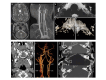
Clinical Description
HOXA1-related disorders are characterized by ocular motility disorder (horizontal gaze palsy with or without Duane syndrome), bilateral sensorineural deafness, variable cerebrovascular malformations (predominantly involving the carotid arteries), motor developmental delay, central hypoventilation, and intellectual disability. To date, 34 individuals have been identified with biallelic pathogenic variants in HOXA1 [Holve et al 2003, Tischfield et al 2005, Bosley et al 2007, Bosley et al 2008, Higley et al 2011, Oystreck et al 2011, Ellsworth et al 2020, Patil et al 2020]. Two overlapping phenotypes have been described: Athabascan brainstem dysgenesis syndrome (ABDS) and Bosley-Salih-Alorainy syndrome (BSAS).
Ocular motility disorder. One of the most common clinical findings among affected individuals with ABDS and BSAS is horizontal gaze palsy with or without Duane syndrome due to underlying abducens nucleus / nerve abnormalities [Holve et al 2003, Tischfield et al 2005, Bosley et al 2007, Bosley et al 2008, Higley et al 2011, Oystreck et al 2011, Ellsworth et al 2020, Patil et al 2020]. Horizontal gaze palsy can be unilateral or bilateral and can be apparent from early infancy. Affected individuals have variable severity of abduction/adduction with or without refractive errors, nystagmus, vertical gaze involvement, strabismus (esotropia), and ptosis. Only two reported individuals with BSAS did not have the typical ocular motility disorder; one had mild ocular motility disorder and the other had normal ocular motility. Untreated eye complications can lead to amblyopia [Bosley et al 2008].
Inner ear anomalies / sensorineural deafness. Structural abnormalities within the inner ear accompanied by congenital profound sensorineural deafness are observed in most affected individuals (32/34 individuals). Structural abnormalities of the inner ear described in individuals with ABDS and BSAS can be unilateral or bilateral, including complete absence of the labyrinthine structure, common cavity deformity, and/or underdevelopment or absence of the cochlea [Holve et al 2003, Tischfield et al 2005, Bosley et al 2007, Bosley et al 2008, Higley et al 2011, Oystreck et al 2011, Ellsworth et al 2020, Patil et al 2020].
Cerebrovascular malformations. Cerebrovascular anomalies affecting the carotid arteries are frequently observed; the internal carotid arteries are most commonly involved [Bosley et al 2007, Bosley et al 2008]. These anomalies may be unilateral or bilateral, including carotid artery aplasia or hypoplasia, early branching of the common carotid artery, anterior cerebral artery aplasia, and duplication of the vertebral artery along with compensatory enlargement of the posterior communicating artery or the vestibulobasilar arterial system. Although a cerebrovascular malformation may increase the risk of stroke, in most individuals with ABDS and BSAS these vascular defects remain silent, except for two individuals. One individual with ABDS had a vertebral artery stroke and another individual with BSAS had a stroke following cardiac surgery [Holve et al 2003, Bosley et al 2008].
Developmental delay. Motor delay is noted in most affected individuals, which can be attributed to the absence of a vestibular system and congenital heart defects in individuals with BSAS rather than a widespread disturbance in the brain [Bosley et al 2008]. Most individuals with BSAS and motor delay achieved independent ambulation [Bosley et al 2008, Patil et al 2020]. However, in individuals with ABDS, unsteady and wide-based gait was reported in those who became ambulatory along with dysmetria possibly related to cerebellar dysfunction [Holve et al 2003].
Learning difficulties / intellectual disability. Cognitive dysfunction is seen in all affected individuals with ABDS and is believed to result from chronic brain oxygen deficiency due to a combination of central hypoventilation, cerebrovascular abnormalities, and the elevated altitude where the Athabascan community resides [Bosley et al 2008]. Two individuals of 14 with ABDS are reported to have relatively mild cognitive impairment, possibly because they were raised at lower altitude.
Individuals with BSAS are reported to have variable developmental abilities and cognitive function ranging from isolated motor delay, learning difficulties, or behavioral issues to global developmental delay and autism spectrum disorder. Often neurologic assessment is confounded by the presence of profound sensorineural deafness and speech issues. Normal neurologic function has been reported in seven of 20 individuals with BSAS [Bosely et al 2007, Bosely et al 2008].
Central hypoventilation is the distinguishing feature in individuals with ABDS. Most children with ABDS had clinical features of central hypoventilation identified in early infancy (age <6 months). All reported individuals required supplemental oxygen either through mechanical ventilation or nasal cannula and aminophylline. Weaning of supplemental oxygen was possible in those who survived beyond infancy with improved respiratory drive with age. Central hypoventilation has been reported to be more severe during sleep. Some individuals only required supplemental oxygen at night [Holve et al 2003, Bosley et al 2008, Ellsworth et al 2020].
None of the individuals with BSAS have central hypoventilation.
Congenital heart disease. The majority of children with ABDS have congenital heart disease commonly involving the cardiac outflow tract including tetralogy of Fallot, coarctation of aorta, double aortic arch, transverse hypoplastic arch, aortic valve stenosis, aberrant left subclavian artery, patent ductus arteriosus, bicuspid aortic valve, ventricular septal defect, and total anomalous pulmonary venous return.
Some individuals with BSAS also have congenital heart disease including double outlet left ventricle, tetralogy of Fallot, and ventricular septal defect. One individual with BSAS was reported to have apparently isolated congenital heart disease without ocular motility disorder or hearing impairment [Bosely et al 2008].
Facial paresis, vocal cord paresis, and swallowing dysfunction are manifestations of hypoplasia and/or aplasia of certain cranial nerves. Variable facial paresis or twitching/spasms (unilateral or bilateral) have been documented in individuals with ABDS and BSAS, which is attributed to hypoplasia or aplasia of cranial nerve VII [Holve et al 2003, Tischfield et al 2005, Bosley et al 2008, Higley et al 2011]. Vocal cord paresis (2/10 individuals) and swallowing dysfunction (6/10 individuals) have also been reported in individuals with ABDS. Often these complications lead to recurrent aspirations requiring hospitalization and gastrostomy tube feeding [Holve er al 2003].
Seizures were reported in two individuals with BSAS and four individuals with ABDS [Holve et al 2003, Bosley et al 2008]. To date, further information regarding type of seizures is not available, except in one individual with ABDS described as having generalized tonic-clonic seizures (onset at age 3 years) [Holve et al 2003].
Other variable, less common clinical features [Bosley et al 2007, Bosley et al 2008]:
External ear minor malformations (e.g., flattened ear helix, low-set ears) in four individuals with BSAS
Clubfoot in three individuals
Chronic constipation in two individuals with BSAS
Frequent grimacing in two individuals with BSAS
Facial asymmetry in one individual with BSAS and one individual with ABDS
Multiple lentigines, hypertrichosis, polydactyly, brachydactyly, and duplex ureteral system with urethral stricture (1 individual each)