Clinical Description
PLPBP deficiency, first identified in 2016, causes a rare, treatable form of vitamin B6-dependent early-onset epileptic encephalopathy [Darin et al 2016].
To date, 56 individuals have been identified with biallelic pathogenic variants in PLPBP [Darin et al 2016, Plecko et al 2017, Kernohan et al 2018, Shiraku et al 2018, Jensen et al 2019, Johannsen et al 2019, Johnstone et al 2019, Koul et al 2019, Ahmed et al 2020, Akiyama et al 2020, Heath et al 2020, Jiao et al 2020, Espinoza et al 2021, Mittal et al 2021, Pal et al 2021, McLean et al 2022]. The following description of the phenotypic features associated with this condition is based on these reports.
In PLPBP deficiency, seizures that have not been responsive – or only partly responsive – to anti-seizure medications (ASMs) show an immediate positive response to vitamin B6 (given as either pyridoxine [PN] or pyridoxal 5'-phosphate [PLP]), a therapy that needs to be continued lifelong. In addition to vitamin B6 treatment, almost 60% of individuals require adjunct ASMs to achieve optimal seizure control [Heath et al 2020]. Although many individuals with PLPBP deficiency have normal motor, speech, and intellectual development, more than 50% have varying degrees of neurodevelopmental issues, including learning difficulties or intellectual disability (varying from mild to severe), delayed or absent speech development, or motor development abnormalities (most commonly mild hypotonia). Brain white matter abnormalities and progressive microcephaly are common in individuals with more severe manifestations.
Classic PLPBP Deficiency
Classic PLPBP deficiency is defined as neonatal onset (i.e., within the first 28 days after birth).
Birth is often at term; however, preterm to late preterm birth is reported. Perinatal distress, reported in some instances, included abnormal intrauterine movements and fetal distress, suggesting fetal seizures [Heath et al 2020].
Seizures. In 43 children with known age of onset of seizures, around 50% of seizures were evident within 24 hours of birth, 23% between age one and seven days, and 14% between age one and four weeks.
The various types of seizures included tonic, clonic, generalized tonic-clonic, myoclonic, lip smacking, and/or grimacing. Initial EEG recordings vary from normal to abnormal, including burst suppression, reduced background activity, focal discharges, and multifocal spikes.
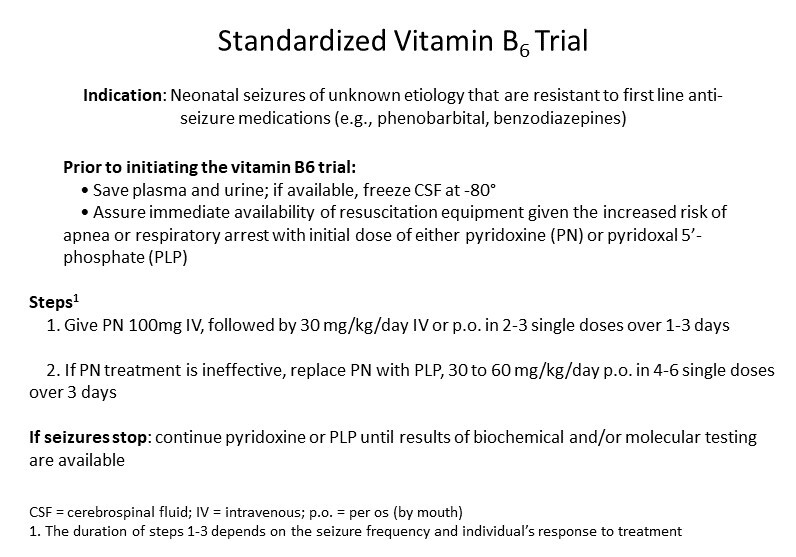
In most instances, seizures that were nonresponsive or only partially responsive to ASMs responded well to PN or PLP. In a few instances, seizure control improved with discontinuation of PN and initiation of PLP [Darin et al 2016, Johnstone et al 2019, Heath et al 2020]. In addition to PN or PLP treatment, the majority of affected individuals still require ASMs.
Withdrawal of PN or PLP (either incidentally or for diagnostic purposes before establishing the diagnosis with molecular genetic testing) led to reoccurrence of seizures, highlighting vitamin B6 dependency in this disorder [Darin et al 2016, Plecko et al 2017, Shiraku et al 2018, Johnstone et al 2019, Jiao et al 2020].
Breakthrough seizures during illness/fever are fairly common.
Neurodevelopmental outcomes vary widely (reviewed in Heath et al [2020]). Although some affected individuals have normal motor, speech, and intellectual development, more than 50% of individuals experience varying degrees of neurodevelopmental issues, including learning difficulties or intellectual disability (varying from mild to severe), delayed or absent speech development, or motor development abnormalities, most commonly (mild) hypotonia [Johnstone et al 2019].
Other features
Head circumference at birth varies widely from low (<10%) to normal. While progressive microcephaly is common, it is not the rule [
Heath et al 2020].
In one individual with an early-onset seizure disorder (at age 15 days), PLPBP deficiency was not diagnosed until age 16 years. His seizures were relatively well controlled with ASMs; he had a mild learning disability. Prior to the diagnosis of PLPBP deficiency and initiation of PN treatment, he experienced excessive seizure clusters and intermittent states of anxiety, suggesting that these may be typical features of untreated PLPBP deficiency in adolescents [
Johannsen et al 2019]. Consistent with this observation, one individual reported hallucinations and panic attacks upon PN withdrawal [
Plecko et al 2017].
Brain imaging findings. Many affected individuals have brain MRI abnormalities including white matter abnormalities (variable T2 hyperintensity and T1 hypointensity, subcortical cystic degeneration), cortical abnormalities (atrophy and/or simplified cortical gyral pattern), periventricular cysts, and/or thinning of the corpus callosum and the posterior limb of the internal capsule (PLIC). Of note, brain MRI findings at seizure onset vary from normal in 12/29 (41%) of individuals to abnormal in 17/29 (59%) (reviewed in Heath et al [2020]). Abnormalities in the 17 individuals were white matter changes (11), simplified sulcation (10), cysts (7), and poor myelination in the PLIC (3).
Late-Onset PLPBP Deficiency
Late-onset PLPBP deficiency is defined as onset after the neonatal period (i.e., after age 28 days).
To date six individuals (14% of affected individuals) have been reported with late-onset PLPBP deficiency, five with seizures [Akiyama et al 2020, Espinoza et al 2021, McLean et al 2022] and one without seizures [Johnstone et al 2019]. The phenotype of these individuals is broad and includes the following observations.
At age four months, a girl presented with paroxysmal episodes of abnormal multidirectional eye-head movements, followed at age five months by almost daily epileptic spasms without hypsarrhythmia [
Kalser et al 2022]. No developmental plateauing or regression was observed. She experienced mild-to-moderate gastroesophageal reflux disease (GERD). After initiation of PN treatment, the seizures and GERD resolved.
At age 14 months, a boy with normal development and a normal brain MRI presented with recurring prolonged myoclonic seizures, after which speech regression was observed [
Espinoza et al 2021]. Subsequently, tonic-clonic seizures occurred with more prominent developmental regression within each seizure cluster. His brain MRI at age 22 months showed bilateral mesial temporal sclerosis. Following diagnosis of PLPBP deficiency at age three years, PN treatment was initiated.
At age three months, a boy presented with focal and generalized tonic seizures and no neurologic abnormalities [
Akiyama et al 2020]. Brain MRI showed structural abnormalities including broad gyri and shallow sulci, underdevelopment of white matter, and microcephaly. At age seven years, he had moderate intellectual disability.
Shiraku et al [2018] described two boys with seizure onset at ages three months and 34 days, respectively. Seizures (including tonic, clonic, generalized tonic-clonic, and myoclonic) were partially responsive to ASMs. Seizures ceased following initiation of PN treatment at ages eight years and five years, five months, respectively. Both boys had delayed motor and speech development and moderate-to-profound intellectual disability. Brain MRI showed broad gyri and shallow sulci, underdevelopment of white matter, and microcephaly.
Other
Biomarkers not effective in identifying PLPBP deficiency. Biochemical alterations previously reported in individuals with PLPBP deficiency are nonspecific, as they are seen in other metabolic and neonatal seizure disorders (see Table 2).
Table 2.
Summary of Nonspecific Biochemical Findings Reported in PLPBP Deficiency
View in own window
| Metabolic Feature 1 | Incidence 2 |
|---|
|
Blood/Plasma
|
| Acidosis | 11/26 |
| High lactate | 16/28 |
| Anemia at birth | 3/12 |
| High glycine | 10/22 |
| High alanine | 4/22 |
| High threonine | 1/22 |
| High PLP (on vitamin B6 treatment) | 4/7 |
| High PL (on vitamin B6 treatment) | 4/7 |
|
CSF
|
| High lactate | 2/6 |
| High glycine | 7/11 |
| High alanine | 2/11 |
| High threonine | 2/11 |
| High tryptophan | 1/11 |
| High tyrosine | 1/11 |
| Low homovanillic acid | 2/10 |
| High 3-O-methyldopa | 3/9 |
| High L-dopa | 1/9 |
| High 5-hydroxytryptophan | 2/9 |
| Low PLP | 2/3 |
| Low PL | 1/1 |
|
Urine
|
| High vanillactic acid | 5/20 |
| High vanilpyruvic acid | 1/20 |
| High N-acetylvanilalanine | 1/20 |
| High lactic acid | 2/20 |
CSF = cerebrospinal fluid
- 1.
Unless otherwise noted, these results are from samples obtained before initiation of vitamin B6 therapy.
- 2.
Refers to number of individuals with specific finding / total number of individuals in whom the analyte was measured
Genotype-Phenotype Correlations
Since the number of individuals with PLPBP deficiency is small, it is difficult to establish true genotype-phenotype correlations. Nonetheless, the following observations about vitamin B6 responsiveness may be helpful in guiding clinical management.
Johnstone et al [2019], who used an adapted clinical severity score to classify phenotypes and variants in 23 individuals with PLPBP deficiency, suggested that severe phenotypes and/or early mortality are usually associated with:
Missense
PLPBP variants that are predicted or experimentally proven to affect residues surrounding the PLP binding sites (e.g.,
c.722G>A [p.Arg241Gln]).
Mild-to-moderate phenotypes may be associated with missense variants that decrease, but do not abolish, PLP binding or protein stability.