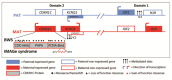
Clinical Description
Thirty-one individuals reported from 19 families have features consistent with the clinical diagnosis of IMAGe syndrome [Vilain et al 1999, Lienhardt et al 2002, Pedreira et al 2004, Bergadá et al 2005, Hutz et al 2006, Tan et al 2006, Ko et al 2007, Amano et al 2008, Balasubramanian et al 2010, Arboleda et al 2012, Hamajima et al 2013, Bodian et al 2014, Kato et al 2014, Bolomiti et al 2021, Çamtosun et al 2021]. Of these 31 individuals, 19 from eleven unrelated families have had the diagnosis confirmed molecularly [Arboleda et al 2012, Hamajima et al 2013, Bolomiti et al 2021]. Of the twelve individuals who have not had confirmatory genetic testing, nearly all have clinical findings that significantly overlap those of the individuals with a molecularly confirmed diagnosis.
A diagnosis of IMAGe syndrome has been considered in other published cases; however, the clinical information was either significantly different from the 31 typical cases or insufficient to determine the diagnosis with certainty, and pathogenic CDKN1C variants were not reported [Blethen et al 1990, Hall & Stelling 1991, Le & Kutteh 1996, Coman et al 2007, McDonald et al 2010, Lindemeyer et al 2014]. Several of these cases are further discussed in Differential Diagnosis.
It is likely that the spectrum and natural history of IMAGe syndrome will be refined as more affected individuals are identified.
Onset. Although intrauterine growth restriction (IUGR) may be detected prenatally, IMAGe syndrome is typically evident at birth with IUGR, mild dysmorphic features, adrenal insufficiency, and (in males) genitourinary abnormalities. Of 23 individuals with information about age and findings at presentation, one was identified prenatally, 13 at birth, four in the first month of life, three from age one month to one year, one at age five years, and one at age 15 years.
Growth. All neonates have had IUGR with birth weights from -2 to -4 SD and birth lengths from -1.8 to -4.5 SD. Of the nine for whom information was available at birth, five had a normal occipitofrontal circumference (OFC) and four had an OFC from -2 to -3 SD.
Individuals with IMAGe syndrome consistently demonstrate continued short stature (height -2.7 to -6.5 SD) and postnatal failure to thrive (weight -2 to -7 SD). Of the nine on whom longitudinal information was available, postnatal OFC was normal in seven and below -2 SD in two individuals.
Skeletal. All affected individuals have had some skeletal abnormality with the most common manifestations being delayed bone age and short stature. Metaphyseal and epiphyseal dysplasia of the long bones are common. The metaphyses are frequently described as striated and the diaphyses as gracile.
A significant degree of age-dependent variation is observed: in some, the metaphyseal dysplasia may be late, mild, and/or easily missed. Most children have radiologic evidence of skeletal abnormality by age five years.
Less common skeletal features include progressive and severe scoliosis with onset before age five years, ovoid-shaped vertebral bodies, short first metatarsals, hallux valgus, and hip dysplasia.
In one individual, fractures of the humerus and tibia were present at birth [Lienhardt et al 2002].
Adrenal insufficiency appears to be universal. Although this may be due to an ascertainment bias for probands, in one family it appeared fully penetrant, with adrenal insufficiency present in 7/7 individuals who possessed a maternally inherited CDKN1C pathogenic variant [Arboleda et al 2012].
Adrenal crisis, presenting with hyponatremia, hyperkalemia, and life-threatening hypotension, can occur within the first month of life, typically within the first week; extremely elevated ACTH levels, frequently above 1000 pg/mL (normal 10-60 pg/mL), can cause severe hyperpigmentation in these infants [Vilain et al 1999].
A few individuals do not have adrenal crisis, but rather milder adrenal insufficiency, presenting with failure to thrive and recurrent vomiting. One child, who experienced recurrent vomiting associated with mild infections from birth, was diagnosed with adrenal insufficiency at age five years following the diagnosis of IMAGe syndrome in her younger brother [Lienhardt et al 2002]. Another affected individual was diagnosed with hypoaldosteronism without glucocorticoid deficiency [Bodian et al 2014]. Hypercalciuria, soft tissue calcification, and renal calculi were noted in one individual [Authors, unpublished observation].
Genitourinary abnormalities. Genital abnormalities, which are nearly universal in males with IMAGe syndrome, have not been reported in females. Reported abnormalities include cryptorchidism (usually bilateral), micropenis, and hypospadias.
Of the 31 individuals reported with IMAGe syndrome 22 are male, which may represent ascertainment bias due to the presence of genital abnormalities in males only. Two females with IMAGe syndrome have had children [Authors, unpublished observations]. No males with IMAGe syndrome are known to have reproduced.
Hydronephrosis has been reported; however, the majority of affected individuals are reported to have normal renal ultrasound examinations.
Neurologic. Developmental outcome is believed to be normal, as 15 of the 16 individuals in whom cognitive outcome was mentioned were reported as normal; the oldest reported was age 26 years.
Hypotonia was reported in six individuals and noted to be absent in four others; some of those reported with developmental delay likely had motor delays secondary to hypotonia.
In one affected individual who had wasting of facial and distal muscles, muscle biopsy showed nonspecific myopathic changes [Lienhardt et al 2002].
Of 31 individuals with IMAGe syndrome, head imaging was reported for seven (3 via cranial ultrasound examination, 1 via head CT, and 3 via brain MRI), all were normal.
Other
Characteristic facial features that have been reported in the vast majority of individuals include frontal bossing, depressed or wide nasal bridge, and small/low-set ears; features are typically appreciated by age one year. The facial profile can be similar to the "triangular" facies seen in
Silver-Russell syndrome. The facial profile may change with time. Micrognathia or retrognathia are also frequently reported. Less common features include cleft palate or cleft uvula, craniosynostosis, short palpebral fissures, smooth philtrum, and microglossia.
Variable hypercalcemia of unclear etiology, occasionally with evidence of soft tissue calcifications, was reported in eight of 16 affected individuals on whom information was available. Several individuals have had nephrocalcinosis-associated hypercalciuria. While hypercalcemia may be a feature of IMAGe syndrome, it may also be secondary to sodium chloride supplementation, which is part of the treatment of the mineralocorticoid deficiency associated with adrenal insufficiency [
Bergadá et al 2005].