open TRPV1 pore holds enough water molecules to form a continuous “water wire,” allowing “proton hopping” along adjacent free water molecules (Hellwig et al., 2004).
Protons influence the cloned TRPV1 in a complex fashion. Lowering the pH sensitizes the responses to capsaicin (Tominaga et al., 1998; Ryu et al., 2003; Reeh and Kress, 2002). This is in accordance with earlier observations, where low pH potentiated responses to low concentrations of capsaicin in sensory neurons in cultures from rats (Petersen and LaMotte, 1993; Kress et al., 1996; Liu and Simon, 2000), rabbits (Martenson et al., 1994), or humans (Baumann et al., 1996). The presence of protons increases the affinity of the receptor for capsaicin and changes the distribution of energy states for capsaicin activation that will bias them toward the open state, thereby affecting the mean open times (Ryu et al., 2003).
Future work will test specific downstream effects of the TRPV1-mediated dual acidification mechanism in nociceptive neurons, but it is clear that low pHs potentiate activation of TRPV1 by capsaicin in cell-free patches where the intracellular machinery of signaling is nonexistent.
Noxious Heat Promotes TRPV1 Activation
The application of temperature ramps revealed that TRPV1 acts as a molecular thermometer. At a holding potential of −60 mV, the inward current abruptly increases at about 43°C (a temperature called the transition temperature). If kept at that temperature (or above) the channels will desensitize. Threshold temperature is reduced at a lower pH and also in the presence of capsaicin (Guenther et al., 1999; Tominaga et al., 1998).
Heat-evoked TRPV1 currents exhibit properties similar to those of capsaicin-evoked currents. Single-channel openings elicited by heat are observed in inside-out membrane patches excised from HEK293 cells expressing TRPV1. Similar to what happens for capsaicin or proton-induced activation of TRPV1, because it can be activated even in excised membrane patches, this channel seems to be itself a heat sensor. It is now known that several TRP family ion channels (TRPV1, TRPV2, TRPV3, TRPV4, TRPM8, TRPA1) are thermosensitive. This suggests that temperature sensor domains must be present in these channels (Patapoutian et al., 2003; Numazaki and Tominaga, 2004).
It has been proposed (Vlachova et al., 2003) and now demonstrated (Brauchi et al., 2006) that the distal half of the TRPV1 C-terminus is reportedly involved in thermal sensitivity. Moreover, it has been shown that certain mutations and phosphorylation by PKA or PKC lead to the reduction of the threshold temperature for TRPV1 activation (Numazaki et al., 2002; Tominaga et al., 2001). Nevertheless, no TRPV1 mutation has been reported to markedly abrogate the response to heat, which suggests more global effects of heat on TRPV1. It is worth mentioning that any results obtained in the future through mutagenesis of the channel have to be carefully analyzed to exclude the possibility that these manipulations affect channel gating by heat in an allosteric fashion.
To date, several TRPV1 splice variants have been identified. In rats, an N-terminal deletion splice variant of TRPV1, VR.5′sv, (Xue et al., 2001; Schumacher et al., 2000) lacks the majority of the N-terminus (amino acids 1–308 and 345–404) and does not form functional channels. More recently, two murine splice variants, mTRPV1α and mTRPV1β, were identified (Wang et al., 2004a). It was found that mTRPV1α (but not mTRPV1β) was activated by capsaicin and protons, suggesting that mTRPV1α is activated in a similar manner to other TRPV1 channels. In contrast, the mTRPV1β subunit, characterized by a ten amino acid deletion near the N-terminus, acts as a naturally occurring dominant negative regulator. The human hTRPV1b splice variant differs from the human TRPV1 in that it contains a sixty amino acid deletion within the N-terminus, and in that it forms functional channels that are activated by noxious heat (threshold, 47°C) but not activated by capsaicin or protons. It has been suggested that it may serve predominantly as a thermal receptor for nociceptive heat stimuli (Lu et al., 2005).
Although TRPV1 is clearly ligand gated, it is also known to have voltage-dependent gating properties (Gunthorpe et al., 2000). Moreover, a recent report has tightly linked temperature sensing in TRPV1 to voltage-dependent gating although this is somewhat controversial. Changes in temperature result in graded shifts in the voltage-dependent activation curve of TRPV1 (Voets et al., 2004). The hypothesis is that there are amino acids responsible for voltage dependence and that these amino acids are also involved in thermosensing (Branchi et al., 2006), even though the fourth TM domain of TRPV1 lacks the multiple positively charged residues typically present in voltage-gated channels (Voets et al., 2004).
MODULATION OF TRPV1 ACTIVITY BY CELLULAR COMPONENTS
In addition, TRPV1’s response to heat can be modified by tyrosine kinases or G-protein-coupled receptors. In these cases, the channel opens even at a normal body temperature (Vellani et al., 2001; Tominaga et al., 2001). Hence, one important aspect of TRPV1 regulation that has received extensive attention concerns the molecular and cellular mechanisms by which the inflammatory mediators in damaged tissues sensitize TRPV1 to its chemical and physical stimuli.
Similar to what happens in other ion channels, TRPV1 binds and is modulated by molecules such as calcium calmodulin (CaM) (Numazaki et al., 2003; Rosenbaum et al., 2004). It can be phosphorylated by kinases including PKA (Vlachova et al., 2003; De Petrocellis et al., 2001; Rathee et al., 2002), PKC (Bhave et al., 2003; Premkumar et al., 2004; Tominaga et al., 2001; Varga et al., 2006), calcium calmodulin–dependent kinase II (CaMKII) (Jung et al., 2004), or Src kinase (Jin et al., 2004).
Further stimulation of TRPV1 activity can be achieved by inflammatory agents such as bradykinin, serotonin, histamine, or prostaglandins, which stimulate TRPV1 either by protein kinase C–dependent pathways (Cesare et al., 1999; Premkumar and Ahern, 2000; Vellani et al., 2001); by releasing the channel from phosphatidylinositol 4,5-bisphosphate-dependent inhibition (Chuang et al., 2001; Prescott and Julius, 2003); by a protein kinase A–mediated recovery from inactivation (Bhave et al., 2002); or by formation of 12-hydroperoxyeicosatetraenoic acid (12-HPETE) (Shin et al., 2002) (see Figure 5.1B).
Phosphorylation by PKA
Inflammatory mediators such as prostaglandins promote the activation of a PKA-dependent pathway influencing capsaicin- or heat-mediated actions of TRPV1 in sensory neurons. Therefore, it seems that PKA plays a crucial role in the development of hyperalgesia and inflammation. Residues Ser 116 and Thr 370 in the amino terminus are phosphorylated by PKA and implicated in desensitization (Bhave et al., 2002; Mohapatra and Nau, 2003). PKA has been shown to phosphorylate residue Ser 116, regulating TRPV1 activity. Finally, residues Thr 144, Thr 370, and Ser 502 have also been implicated in sensitization of heat-evoked TRPV1 responses when phosphorylated by PKA (Rathee et al., 2002).
Phosphorylation by PKC
Phosphorylation of TRPV1 by PKC is a downstream event from activation of Gq-coupled receptors by several inflammatory mediators such as ATP, bradykinin, prostaglandins, and trypsin or tryptase (Moriyama et al., 2005; Moriyama et al., 2003; Tominaga et al., 1998; Cortright and Szallasi, 2004). Phosphorylation of TRPV1 by PKC acts to potentiate capsaicin- or proton-evoked responses and reduces the temperature threshold for TRPV1 activation.
Direct phosphorylation of TRPV1 by PKC has been demonstrated (Numazaki et al., 2002), and two target Ser residues (Ser 502 and Ser 800) have been identified (Bhave et al., 2003; Numazaki and Tominaga, 2004). When these two Ser residues are replaced with Ala, TRPV1 activity induced by capsaicin, protons, or heat is eliminated. These two serines are also implicated in potentiation of endovanilloid/endocannabinoid N-Arachidonoyldopamine (NADA)-induced TRPV1 activation (Premkumar et al., 2004), oleoylethanolamide (OEA)-induced TRPV1 activation (Ahern, 2003), and rephosphorylation of TRPV1 after desensitization in the presence of Ca2+ (Mandadi et al., 2004). While some investigators have provided support for the involvement of PKC (Cesare et al., 1999; Khasar et al., 1999; Numazaki et al., 2002), others have suggested that isoforms of PKCɛ (Olah et al., 2002) or PKCμ (Wang et al., 2004b) are responsible for the effects described above.
Phorbol esters have also been implicated in TRPV1 activation. For example, it has been shown that a PKC-activating phorbol 12-myristate 13-acetate (PMA) decreases binding of [3H] RTX to TRPV1 (Chuang et al., 2001). Moreover, when Tyr 704 in the C-terminus is replaced with Ala, direct activation of TRPV1 by PMA is dramatically reduced (Bhave et al., 2003).
Phosphorylation by CaMKII and Binding and Modulation Of TRPV1 Activity by CaM
The phosphatase calcineurin inhibits desensitization of TRPV1, demonstrating that a phosphorylation/dephosphorylation process is pivotal for TRPV1 activity (Docherty et al., 1996). CaMKII controls TRPV1 activity through phosphorylation of Ser 502 and Thr 704 by regulating capsaicin binding (Jung et al., 2004). Consequently, phosphorylation of TRPV1 by three different kinases controls TRPV1 activity by means of an intricate balance between phosphorylation and dephosphorylation of this channel.
It is known that desensitization of the TRPV1 channel depends on the presence of intracellular Ca2+, and it has recently been shown that a 35 amino acid region in the COOH-terminal region of TRPV1 (residues 767–801) binds CaM in an in vitro assay in a Ca2+-dependent manner (Numazaki and Tominaga, 2004). However, mutant channels in which this region has been deleted continued to show desensitization in whole-cell experiments, albeit with altered kinetics.
In contrast, in experiments using excised membrane patches with heterologously expressed TRPV1, the application of Ca2+ with CaM produced a large reduction in current. It was also found that overexpression of CaM, together with the TRPV1 channel in this system, potentiates the inhibitory effects of Ca2+ alone, whereas coexpression of a mutant inactive form of CaM with TRPV1 does not produce a Ca2+-mediated inhibition (Rosenbaum et al., 2004). Using GST-fusion proteins corresponding to regions of the TRPV1 NH2-terminus, a binding site for CaM was localized in the region including amino acids 189–222 (Rosenbaum et al., 2004).
Modulation by Lipids
Membrane-derived lipids regulate the function of some ion channels, including TRPV1. For instance, TRPV1 is activated by OEA, anandamide, and some lipoxygenase products (Ahern, 2003; Hwang et al., 2000; Zygmunt et al., 1999). It has been proposed that phosphatidylinositol 4,5-bisphosphate (PIP2) is constitutively associated with TRPV1, promoting its inhibition. PIP2-mediated inhibition of TRPV1 can be released upon PLC activation by activation of metabotropic receptors and by the resulting hydrolysis of PIP2 to diacylglycerol and inositol (1,4,5) trisphosphate. Removal of PIP2 from TRPV1 by means of cleavage by PLC causes channel activation (Chuang et al., 2001). A region formed by amino acids 777–820 of TRPV1, which includes eight positively charged residues, has been proposed as a motif that regulates PIP2 (Prescott and Julius, 2003). The region in question includes Ser 800, substrates for PKC-dependent phosphorylation, and also overlaps with the C-terminus CaM-binding site.
Moreover, metabolic products of LOXs, such as 12- and 15-HPETEs and 5- and 15-HETEs, are capable of activating TRPV1 (Hwang et al., 2000; see Figure 5.1B). Interestingly, bradykinin, an inflammatory response mediator, seems to produce nociceptor excitation by stimulating PLC, which, in turn, results in the release of inositol (1,4,5)-trisphosphate and 1,2-diacylglycerol in sensory neurons (Burgess et al., 1989; Thayer et al., 1988). Bradykinin also releases arachidonic acid (AA), a substrate for LOX, which produces excitation of sensory neurons via the phospholipase A2 (PLA2)/LOX pathway (Shin et al., 2002; Suh and Oh, 2005).
Effects Of Reducing Agents On TRPV1 Activity
Agents that promote reduction or oxidation of –SH groups of cysteines influence membrane currents induced by noxious heat or capsaicin. For example, dithiothreitol (DTT), an agent that maintains –SH groups of Cys in a reduced state, facilitates membrane currents through TRPV1 when applied from the extracellular face of the channel by interacting with Cys located at positions 616, 621, and 634 in the loop between the fifth and sixth transmembranal domains (Tousova et al., 2004). These results suggest that the sensitivity of TRPV1 to heat and capsaicin also depends on the reduced and oxidized states of the sulphydryl groups of the cysteines of the TRPV1 channel.
The structural regions in TRPV1 necessary for agonist binding and modulation mentioned above are shown in Figure 5.1A. Although some regions and residues implicated in TRPV1 function have been identified, further work on the relationship between the structure and the function of this channel is required. The next few years will prove to be important in the advancement of our knowledge of the molecular mechanisms that underlie our perception of noxious stimuli.
ACKNOWLEDGMENTS
This work is supported in part by DC-01065, GM-63577, GM-27278, and Philip Morris USA Inc. and Philip Morris International, and by DGAPA IN201705 and CONACyT 46004, México.
REFERENCES
- Acs G, Palkovits M, Blumberg PM. Comparison of [3H]resiniferatoxin binding by the vanilloid (capsaicin) receptor in dorsal root ganglia, spinal cord, dorsal vagal complex, sciatic and vagal nerve and urinary bladder of the rat. Life Sci. 1994;55:1017–1026. [PubMed: 8084206]
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. [PubMed: 12761211]
- Ahluwalia J, Yaqoob M, Urban L, Bevan S, Nagy I. Activation of capsaicin-sensitive primary sensory neurons induces anandamide production and release. J Neurochem. 2003;84:585–591. [PubMed: 12558978]
- Appendino G, Szallasi A. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60:681–696. [PubMed: 9064473]
- Baumann TK, Burchiel KJ, Ingram SL, Martenson ME. Responses of adult human dorsal root ganglion neurons in culture to capsaicin and low pH. Pain. 1996;65:31–38. [PubMed: 8826487]
- Bevan S, Yeats J. Protons activate a cation conductance in a subpopulation of rat dorsal root ganglion neurons. J Physiol (Lond). 1991;433:145–161. [PMC free article: PMC1181364] [PubMed: 1726795]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci USA. 2003;100:12480–12485. [PMC free article: PMC218783] [PubMed: 14523239]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. [PubMed: 12194871]
- Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron. 2002;34:337–340. [PubMed: 11988165]
- Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. [PMC free article: PMC6674176] [PubMed: 16672657]
- Burgess GM, Mullaney I, McNeill M, Dunn PM, Rang HP. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci. 1989;9:3314–3325. [PMC free article: PMC6569669] [PubMed: 2552043]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. [PubMed: 11283319]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. [PubMed: 9349813]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. [PubMed: 10433272]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. [PubMed: 11418861]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. [PubMed: 14654832]
- Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–1819. [PubMed: 15128291]
- Costigan M, Woolf CJ. Pain: Molecular mechanisms. Pain. 2000;1:35–44. [PubMed: 14622841]
- Craib SJ, Ellington HC, Pertwee RG, Ross RA. A possible role of lipoxygenase in the activation of vanilloid receptors by anandamide in the guinea-pig bronchus. Br J Pharmacol. 2001;134:30–37. [PMC free article: PMC1572923] [PubMed: 11522594]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. [PMC free article: PMC6729433] [PubMed: 15128843]
- De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppetti P, Di Marzo V. The vanilloid receptor (VR1)–mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem. 2001;77:1660–1663. [PubMed: 11413249]
- De Petrocellis L, Orlando P, DiMarzo V. Anandamide, an endogenous cannabinomimetic substance, modulates rat brain protein kinase C in vitro. Biochem Mol Biol Int. 1995;36:1127–1133. [PubMed: 8535283]
- DiMarzo V, Blumberg P, Szallasi A. Endovanilloid signalling in pain. Curr Opinion in Neurobiol. 2002;12:372–379. [PubMed: 12139983]
- DiMarzo V, Fontana A, Cadas H, Schinellis S, Cimino G, Schwartz JC, Pimellid D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. [PubMed: 7990962]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HWGM. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurons from adult rats. Pflügers Arch. 1996;431:828–837. [PubMed: 8927498]
- Dray A, Dickenson A. Systemic capsaicin and olvanil reduce the acute algogenic and the late inflammatory phase following formalin injection into rodent paw. Pain. 1991;47:79–83. [PubMed: 1771095]
- Farkas-Szallasi T, Lundberg JM, Wiesenfeld HZ, Hokfelt T, Szallasi A. Increased levels of GMP, VIP, and nitric oxide synthase, and their mRNAs, in lumbar dorsal root ganglia of the rat following systemic resiniferatoxin treatment. Neuroreport. 1995;6:2230–2234. [PubMed: 8595209]
- Ferrer-Montiel A, Garcia-Martinez C, Morenilla-Palao C, Garcia-Sanz N, Fernandez-Carvajal A, Fernandez-Ballester G, Planells-Cases R. Molecular architecture of the vanilloid receptor. Insights for drug design. Eur J Biochem. 2004;271:1820–1826. [PubMed: 15128292]
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. [PubMed: 14996838]
- Goso C, Evangelista S, Tramontana M, Manzini S, Blumberg PM, Szallasi A. Topical capsaicin administration protects against trinitrobenzene sulfonic acid-induced colitis in the rat. Eur J Pharmacol. 1993;249:185–190. [PubMed: 8287899]
- Guenther S, Reeh PW, Kress M. Rises in [Ca2+]i mediate capsaicin- and proton-induced heat sensitization of rat primary nociceptive neurons. Europ J Neurosci. 1999;11:3143–3150. [PubMed: 10510178]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. [PubMed: 11931994]
- Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A. Voltage-and time-dependent properties of the recombinant rat vanilloid receptor (rVR1). J Physiol. 2000;525(Pt 3):747–759. [PMC free article: PMC2269971] [PubMed: 10856126]
- Hellwig N, Plant TD, Janson W, Schafer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem. 2004;279:34553–34561. [PubMed: 15173182]
- Hergenhahn M, Kusumoto S, Hecker E. On the active principles of the spurge family (Euphorbiaceae). V. Extremely skin-irritant and moderately tumor-promoting diterpene esters from Euphorbia resinifera Berg. J Cancer Res Clin Oncol. 1984;108:98–109. [PubMed: 6746723]
- Hermann H, De Petrocellis L, Bisogno T, Schiano Moriello A, Lutz B, DiMarzo V. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol Life Sci. 2003;60:607–616. [PubMed: 12737320]
- Horie S, Michael GJ, Priestley JV. Co-localization of TRPV1-expressing nerve fibers with calcitonin-gene-related peptide and substance P in fundus of rat stomach. Inflammopharmacology. 2005;13:127–137. [PubMed: 16259734]
- Hui K, Liu B, Qin F. Capsaicin activation of the pain receptor, VR1: multiple open states from both partial and full binding. J Gen Physiol. 2003;84:2957–2968. [PMC free article: PMC1302858] [PubMed: 12719227]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. [PMC free article: PMC18574] [PubMed: 10823958]
- Jin X, Morsy N, Winston J, Pasricha PJ, Garrett K, Akbarali HI. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am J Physiol Cell Physiol. 2004;287:C558–C563. [PubMed: 15084474]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to ‘‘hot’’ chili peppers. Cell. 2002;108:421–430. [PubMed: 11853675]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA. 2000;97:8134–8139. [PMC free article: PMC16682] [PubMed: 10859346]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. [PubMed: 11557989]
- Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. [PMC free article: PMC6782213] [PubMed: 9880573]
- Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. [PubMed: 12228246]
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. [PubMed: 14630912]
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. [PubMed: 11358970]
- Kellenberger S, Gautschi I, Schild L. An external site controls closing of the epithelial Na+ channel ENaC. J Physiol. 2002;543:413–424. [PMC free article: PMC2290510] [PubMed: 12205178]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, et al. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. [PubMed: 10677042]
- Kim S, Kang C, Shin CY, Hwang SW, Yang YD, Shim WS, Park MY, Kim E, Kim M, Kim BM, Cho H, Shin Y, Oh U. TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J Neurosci. 2006;26:2403–2412. [PMC free article: PMC6793661] [PubMed: 16510717]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. [PMC free article: PMC6573672] [PubMed: 9133377]
- Krause JE, Chenard BL, Cortright DN. Transient receptor potential ion channels as targets for the discovery of pain therapeutics. Current Opinion in Investigational Drugs. 2005;6:48–57. [PubMed: 15675603]
- Kress M, Fetzer S, Reeh PW, Vyklicky L. Low pH facilitates capsaicin responses in isolated sensory neurons of the rat. Neurosci Lett. 1996;211:5–8. [PubMed: 8809834]
- Kuzhikandathil EV, Wang H, Szabo T, Morozova N, Blumberg PM, Oxford GS. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. J Neurosci. 2001;15:8697–8706. [PMC free article: PMC6762288] [PubMed: 11698581]
- Liu L, Lo Y, Chen I, Simon SA. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J Neurosci. 1997;17:4101–4111. [PMC free article: PMC6573573] [PubMed: 9151727]
- Liu L, Simon SA. Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J Neurophysiol. 1996;76:1858–1869. [PubMed: 8890298]
- Liu L, Simon SA. Capsacin, acid, and heat–evoked currents in rat trigeminal ganglion neurons: relationships to functional VR1 receptors. Physiol Behav. 2000;69:363–378. [PubMed: 10869604]
- Lu G, Henderson D, Liu L, Reinhart PH, Simon SA. Cloning and functional characterization of TRPV1b, a new human vanilloid receptor. Molecular Pharmacology. 2005;67:1119–1127. [PubMed: 15644492]
- Maccarone M, Salvati S, Bari M, Finazzi-Agro A. Anandamide and 2-arachidonoylglycerol inhibit fatty acid amide hydrolase by activating the liproxygenase pathway of the arachidonate cascade. Biochemical and Biophysical Research Communications. 2000;278:576–583. [PubMed: 11095952]
- Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium. 2004;35:471–478. [PubMed: 15003856]
- Martenson ME, Ingram SL, Baumann TK. Potentiation of rabbit trigeminal responses to capsaicin in a low pH environment. Brain Res. 1994;651:143–147. [PubMed: 7922561]
- McNamara FN, Randall A, Gunthorpe MJ. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br J Pharmacol. 2005;144:781–790. [PMC free article: PMC1576058] [PubMed: 15685214]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. [PMC free article: PMC16295] [PubMed: 10725386]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. [PubMed: 14506258]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. [PMC free article: PMC1074353] [PubMed: 15813989]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. [PMC free article: PMC6740351] [PubMed: 12853424]
- Numazaki M, Tominaga M. Nociception and TRP channels. Curr Drug Targets CNS Neurol Disord. 2004;3:479–485. [PubMed: 15578965]
- Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100:8002–8006. [PMC free article: PMC164702] [PubMed: 12808128]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase C epsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. [PubMed: 11884385]
- Oh US, Hwang W, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996;16:1659–1667. [PMC free article: PMC6578684] [PubMed: 8774434]
- Olah Z, Karai L, Iadarola MJ. Protein kinase C (alpha) is required for vanilloid receptor 1 activation. Evidence for multiple signaling pathways. J Biol Chem. 2002;277:35752–35759. [PubMed: 12095983]
- Parlani M, Conte B, Goso C, Szallasi A, Manzini S. Capsaicin-induced relaxation in the rat isolated external urethral sphincter: characterization of the vanilloid receptor and mediation by CGRP. Br J Pharmacol. 1993;110:989–994. [PMC free article: PMC2175827] [PubMed: 7905345]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. [PubMed: 12838328]
- Petersen M, LaMotte RH. Effect of protons on the inward current evoked by capsaicin in isolated dorsal root ganglion cells. Pain. 1993;54:37–42. [PubMed: 7690929]
- Phillips E, Reeve A, Bevan S, McIntyre P. Identification of species-specific determinants of the action of the antagonist capsazepine and the agonist PPAHV on TRPV1. J Biol Chem. 2004;279:17165–17172. [PubMed: 14960593]
- Piper AS, Yeats JC, Bevan S, Docherty RJ. A study of the voltage-dependence of capsaicin-sensitive membrane currents in rat sensory neurons before and after acute desensitization. J Physiol. 1999;518:721–733. [PMC free article: PMC2269463] [PubMed: 10420009]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. [PubMed: 11140687]
- Premkumar LS, Qi ZH, Van Buren J, Raisinghani M. Enhancement of potency and efficacy of NADA by PKC-mediated phosphorylation of vanilloid receptor. J Neurophysiol. 2004;91:1442–1449. [PubMed: 14973326]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. [PubMed: 12764195]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. [PubMed: 10846154]
- Purkiss JRMJ, Welch S, Doward K, Foster A. Capsaicin stimulates release of substance P from dorsal root ganglion neurons via two distinct mechanisms. Biochem Soc Trans. 1997;25:542S. [PubMed: 9388756]
- Rami HK, Gunthorpe MJ. The therapeutic potential of TRPV1 (VR1) antagonists: clinical answers await. Drug Discovery Today: Therapeutic Strategies. 2004;1:97–104.
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. [PMC free article: PMC6758778] [PubMed: 12040081]
- Reeh PW, Kress M. Molecular physiology of proton transduction in nociceptors. Current Opinion in Pharmacology. 2002;1:45–51. [PubMed: 11712534]
- Rinder J, Szallasi A, Lundberg JM. Capsaicin-, resiniferatoxin-, and lactic acid–evoked vascular effects in the pig nasal mucosa in vivo with reference to characterization of the vanilloid receptor. Pharmacol Toxicol. 1996;78:327–335. [PubMed: 8737969]
- Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. [PMC free article: PMC2217413] [PubMed: 14699077]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. [PMC free article: PMC1574087] [PubMed: 14517174]
- Ryu S, Liu B, Qin F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol. 2003;122:45–61. [PMC free article: PMC2234467] [PubMed: 12835470]
- Schumacher MA, Moff I, Samndanagunda SP, Levine JD. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. J Biol Chem. 2000;275:2756–2762. [PubMed: 10644739]
- Shin HJ, Gye MH, Chung KH, Yoo BS. Activity of protein kinase C modulates the apoptosis induced by polychlorinated biphenyls in human leukemic HL-60 cells. Toxicol Lett. 2002;135:25–31. [PubMed: 12243861]
- Spath E, Darling SF. Synthesis of capsaicin. Ber Chem Ges. 1930;63B:737–740.
- Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Current Pharmaceutical Design. 2005;11:2687–2698. [PubMed: 16101449]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed: 10353985]
- Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. [PubMed: 2747924]
- Szallasi A, Goso C, Blumberg PM, Manzini S. Competitive inhibition by capsazepine of [3H]resiniferatoxin binding to central (spinal cord and dorsal root ganglia) and peripheral (urinary bladder and airways) vanilloid (capsaicin) receptors in the rat. J Pharmacol Exp Ther. 1993;267:728–733. [PubMed: 8246148]
- Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 1990;255:923–928. [PubMed: 2243359]
- Thayer SA, Perney TM, Miller RJ. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988;8:4089–4097. [PMC free article: PMC6569470] [PubMed: 3183714]
- Thresh LT. Isolation of capsaicin. Pharm J. 1846;6:941–947.
- Tominaga M, Tominaga T. Structure and function of TRPV1. Pflügers Arch. 2005;451:143–150. [PubMed: 15971082]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. [PubMed: 9768840]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. [PMC free article: PMC34459] [PubMed: 11371611]
- Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. [PubMed: 15857679]
- Tousova K, Susankova K, Teisinger J, Vyklicky L, Vlachova V. Oxidizing reagent copper-o-phenanthroline is an open channel blocker of the vanilloid receptor TRPV1. Neuropharmacology. 2004;47:273–285. [PubMed: 15223306]
- Varga A, Bolcskei K, Szoke E, Almasi R, Czeh G, Szolcsanyi J, Petho G. Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo. Neuroscience. 2006;140:645–657. [PubMed: 16564637]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat, and anandamide. J Physiol. 2001;534:813–825. [PMC free article: PMC2278732] [PubMed: 11483711]
- Vlachova V, Teisinger J, Susankova K, Lyfenko A, Ettrich R, Vyklicky L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci. 2003;23:1340–1350. [PMC free article: PMC6742269] [PubMed: 12598622]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. [PubMed: 15306801]
- Waldmann R, Champigny G, Lingueglia E, DeWeille JR, Heurteaux C, Lazdunski M. H(+)-gated ion channels. Ann NY Acad Sci. 1999;868:67–76. [PubMed: 10414282]
- Walpole CS, Wrigglesworth R, Bevan S, Campbell EA, Dray A, James IF, Masdin KJ, Perkins MN, Winter J. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 3. The hydrophobic side-chain ‘‘C-region.’’ J Med Chem. 1993;36:2381–2389. [PubMed: 8360883]
- Wang C, Hu HZ, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine Trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem. 2004a;27:37423–37430. [PubMed: 15234965]
- Wang Y, Kedei N, Wang M, Wang QJ, Huppler AR, Toth A, Tran R, Blumberg PM. Interaction between protein kinase C mu and the vanilloid receptor type 1. J Biol Chem. 2004b;279:53674–53682. [PubMed: 15471852]
- Welch JM, Simon SA, Reinhart PH. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci USA. 2000;97:13889–13894. [PMC free article: PMC17671] [PubMed: 11095706]
- Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. [PMC free article: PMC6569450] [PubMed: 3171675]
- Xue Q, Yu Y, Trik SL, Jong BE, Schumacher MA. The genomic organization of the gene encoding the vanilloid receptor: evidence for multiple splice variants. Genomics. 2001;76:14–20. [PubMed: 11549313]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. [PubMed: 10440374]
- Review Structure and function of TRPV1.[Pflugers Arch. 2005]Review Structure and function of TRPV1.Tominaga M, Tominaga T. Pflugers Arch. 2005 Oct; 451(1):143-50. Epub 2005 Jun 22.
- Review TRP Channels and Pain.[Neurobiology of TRP Channels. ...]Review TRP Channels and Pain.González-Ramírez R, Chen Y, Liedtke WB, Morales-Lázaro SL. Neurobiology of TRP Channels. 2017
- Review ThermoTRP channels as modular proteins with allosteric gating.[Cell Calcium. 2007]Review ThermoTRP channels as modular proteins with allosteric gating.Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. Cell Calcium. 2007 Oct-Nov; 42(4-5):427-38. Epub 2007 May 17.
- Ca2+/calmodulin modulates TRPV1 activation by capsaicin.[J Gen Physiol. 2004]Ca2+/calmodulin modulates TRPV1 activation by capsaicin.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. J Gen Physiol. 2004 Jan; 123(1):53-62.
- Review TRP Channels at the Periphery of the Taste and Trigeminal Systems.[Neurobiology of TRP Channels. ...]Review TRP Channels at the Periphery of the Taste and Trigeminal Systems.Simon SA, Gutierrez R. Neurobiology of TRP Channels. 2017
- TRPV1 Receptors and Signal Transduction - TRP Ion Channel Function in Sensory Tr...TRPV1 Receptors and Signal Transduction - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
Your browsing activity is empty.
Activity recording is turned off.
See more...