NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Food and Nutrition Board; Board on Agriculture and Natural Resources; Committee on Exploring Linkages Between Soil Health and Human Health. Exploring Linkages Between Soil Health and Human Health. Washington (DC): National Academies Press (US); 2024 Sep 19.

Exploring Linkages Between Soil Health and Human Health.
Show detailsChemical contaminants in soil and other ecosystems are a significant concern for current and future security of the food system. They are also a parameter highlighted by the Planetary Boundaries Framework (PBF), which establishes a set of environmental boundaries that define the safe operating limits for humanity to maintain a stable and habitable planet (Rockström et al. 2009; Steffen et al. 2015). These boundaries represent the critical thresholds, including those related to climate change, biodiversity loss, and chemical pollution, beyond which the environment faces irreversible harm. By identifying these limits, the PBF plays a crucial role in guiding sustainable development and informing environmental policies aimed at ensuring the long-term well-being of the planet and future generations (Rockström et al. 2009).
Regarding contaminants, the PBF is concerned about the “amount emitted to, or concentration of persistent organic pollutants, plastics, endocrine disrupters, heavy metals and nuclear waste in the global environment, or the effects on ecosystem and function of Earth system thereof” (Rockström et al. 2009, 473). Initially referred to in the literature as “chemical pollution,” this planetary boundary category has been further expanded to “novel entities” (Steffen et al. 2015), which include newly developed chemicals, engineered materials, and previously undiscovered organisms within the earth system as well as naturally occurring substances such as heavy metals that are released into the environment due to human activities. Persson et al. (2022) argued that humanity has exceeded its planetary boundary concerning contaminants and novel entities. This assessment is attributed to the escalating production and release of contaminants, coupled with the challenges in evaluating and monitoring associated risks. In the context of soil, a significant repository for pollutants, chemical contaminants pose substantial threats to both human health and the environment, as summarized in Figure 6-1.

FIGURE 6-1
Impacts of soil contaminants on key soil functions. SOURCE: Food and Agriculture Organization of the United Nations. Reproduced with permission.
Each year, a vast and diverse array of chemicals enters the soil. These contaminants are often in complex mixtures, which significantly complicates the assessment of potential exposure and associated health risks, as highlighted by Persson et al. (2022). These inorganic and organic chemicals—varying in chemical composition, concentration, and behaviors—enter the soil through routine agricultural practices, industrial activities, waste disposal, atmospheric deposition, and accidental spills. Among these releases, some are intentional, such as the application of pesticides and fertilizers, while others are unintentional, leading to severe negative consequences, as seen with microplastics and per- and polyfluoroalkyl substances (PFAS).
This chapter delves into the interactions between soil processes and contaminants, exploring their impact on exposure and how soil health influences these processes. The committee recognizes that contaminants affect all soil, not just soil used to grow crops. The committee is also aware that “chemical pollution” or “novel entities,” as defined by the PBF, encompass hundreds of thousands of substances (Steffen et al. 2015). It is not within the committee’s capacity to review the interaction of the soil microbiome with all soil contaminants. Some sources of contamination, such as pesticides, are discussed briefly but are primarily addressed in Chapter 4. In this chapter, after reviewing how humans may be exposed to contaminants in soil and how, in general, soil health can mitigate exposure, the committee provides details about three major classes of contaminants: heavy metals, plastics, and PFAS. The committee selected these three classes as case studies because heavy metals have been a long-standing concern in food production and because plastics and PFAS are emerging soil contaminants with potential repercussions for food production and beyond. The case studies provide overviews of the contaminants’ sources, their fate and transport, their repercussions on human health and soil ecosystems, and strategies for mitigation and remediation. Although these contaminants enter the soil (or may be magnified in the soil) through agricultural production, the committee notes that contact with soil, crop production, and food consumption are not the only routes of exposure for all the contaminants discussed.
HUMAN EXPOSURE TO CONTAMINANTS IN SOIL
Contaminant Fate and Transport in Soil
Once chemicals enter the soil, their movements and potential impacts are determined by a range of physicochemical characteristics, such as solubility, volatility, and sorption potential, as well as their interaction with specific soil properties. These interactions and processes (Figure 6-2) govern whether contaminants will migrate deeper into the soil through percolation, escape into the atmosphere via volatilization, or remain bound to soil particles (Jury and Godhrati 1989; Linn et al. 1993; Schnoor 1996).
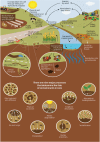
FIGURE 6-2
The route of entrance and fate of contaminants in soils, and nine major soil processes determining the fate of soil contaminants. SOURCE: Food and Agriculture Organization of the United Nations. Reproduced with permission. Adapted from Current Opinion (more...)
Transformation of contaminants—loss or removal of certain substances (e.g., reactants) and the generation or formation of new substances (e.g., products)—can occur via both abiotic and biotic processes (Linn et al. 1993; Scow and Johnson 1996; Al-Mamun 2017). Physical and chemical transformation processes include photolysis and hydrolysis reactions (Figure 6-3). Coupling or polymerization reactions, mediated by certain mineral oxides and fungal extracellular enzymes, can also transform and alter the availability of some organic contaminants (Linn et al. 1993). Biodegradation specifically refers to the transformation of a contaminant by metabolic reactions carried out by the soil organisms, primarily the microbiome. Given that the soil microbiome, with its strong connection to soil health, is a major focus of this report, the committee chose to focus on biodegradation and its role in contaminant removal.
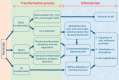
FIGURE 6-3
Transformation processes affecting the fate of pesticides and their degradation products in the environment. NOTE: In this example, a pesticide stands in as an example of an organic contaminant. SOURCE: Adaptation used with permission of Spring Nature (more...)
Biodegradation
Biodegradation is defined as the alteration of the chemical structure of a compound by a biological process (Scow and Johnson 1996; Al-Mamun 2017) and often provides a source of carbon and energy to the organisms responsible. In soil, these primarily microbial processes lead to the destruction, and thus either complete removal or change in chemical composition of the original contaminant. Many organic contaminants can be metabolized by the soil microbiome in a process known as mineralization, in which case the contaminant is converted into harmless byproducts, such as carbon dioxide (CO2) and nutrients, and into microbial biomass (Scow et al. 1995; Scow and Johnson 1996; Figure 6-3). Examples of mineralizable contaminants include many pesticides and petroleum products. In other cases, however, a contaminant may be chemically transformed into other chemical forms, some of which may persist in the environment (Scow et al. 1995). Often, metabolites are less toxic than the parent compound. Sometimes, however, metabolites are more toxic than the parent compounds (e.g., trichloroethylene to vinyl chloride) (Yoshikawa et al. 2017).
Numerous metabolic pathways for contaminants have been identified, some very specific to particular chemicals (Kolvenbach et al. 2014). The University of Minnesota Biocatalysis/Biodegradation Database (Gao et al. 2010) is a comprehensive, publicly accessible resource that provides information on the biodegradation and biotransformation of organic compounds, including many contaminants, by bacteria and fungi (Ellis and Wackett 2012). Among the data compiled are enzymatic pathways, metabolites of degradation, and organisms involved. The database is valuable for bioremediation research, assessment of contaminant degradation potential, and the study of microbial metabolic pathways (Gao et al. 2010).
Human Exposure to Contaminants in Soil
The level of threat that a contaminant poses to human health is thus determined by the complex interplay between the contaminant and the unique soil conditions at a particular location. In general, the enhancement of soil quality (for instance, increasing the level of soil organic matter) can serve as a means to mitigate the adverse effects of soil contamination. The overall health of the soil plays a pivotal role in determining the extent to which contaminants may pose harm to human health.
Exposure assessments (NRC 1991; Swartjes 2015) are frameworks used to identify potential exposure pathways for humans for specific chemicals. An important part is evaluation of how contaminants move and persist and where they end up (e.g., in water, air, or soil) after they enter the environment. From this information, the most likely pathways for human exposure can be identified (Figure 6-4). There are multiple routes by which soil is involved in human exposure (NRC 1991; Swartjes 2015). For instance, individuals near contaminated areas can be exposed directly through dermal contact of the skin with soil or through ingestion of soil, which may occur from incidental ingestion, from consumption of soil particles remaining on foods, or through consumption of contaminants concentrated in foods and animal products. Apart from these direct health effects, contaminants may also be leached through the soil into groundwater and surface water, which can affect drinking water quality and accumulate in seafood. Finally, contaminants on the soil surface may be aerosolized, leading to exposure through inhalation. Whatever the route, estimates are made of contaminant concentrations in exposure media using monitoring data, environmental fate models, or both. These estimates are then used to gauge the potential contaminant intake by individuals, considering factors such as ingestion and inhalation rates (NRC 1991; Swartjes 2015).

FIGURE 6-4
An example of contaminant source, fate, transport, and exposure setting. NOTE: Leaking drums are the source of contamination in this figure. Chemicals are released into the air via volatilization, soil via leakage, and water via leaching. The chemicals (more...)
Bioavailability of Soil Contaminants
Bioavailability refers to the propensity of a contaminant to be taken up (e.g., absorbed by various biota, including plants, microorganisms, and human receptors. Bioavailability in soil is a multifaceted concept influenced by physical, chemical, and biological factors, as examined by the National Research Council (NRC 2003). These factors encompass a wide range of interactions that include soil properties (e.g., texture and pH), contaminant properties (e.g., chemical form and solubility), and biological factors, notably microbial activity. Bioavailability can vary over time due to evolving environmental conditions and processes. Considering bioavailability is paramount in the assessment of risks at contaminated sites and in the development of effective remediation strategies.
Barriers presented by soil and plants can diminish the transmission of contaminants to humans. The “soil–plant barrier” concept (Chaney and Ryan 1994; Basta et al. 2005) aids in discerning the relative significance of food chain versus soil ingestion exposure pathways, particularly for contaminants such as metals. The soil barrier, for instance, can reduce contaminant bioavailability through robust absorption mechanisms. Conversely, for the majority of plants, the plant barrier comes into play when contaminants exhibit phytotoxicity, potentially harming food crops before reaching levels detrimental to human health. Contaminants strongly adsorbed to soil, such as lead, encounter the soil barrier and do not pose a risk via the food chain; instead, ingestion of contaminated soil or dust becomes the primary risk driver. Conversely, highly mobile and weakly adsorbed contaminants such as cadmium bypass the soil–plant barrier, contaminating food crops and elevating the risk associated with the food chain pathway.
Soil properties and components, such as clay and organic matter content, exert substantial influence on contaminant solubility, mobility, and bioavailability. Soil pH, in particular, can modify contaminant bioavailability and mobility by regulating heavy metal dissolution/precipitation, by influencing the ionization of pH-dependent ion exchange sites on organic matter and metal oxide clay minerals, and by regulating strong adsorption of heavy metals and oxyanion metalloids (e.g., arsenate, arsenite) on soil clay minerals. Furthermore, pH-dependent functional groups on soil organic matter affect the formation of stable organometallic complexes. For organic contaminants such as pesticides, bioavailability varies, with nonpolar organic chemicals primarily adsorbed via partitioning to soil organic matter. Polar organic compounds, for example, perfluoroalkyl acids (a type of PFAS), exhibit anionic characteristics within the environmentally relevant pH range. The length of the hydrophobic chain controls the extent to which PFAS contaminants are adsorbed by soil organic matter.
The type of adsorption significantly shapes the environmental fate and long-term exposure of contaminants (Table 6-1). Significant advances have been made to characterize the type of metal contaminant adsorption to soil. Application of synchrotron spectroscopy including X-ray absorption near edge structure and extended X-Ray absorption fine structure for lead and arsenic have been reviewed (Sparks 2013). Limited studies have reported the relationship between metal speciation in soil and their bioavailability or bioaccessibility for lead or arsenic (Scheckel et al. 2009; Noerpel et al. 2020). Over the last two decades, in vitro gastrointestinal (IVG) soil extraction methods have been reported to be highly predictive of lead and arsenic bioavailability (Drexler and Brattin 2007; Basta and Juhasz 2014; Diamond et al. 2016; Whitacre et al. 2017). The IVG extraction methods have been shown to be more predictive of bioavailability than chemical speciation methods via synchrotron spectroscopy (Stevens et al. 2018). Weak adsorption processes, such as electrostatic and hydrophobic partitioning to organic matter, reduce bioavailability and mobility in the short term but not over the long term. In contrast, strong and irreversible sorption is desirable for persistent contaminants like heavy metals and PFAS, effectively reducing long-term human exposure.
TABLE 6-1
Bioavailability and Strength of Adsorption of Select Contaminants to Soil Adsorbent Phases.
In addition to adsorption reactions, metal contaminants can be sequestered as minerals or in soil minerals. Formation of double layer hydroxides (LDH) have been shown to sequester metals including cobalt, nickel, and zinc. Sequestration of these metals in LDH has been reviewed (Siebecker et al. 2018). This sequestration mechanism has not been reported for the metals of concern (lead, arsenic, and cadmium) in this chapter.
Role of Soil Health in Remediation and Mitigation of Contaminants
Remediation strategies encompass a wide range of methods crucial for managing contaminated soil environments. These approaches play a pivotal role in reducing the concentrations and accessibility of harmful contaminants, thus mitigating potential harm to human and ecosystem health. Remediation, as a comprehensive term, refers to processes directed at lessening the presence, bioavailability, mobility, and potential human exposure to contaminants. It includes a spectrum of approaches, from complete removal to reducing contaminant bioavailability and mobility. Strategies primarily focus on reducing the bioavailability of contaminants in soil, making them less accessible to organisms, especially humans who may be exposed. These approaches often involve altering the chemical and physical properties of the soil to decrease contaminant mobility and uptake.
Bioremediation is defined as “the intentional use of biodegradation processes to eliminate environmental pollutants from sites where they have been intentionally or inadvertently released” (Madsen 1997). In most cases, the treatment process harnesses the metabolic activities of indigenous microbial populations to break down contaminants into less harmful forms (Seagren 2024). Biostimulation involves adding limiting nutrients to support indigenous microbial communities in metabolizing contaminants, while bioaugmentation introduces microbial strains with the potential to degrade specific contaminants (Scow and Hicks 2005).
Chemical remediation methods, such as adsorption and precipitation, aim to reduce bioavailability by binding contaminants to soil particles or transforming them into less mobile forms; these practices are commonly used for metals and inorganic contaminants (Scheckel et al. 2009). For metals (and some organic pollutants), immobilization is the only option since complete removal is not possible. This strategy involves transforming contaminants into forms (e.g., changing oxidation state, solubility) that form strong, irreversible chemical bonds that remain stable in the environment. Contaminant aging is a natural process where contaminants become more strongly sorbed to soil particles over time, making them less bioavailable, involving both fast, reversible sorption reactions and slow, irreversible sorption reactions.
Incorporating organic matter into the soil, such as plant residues, animal manures, and biochar, improves soil physical, chemical, and biological properties (Attanayake et al. 2015). Organic inputs stimulate microbial activity, supporting the biodegradation of organic contaminants and enhancing soil health (Kästner and Miltner 2016). Soil organic matter, influenced by organic amendments, can adsorb metals and organic chemical contaminants, reducing their bioavailability. Additionally, soil pH adjustment resulting from organic matter additions can decrease the bioavailability of metal contaminants (Brown et al. 2004). The use of biochar can be particularly valuable for immobilizing metals and organic pollutants, contributing to the overall effectiveness of contaminant mitigation strategies (Zhang et al. 2013).
In summary, practices that improve soil health not only enhance the overall health of the soil but also play a crucial role in facilitating remediation and mitigation efforts. Improved soil health can effectively reduce the bioavailability of contaminants, ultimately protecting human and ecosystem health. In the “Contaminant Case Studies” section below, greater detail regarding the potential for remediation in soil is provided for various contaminant classes.
CONTAMINANT CASE STUDIES
In this section, the source, fate and transport, exposure pathway, and impacts of major classes of soil contaminants on soil ecosystems and human health are summarized. Although not exhaustive, these examples represent some of the most pressing soil contamination issues that pose a risk to human health. While contaminant sources and exposure routes are not exclusive to agriculture production, both the sources and solutions have, in many cases, a direct connection to agricultural management practices and soil health.
Heavy Metals and Metalloids
Many heavy metals and metalloids can be toxic to humans and the environment, depending on exposure routes, concentrations, and bioavailability. Lead and arsenic are ranked as the top two substances found at national priorities sites determined to pose the most significant potential threat to human health due to their known or suspected toxicity and potential for human exposure.1 Lead is the most common heavy metal soil contaminant worldwide (Hettiarachchi et al. 2023). The environmental chemistry of lead (a cation) and arsenic (usually an oxyanion) are used here to demonstrate behavior and health impacts of these toxic trace elements. Cadmium is included as an example because it is a highly mobile toxic metal that concentrates in food crops.
Sources of Lead in the Soil Environment
Lead occurs naturally in soils, but most soil lead contamination is a consequence of anthropogenic activities. Mining, smelting, refining activities, and coal combustion have resulted in widespread contamination of soil (Rieuwerts et al. 1998; Tchounwou et al. 2012; ATSDR 2020). Leaded gasoline and paint are also major sources of soil contamination (ATSDR 2020). Ash deposits from solid waste incineration prior to the U.S. Solid Waste Disposal Act of 1965 have been found to be a source of lead contamination in soil as well (Bihari et al. 2023).
In agriculture, lead-containing inorganic pesticides such as lead arsenate (PbHAsO4) were extensively used because of its immediate effectiveness, low cost, and easy handling (Hettiarachchi et al. 2023). Lead arsenate was widely used in U.S. agriculture until the 1950s; its heavy use in orchards over many years caused the buildup of lead as well as arsenic in the soil environment (Schooley et al. 2008). Inorganic fertilizers, manure, compost, and biosolids are alternative sources of accumulation of lead, either in soil or plants (Alengebawy et al. 2021).
Sources of Arsenic in the Soil Environment
Similar to lead, arsenic is found naturally in soil, but most contamination of soil with arsenic is primarily related to anthropogenic sources. These sources include nonferrous metal mining and smelting, wood and coal combustion, wood preservation, pesticide application, and waste incineration (ATSDR 2007). Irrigation water containing high concentrations of arsenic can also be a significant source of soil contamination in India, Bangladesh, and east Asian countries (e.g., Vietnam, China) (Mitra et al. 2017). Arsenic contamination of rice, especially brown rice, from arsenic-tainted irrigation water has been reported in the U.S. Southeast (Meharg et al. 2009) and in California (Carrijo et al. 2022). Arsenic contamination in the southeastern soils is likely from the historical use of arsenical pesticides for cotton production before these pesticides were banned in the 1980s and 1990s; conversion of land from cotton production to rice production has resulted in elevated levels of arsenic in rice in the Mississippi Delta and Texas (Zavala and Duxbury 2008).
Sources of Cadmium in the Soil Environment
Cadmium is one of the most toxic heavy metals and negatively affects essential biological processes of humans, plants, and animals (Kabata-Pendias 2010). Major sources of cadmium pollution in soil include zinc mining, processing, and smelting and the use of phosphate fertilizer produced from rock phosphate ore with elevated cadmium (Kabata-Pendias 2010). Smelter emissions from zinc and cadmium production facilities have contaminated downwind environments (Chaney and Ryan 1994; Zhou et al. 2022). Land application of phosphate fertilizer can result in significant soil pollution with cadmium that impairs crop quality (Kabata-Pendias 2010). However, long-term application of phosphate fertilizer with low cadmium content does not increase soil cadmium or impair wheat grain quality (Basta et al. 1998). Cadmium can also reach soil through land-applied biosolids (ATSDR 2012).
Impact on Soil Ecosystems
Heavy metals change soil microorganism community structures and diversity (Konopka et al. 1999; Khan et al. 2010; Rodríguez Martín et al. 2014; Gutiérrez et al. 2016). Khan et al. (2010) determined that the high levels of cadmium and lead in soils caused decreases in microbial biomass carbon, inhibited acid phosphatase and urease enzymatic activity, and changed community structure based on denaturing gradient gel electrophoresis banding patterns. They also found that bacteria are more sensitive to heavy metal concentrations than actinomycetes and fungi. Konopka et al. (1999) found lead soil content to correlate with microbial biomass and determined that some bacteria populations contained lead-resistance genes. They noted that heavy metal–contaminated soils also have reduced plant biomass that could affect microbial community composition and activity. Others have noted that the diversity and function of soil invertebrates, such as earthworms and nematodes, can be altered by cadmium and lead (Žaltauskaitė and Sodienė 2010; Rodríguez Martín et al. 2014; Gutiérrez et al. 2016; Kavehei et al. 2018).
Fate and Transport: Human Exposure Pathways
Fate and transport of heavy metals is complex and varies greatly between metals. Three exposure pathways important to human health are: soil/dust ingestion and inhalation, ingestion of contaminated food, and ingestion of contaminated drinking water. The first two pathways, which relate to soil, are reviewed here.
Soil and dust ingestion
One of the major exposure pathways for lead, arsenic, and other heavy metals to humans is through the incidental ingestion of soil or dust. Ingestion is of special concern for children due to their increased hand-to-mouth activity and enhanced pharmacokinetics. Many heavy metals, including lead and arsenic, often have low water solubility in the environment. Low solubility reduces the transport of metals from soil to crops and from soil to source waters. The exception is cadmium, which escapes the soil–plant barrier and easily contaminates food crops (Basta et al. 2005). Many of the heavy metals in ingested dust or soil are dissolved in the acidic conditions (e.g., pH 1.5 to 2.5) of the upper gastrointestinal tract.
Soil properties and agricultural management practices can affect bioavailability and exposure to lead and arsenic. Lake et al. (2021) reported key soil properties reduced the bioavailability of arsenic by 17–96.5 percent and of lead by 1.3–38.9 percent associated with soil human ingestion. For both arsenic and lead, bioavailability decreased with increasing content of aluminum oxide and iron oxide. Soil amendments that add aluminum oxide and iron oxide to soil (e.g., via biosolids) will decrease arsenic and lead bioavailability.
Contaminant transport from soil to the food chain
In general, transport of many heavy metals from contaminated soil to plants to humans is small when compared to incidental ingestion (Chaney and Ryan 1994; Attanayake et al. 2015). However, small amounts of heavy metal absorption into crops that are diet staples (e.g., wheat, rice) can result in significant human exposure. For example, root crops such as carrots, radishes, and beets can accumulate soil lead, and surface contamination of crops with soil can affect human exposure (Attanayake et al. 2014, 2021). Human exposure from ingestion of soil adhered to vegetables has been found to be greater than amounts in those vegetables. Best management practices to reduce heavy metal exposure include washing crops thoroughly before consumption to get rid of adhering soil particles.
Diet is the largest source of exposure to arsenic, largely from grains, produce, seafood, and drinking water. Rice, in particular, is an important source of dietary arsenic because it is cultivated under flooded conditions leading to an anoxic soil environment that liberates arsenic from its minerally bound form and allows it to be taken up and concentrated in the grain. Notably, a recent study found that arsenic availability, uptake, and allocation in rice increases in warmer temperatures; therefore, global warming may increase the risk of arsenic exposure through consumption of rice in production systems that were previously considered low risk (Farhat et al. 2021).
For non-smokers, diet is the largest exposure route for cadmium (ATSDR 2012). However, the concentration of cadmium in the soil is not predictive of the concentration of cadmium in food grown in the soil; soil properties, the type of crop and its genetics, crop rotation, and fertilizer and irrigation management practices all affect the ultimate concentration of cadmium in food (Schaefer et al. 2020).
Impacts on Human Health
Lead exposure primarily occurs through incidental ingestion of contaminated soil and dust, with children being particularly vulnerable. Unlike respiratory and dermal pathways, incidental ingestion involves unintentional swallowing due to hand-to-mouth contact. Studies have consistently highlighted the significance of this pathway, with exposure typically occurring through hand-to-mouth transfer of contaminated soil or dust (Mielke and Reagan 1998; Glorennec et al. 2010; Oulhote et al. 2011; Zahran et al. 2013; Henry et al. 2015). Once lead is absorbed into the body and enters the bloodstream, it can rapidly distribute to the kidneys and liver or be slowly absorbed by soft tissues. The majority of the body’s lead burden is stored in teeth and bones. Lead is highly toxic and can affect nearly every organ, especially the nervous system. At elevated exposure levels, lead can lead to comas, convulsions, and even fatalities. There are no safe blood lead levels, and any concentration of lead can be associated with decreased IQ levels in children and behavioral challenges. These effects are considered permanent. Additionally, lead exposure can cause symptoms such as dizziness, irritability, fatigue, impaired cognition, anemia, hypertension, kidney damage, and reproductive organ toxicity (Wani et al. 2016; ATSDR 2020). Lead exposure during pregnancy has been linked to maternal health risks, including hypertension and pre-eclampsia. One study found that women living in areas with higher soil lead levels were more likely to suffer an eclampsia event (Zahran et al. 2014). Even low levels of exposure during pregnancy have been found to lead to poor birth outcomes including low birthweight and preterm birth (Bellinger 2005).
Inorganic forms of arsenic like As(III) (i.e., arsenite) and As(V) (i.e., arsenate) are associated with more severe health effects from exposure as compared exposure to organic arsenic. Ingestion of arsenic can cause gastrointestinal symptoms, decreased production of white and red blood cells leading to bruising, impaired nerve function leading to burning sensations in the hands and feet, and skin changes (Mandal and Suzuki 2002; ATSDR 2007). The U.S. Environmental Protection Agency (EPA) and the International Agency for Research on Cancer have also listed arsenic as a human carcinogen because it has been linked to cancer of the liver, skin, bladder, and lung. Fetuses, infants, and children are particularly vulnerable to the potential harmful effects from arsenic exposure, and exposure during times of active brain development has been linked to learning disabilities, behavioral problems, and lowered IQ.
As described above, the impact on human health can vary based on the timing duration, route of exposure, and host variability. Notably, recent research has shown that gut microbiota can increase the bioavailability of soil-bound arsenic thereby enhancing its toxicity (Yin et al. 2017; McDermott et al. 2020; Griggs et al. 2022). While research in humans is limited, studies using in vitro models such as the Simulator of the Human Intestinal Ecosystem have shown that human gut microbiota may increase the bioavailability of arsenic in contaminated soil samples (Laird et al. 2007; Van de Wiele et al. 2010). Despite this individual variability, the U.S. Food and Drug Administration has issued guidance to industry on acceptable levels of arsenic in foods often consumed by infants and children. It recommends that inorganic arsenic levels do not exceed 100 parts per billion (ppb) in infant rice cereal (FDA 2020) and 10 ppb of inorganic arsenic in single-strength (ready-to-drink) apple juices (FDA 2023).
Recent research has also described the impact of heavy metals and metalloids on the gut microbiome, which is important for human health. Studies using animal model systems have shown significant alterations of gut microbiomes to arsenic exposure (K. Lu et al. 2014; Gokulan et al. 2018). One study in mice showed that arsenic exposure not only caused shifts in the composition of the gut microbiota but also led to impairment of the immune response and higher inflammation. Further, these changes were dose-dependent and differed according to the age of the animals (Gokulan et al. 2018). The function of the gut microbiome also is impacted by arsenic exposure, which in one study inhibited the fermentation rate of rumen bacteria by almost one third (Forsberg 1978).
Information about the effect of arsenic on the human microbiome is scarce, but vast shifts in the composition of gut microbial communities have been observed in populations exposed to arsenic (McDermott et al. 2020). For example, in Bangladesh, there was a significantly higher abundance of Proteobacteria as well as multidrug and arsenic resistance microbial genes in children exposed to arsenic than their unexposed counterparts (Dong et al. 2017). In the United States, urinary arsenic was correlated with several taxonomic groups in naturally exposed infants, although none of them were in the Proteobacteria phylum (Hoen et al. 2018). Significant compositional shifts were also observed in a mining community in China with prolonged exposure to heavy metal contamination when compared to a community with little exposure to these pollutants. Paradoxically, metal exposure was also correlated with microbial richness in the human gut (Shao and Zhu 2020).
Evidence supports adverse effects on the kidneys and bone density from chronic dietary cadmium exposure, although in some studies, the populations also lived in areas polluted with cadmium. Studies of the effects of dietary cadmium exposure on human health have found mixed results on cardiovascular disease. Consumption of cadmium at high concentrations is known to cause gastrointestinal distress (ATSDR 2012).
Mitigation and Remediation
Remedial approaches for lead are based on removal or reducing the bioavailability of lead. Bioavailability-based remediation is a cost-effective in situ approach that does not remove the contaminant but does reduce the contaminant’s bioavailability. The use of soil amendments to remediate lead-contaminated soils focuses mainly on changing existing soil lead chemistry in situ. It is done by inducing the formation of sparingly soluble lead solids or enhancing chemisorption. Many soil amendments have been used successfully for bioavailability-based remediation (Henry et al. 2015; Wang et al. 2021; Hettiarachchi et al. 2023). Commonly used soil amendments reported to reduce lead bioavailability are summarized in Table 6-2. Other amendments are in development including formation of plumbojarosite (Sowers et al. 2023) and the use of modified biochars (Yang et al. 2019).
TABLE 6-2
Commonly Used Soil Amendments for Bioavailability-Based Remediation of Lead-Contaminated Soil.
Several of the soil amendments are applied to agricultural fields through additions of phosphorus fertilizer, manures, compost, biochar, and liming materials. These amendments decrease lead bioavailability and exposure to human and ecosystem receptors. Many amendments are green and sustainable remediation (GSR) strategies, which are holistic approaches that maximize environmental, social, and economic benefits (Wang et al. 2021).
Phytoextraction is using hyperaccumulator plants that absorb metals (e.g., more than 10,000 mg/kg) from soil followed by harvesting of the hyperaccumulator plants. Phytoremediation of cadmium, zinc, selenium, and arsenic from contamination soil has been reviewed (Chaney et al. 2014). Hyperaccumulator plants for lead do not exist. Application of chelating ethylenediaminetetraacetic acid solution to lead-contaminated soil planted with Brassica juncea (e.g., Indian mustard) was able to accumulate more than 1 percent of the lead in plant tissue (Blaylock et al. 1997). However, this practice contaminated groundwater (Nowack et al. 2006).
Remediation strategies vary depending on the form of arsenic in the environment. Often, the dominant form of arsenic contamination in aerobic soil is arsenate. The majority of in situ remediation methods are designed to adsorb or precipitate arsenate and reduce its bioavailability and mobility (Gong et al. 2018). In particular, iron oxide clay minerals strongly adsorb arsenate and reduce arsenate bioavailability (Violante et al. 2010). More recently, GSR soil amendments for remediation or arsenic-contaminated soils include biochar (Wang et al. 2021) and biochar modified with iron oxide (Yang et al. 2021). These treatments improve soil health while remediating arsenic.
Mitigating cadmium in the food supply is complicated by the properties of the soil the food is grown in, the type of amendments that may be applied to the crop, and the genetics of the crop. Cadmium can also be introduced to the food supply from utensils, plastics, and cookware that contain cadmium. When it comes to steps to reduce cadmium uptake in the field, better understanding of geogenic sources and specific plant uptake would help mitigate cadmium in food, as would minimizing the application of phosphate fertilizers and irrigation water that contains cadmium (Schaefer et al. 2020). Producers would benefit from research that provides evidence for management strategies tailored to specific soils and specific crops that prevents plant uptake of cadmium (Schaefer et al. 2020).
Microplastics
Microplastics (MPs) are defined as small plastic particles measuring less than 5 mm in size (Masura et al. 2015). MP pollution includes primary MPs (e.g., microfibers, textiles) and secondary MPs, which are defined as degradation products of larger plastic products (Petersen and Hubbart 2021). Approximately 90 percent of synthetic polymers produced are polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), or polyethylene terephthalate (PET) (Andrady and Neal 2009). MPs exist in a variety of physical forms that include fibers, foam, film, and particles (Rillig et al. 2019a). Found in diverse ecosystems worldwide, MPs have become a pervasive and global environmental issue (Bolan et al. 2020; Büks and Kaupenjohann 2020) and a major reason the planetary boundary for the category of contaminants (i.e., novel entities) is being exceeded (Persson et al. 2022).
Sources
Microplastics reach soil through direct and indirect pathways, which vary based on location, land use, and management practices (Figure 6-5; Yadav et al. 2022). In agriculture, the primary sources are compost (Bläsing and Amelung 2018), biosolids (Bläsing and Amelung 2018; Crossman et al. 2020), irrigation with untreated wastewater (Bläsing and Amelung 2018; He et al. 2018), and polymer coatings of slow-releasefertilizers (Lian et al. 2021). Worldwide, plastic mulch use in agricultural production is a growing source as well (He et al. 2018).

FIGURE 6-5
The source, movement, and fate of microplastics in the environment. SOURCE: Used with permission of Springer Nature BV, from “Unravelling the Emerging Threats of Microplastics to Agroecosystems”, Yadav et al., Reviewers in Environmental (more...)
Once in soil, MPs tend to accumulate near the soil surface due to their low density and small particle size. MPs can also become incorporated into soil aggregates or immobilized on to soil particles as a function of the particular soil’s texture, organic matter content, and moisture levels (Rillig and Lehmann 2020). MPs can be transported within and out of soil through processes such as surface runoff, erosion events, and leaching to groundwater and via biological activities such as earthworm burrowing and root growth.
Fate and Transport
The persistence and degradation of plastics and MPs in soil environments have been reviewed by Restrepo-Flórez et al. (2014), Krueger et al. (2015), and Bläsing and Amelung (2018). Plastics, synthesized to be durable and withstand deterioration, accumulate in soil (Bläsing and Amelung 2018) despite being susceptible to degradation by abiotic and biotic processes (Figure 6-5). Degradation rates are dependent on the type of MPs, location in soil, management practices, climate, and other factors. Degradation pathways via ultraviolet (UV) radiation are known, particularly in aquatic ecosystems, but in soil only materials near the surface are susceptible and degradation rates are generally slow. Over time, UV exposure and weathering processes cause larger MPs to fragment into smaller particles, which then move through soil and become more susceptible to both uptake and degradation (Hooge et al. 2023).
With respect to biodegradation, a microbial process, most plastics degrade very slowly (if at all) in soil, with persistence estimated to range from several years to several thousand years (Chamas et al. 2020). The usually low surface-to-volume ratio of most forms of microplastics physically limits their bioavailability to microorganisms, who need to maintain close proximity to surfaces for reactions (Hooge et al. 2023). Field studies reviewed by Bläsing and Amelung (2018) indicate minimal soil degradation of MPs with far less than 1-percent loss by weight of PE over 2.5 year (Albertsson 1980) and PP after 1 year (Arkatkar et al. 2009) and no detectable PVC degradation after 10–35 years in soil (Otake et al. 1995; Santana et al. 2012; Ali et al. 2014). Thus, although large and visible pieces of plastics may fragment over time, most data confirm that MPs are persistent in soil (Krueger et al. 2015; Bläsing and Amelung 2018). Unfortunately, rates of inputs of MPs greatly exceed their rates of removal in soil (Petersen and Hubbert 2021).
Impact on Soil Ecosystems
Physical–chemical
MPs are redistributed throughout the soil by tillage, by physical mixing from wet-dry or freeze-thaw cycles, or by soil organisms (Hooge et al. 2023). MPs can affect soil structure and porosity, leading to changes in water infiltration, retention, and drainage (Figure 6-6; de Souza et al. 2019; Lehmann et al. 2019) and, in turn, influence nutrient availability, root growth, and plant productivity. Due to their larger size (around 2 mm), fiber, foam, and film MPs can potentially increase soil aeration and microporosity (Sun et al. 2022). MP microfibers can decrease the water stable macroaggregates via both physical effects and biological effects on soil biota that contribute to soil aggregation (Boots et al. 2019). MPs can also modify soil chemical properties by adsorbing and releasing contaminants, such as heavy metals and organic pollutants, thereby influencing their bioavailability and potential toxicity to soil organisms and plants (Sajjad et al. 2022).

FIGURE 6-6
A schematic diagram of microplastic interaction with plant–soil systems. SOURCE: Used with permission of Springer Nature BV, from “Unravelling the Emerging Threats of Microplastics to Agroecosystems”, Yadav et al., Reviewers in (more...)
Impacts on soil communities
MPs have been documented to change the diversity, abundance, and functions of soil microbial communities (Rillig et al. 2021b); the extent of impact is often proportional to concentration (Sun et al. 2022). Overall effects on soil biota can be negative, positive, or neutral and occur via both direct (e.g., absorption of MP nanoparticles) and indirect (e.g., altering soil structure) pathways. Though not well understood, the impacts are many (Rillig et al. 2021b), such as shifting the composition of microbial communities and altering microbial processes, including carbon storage, organic matter decomposition, and greenhouse gas emissions (Rillig et al. 2021a,b). MPs are made up primarily of recalcitrant carbon and can slow down overall organic matter decomposition rates (Rillig et al. 2021a). MP fibers in soil resulted in an increase in CO2 fluxes and a decrease in nitrous oxide emissions, especially after urea fertilizer application, which is attributed to increased soil aggregation and air permeability (Rillig et al. 2021a).
The plastisphere—defined as the soil surfaces influenced by plastic—has a different microbial community than bulk soil. For example, more pathogens and antibiotic resistance genes are present as compared to bulk soil (Zhu et al. 2022; Rillig et al. 2024). Furthermore, plastics have been found to change soil bacteria community structure and interfere with microbial lipid metabolisms and the biosynthesis of secondary metabolites (Wu et al. 2022). MPs can detrimentally affect the growth, reproduction, lifespan, and overall survival of soil fauna through various mechanisms, including ingestion, bioaccumulation, oxidative stress, reproductive and neurotoxic effects, metabolic disruptions, and gut microbiota imbalances (Wang et al. 2022). Some soil fauna play a role in the formation and degradation of MPs, modify their movement in soil, and can potentially transfer accumulated MPs up the food chain (Helmberger et al. 2020; Wang et al. 2022).
The indirect effects of MPs on plant growth include alteration of soil structure, bulk density, and water-holding capacity, which in turn influences plant development (Helmberger et al. 2020). These effects are varied and can be both positive and negative, heavily dependent on the characteristics of the MPs (like their chemical additives) and the specific types of soil and plants in the environment (Rillig et al. 2021b). MPs can impair plant seed germination, reduce root elongation, and negatively affect plant biomass and reproductive capacity (Boots et al. 2019; de Souza et al. 2019). MPs may also interfere with plant nutrient uptake and induce oxidative stress, leading to physiological disorders and decreased overall plant performance (Rillig et al. 2019b). These impacts could have a cascading effect on other biophysical processes in the soil and may pose a threat to soil biodiversity and ecosystem functioning (Boots et al. 2019).
Human Exposure Pathways
MPs in soils can pose potential risks to human health through several exposure pathways such as direct soil ingestion, particularly by children during outdoor activities as well as inhalation of MP particles suspended in the air due to wind erosion or dust generation from contaminated soils. Consumption of contaminated foods is also possible; most research to date has focused on MPs in food that is not directly related to soil, such as seafood, fish, salt, honey, and water (Karbalaei et al. 2018). One study that investigated the quantity of MPs in fruits and vegetables found that the median distribution of MPs <10 micron in size ranged by 52,050 to 233,000 particles/g and that apples were the most contaminated samples (Conti et al. 2020). The estimated daily intakes associated with that study are smaller than the estimated daily intakes from plastic bottled mineral water (Zuccarello et al. 2019), therefore the committee concluded that consumption from soil-related agricultural produce may be less of a concern.
Impacts on Human Health
The human health effects of MPs can be categorized into chemical, physical, and biological effects, as illustrated in Figure 6-7. Chemical effects refer to the impact of additives and dyes that may be toxic, teratogenic, or carcinogenic. For example, phthalates are commonly used as plasticizers to provide flexibility to plastics. Because they are additives, they are not chemically bound (covalently bonded) and are more likely to be released and transferred to the environment (Blackburn and Green 2022). Similarly, polybrominated diphenyl ethers are used as flame retardants in many commercial products and, because they are not chemically bound, can leach during production and recycling, bioaccumulate, and cause endocrine disruption and impaired neurological development in animal models. Secondary toxins include persistent organic pollutants that can lead to immunotoxicity and secondary toxicity through interaction with other chemical pollutants. For example, plastic mulching sheets used in agriculture can both absorb pesticides and increase risks for human exposure (Huang et al. 2020, Wang et al. 2020).
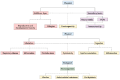
FIGURE 6-7
A flow diagram illustrating the potential human health effects of microplastics. NOTE: The dotted lines represent current speculative research. SOURCE: Used with permission of Springer Nature BV, from “The Potential Effects of Microplastics on (more...)
Physical effects include inhalation and ingestion of MPs and subsequent immunotoxicity. Small MPs are common in the atmosphere and can be readily inhaled; however, the health effects remain poorly understood. Eyles et al. (2001) found that, once inhaled or ingested, MPs could also translocate to other tissues; fluorescent polystyrene microspheres delivered intranasally to mice were found in the spleen 10 days later. MPs have also been shown to impair gastrointestinal function and alteration of hepatic lipid metabolism in animal models (Y. Jin et al. 2019; L. Lu et al. 2019); this may also be the case for humans.
Finally, there are biological effects that primarily refer to the impact on the microbiome. Evidence thus far, mostly stemming from animal models, has shown that nano and microplastics alter microbial community structures in the gut (both alpha and beta diversity), which may in turn underlie immune impairment, as previously reviewed (Fackelmann and Sommer 2019; L. Lu et al. 2019; Santos et al. 2022).
As an additional burden, microplastics from soil may carry pathogens and other pollutants that pose a threat to human health potentially through alterations in gut microbial communities (Fackelmann and Sommer 2019). A mice study found that when co-ingested with polyethylene particles, the bioavailability of arsenate increased, and this seemed to be mediated by the metabolite output of the gut microbiota (Chen et al. 2023). Also, impacts of MPs on soil communities may translate into effects on the food system and have negative consequences for human health (Daghighi et al. 2023).
Few studies have comprehensively assessed the impact of microplastics on health concurrently with their presence in the environment. A recent study compared the soil, air, nasal, and gastrointestinal microbiomes of two populations, one with high exposure to microplastic pollution and the other with low exposure. They found overlap in the microplastic species found in gut microbiome samples and soil samples in the high exposure area, along with inverse correlations between microplastic exposure and the abundance of taxonomic groups that may be beneficial for human health (Zhang et al. 2022).
Mitigation and Remediation
The reduction of plastic use in agriculture is an essential step toward more sustainable and environmentally friendly farm management. To address these issues, the “3 R” waste hierarchy concept of reducing, reusing, and recycling plastics, before considering disposal, is crucial (Hofmann et al. 2023). Similarly, the United Nations’ Food and Agriculture Organization (FAO) has promoted the 6R model (Refuse, Redesign, Reduce, Reuse, Recycle, and Recover) for reduction of use of plastics in agriculture (FAO 2021). Both approaches advocate for plastic applications that have circular end-of-life treatment options and stress the importance of innovative material design to ensure plastics can be completely collected, recycled, and reused. Use of non-biodegradable polymer-coated fertilizers and mulching films, in particular, should be minimized and, if used, be collected after use. Additionally, in situations where plastics cannot be collected after use, or where recovered plastic is too degraded or soiled to be reused or recycled, polymers that are more biodegradable or less toxic should replace conventional persistent polymers (Galati and Scalenghe 2021; Hofmann et al. 2023).
Biodegradable polymers offer potential solutions for reducing environmental impacts of plastics for short-term uses (e.g., cutlery). Biodegradable plastics can break down through composting or exposure to UV radiation. However, uncertainties concerning biodegradable polymers involve their complex waste management, including requirement for specific collection and composting facilities, and the low volumes produced that may not justify the waste management efforts (Prata et al. 2019). Additionally, some degradable plastics produce non-degradable by products (Prata et al. 2019).
The possibility of significant and consistent biodegradation of conventional plastics in the environment remains uncertain (Krueger et al. 2015). Some strains of bacteria and fungi have been demonstrated in the laboratory to be capable of degrading many polymers via enzymatic hydrolysis or oxidation (Sivan 2011; Krueger et al. 2015). Some conventional plastics can biodegrade under lab conditions when exposed to specific plastic-degrading organisms, such as Zalerion maritimum (Paço et al. 2017); how successful these organisms would be in soil is not known.
PFAS
Per- and polyfluoroalkyl substances are a large group of synthetic, organofluorine chemicals that are of increasing concern as environmental contaminants. Their characteristic carbon–fluorine bond is one of the strongest single bonds in chemistry, so PFAS molecules are extremely hard to break down. Thus, they are highly persistent in the environment and are often referred to as “forever chemicals.” There are thousands of PFAS compounds2 of concern with respect to the health of people and the environment.
PFAS give desirable properties to industrial and consumer products because they resist water, oil, and heat. They are used to make waterproof and stain-resistant garments, nonstick pans, and oil-resistant containers. Teflon is a well-known example. They are also used to make firefighting foams, fabrics that resist fire and stains, and a great many other products and industrial processes.
The different PFAS species vary in their length of the carbon chain, their branching structure, the number of fluorine molecules and their positions, attached functional groups, and so on (Buck et al. 2011). The large and diverse PFAS family of molecules can be divided into the polymer and non-polymer classes, with the latter most commonly found in biological and environmental samples. This non-polymer class includes per- and polyfluoroalkyl substances; the carbon chains of the perfluoroalkyl substances are fully fluorinated, while the carbon chains of the polyfluoroalkyl substances are not fully saturated with fluorine atoms. The perfluoroalkyl acids (PFAAs) are one major group of the perfluorinated subclass of the non-polymer class of PFAS. Because of their saturation, they are the most stable and thus most persistent in the environment. Their non-saturated precursors can be transformed into PFAAs through biological or chemical cleavage of their non-fluorinated moieties.
Sources
PFAS molecules are created through industrial processes. They have been manufactured since the 1940s and widely used since the 1950s. They are spread from both point sources (where they are made or used) and diffuse sources (such as water, soil, and air). Point sources include industrial sites where PFAS is made and used, such as fluorochemical production plants (Gebbink and van Leeuwen 2020).
Several studies have found soil to be a major environmental reservoir of PFAS (Strynar et al. 2012; Brusseau et al. 2020). Soils can receive PFAS from applied biosolids, contaminated irrigation water (Pepper et al. 2021), aqueous film-forming foam used extensively at military bases and airports (Yan et al. 2024), and rainfall (Pfotenhauer et al. 2022). PFAS in biosolids can come from many sources, including landfill leachate. Landfill leachate is a rich source of PFAS because of the discarded domestic products (e.g., cookware, clothing, carpets, and furniture) and industrial products deposited in these sites (Lang et al. 2017; Capozzi et al. 2023). Landfill leachate is typically treated at the municipal wastewater treatment plants where other wastes, including excreta and industrial wastes, are processed (Masoner et al. 2020; Helmer et al. 2022).
Because the biological and other treatment processes implemented through conventional wastewater treatment do not destroy PFAS, biosolids (the stabilized solids that result from municipal wastewater treatment, also known as sewer sludge) may be contaminated. Longer-chain PFAS molecules (e.g., those with an 8-carbon backbone or C8) are typically enriched in biosolids relative to liquid effluent (Helmer et al. 2022).
Fate and Transport
PFAS are highly mobile in the environment (Brunn et al. 2023). As noted above, their forms can change in the environment as non-fluorinated moieties are cleaved, yielding stable (saturated) PFAAs (Evich et al. 2022). Greater amounts of some PFAS molecules are thus found in the effluent from wastewater treatment plants than in the influent (Coggan et al. 2019; Thompson et al. 2023). Short-chain PFAS are more mobile in soils than longer-chain forms (Brusseau et al. 2020). General pathways are indicated in Figure 6-8.
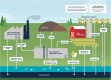
FIGURE 6-8
A diagram illustrating the sources of PFAS and pathways for their release into the water cycle and the environment. SOURCE: Diagram courtesy of Michigan Department of Environment, Great Lakes, and Energy.
Biosolids have long been used as a soil amendment, adding carbon and nutrients to agricultural and other lands (Lu et al. 2012). While it is desirable to regard organic wastes as resources, concern is rising about PFAS contamination of biosolids leading to contamination of farmland (Lowman et al. 2013; Mason-Renton and Luginaah 2018). Plants can take up PFAS, with short-chain forms showing greater mobility in plant tissues than longer-chain forms (Costello and Lee 2020). Maine recently banned the land application of biosolids after some farms were found to have high levels of contamination (Perkins 2022).
Impact on Soil Ecosystems
Studies of the effects of PFAS on soil microbiomes have shown that PFAS changes community composition and metabolite production (Senevirathna et al. 2022; Xu et al. 2022; Wu et al. 2023). Several studies have reported that PFAS reduce microbial abundance, including in laboratory experiments (Xu et al. 2022) and field studies comparing contaminated and noncontaminated sites (Senevirathna et al. 2022). Xu et al. (2022) found that soil treated with the PFAS compounds perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) had bacterial reduced gene abundance as compared to the control treatment and that PFAS exposure altered soil microbial composition. The researchers speculated that the increase in Proteobacteria was due to Proteobacteria’s greater tolerance for PFAS (Xu et al. 2022). Increases in Proteobacteria have also been observed in other studies (Wu et al. 2023). Modeling also predicted that exposure to PFAS inhibited numerous microbial metabolism processes in soil. Wu et al. (2023) found that soil microorganisms downstream of a Teflon production plant had reduced lipid biosynthesis and possibly decreased metabolic activity.
Findings with regard to effects of PFAS on soil pH and microbial diversity have varied across studies. Xu et al. (2022) did not find a change in soil pH between soil treated with PFOA and PFOS and a control sample, while Xu et al. (2023) found a significant increase in soil pH in another laboratory experiment. The latter results were likely because PFAS increased litter decomposition rather than PFAS having a direct effect on soil pH. The latter researchers also found that soil aggregate stability was not affected by PFAS but water-stable aggregates decreased.
The direction of change in microbial diversity and richness of soil microorganisms exposed to PFAS is unclear, possibly due to differences in PFAS concentration and species across studies. Xu et al. (2022) found that microbial diversity and richness of soil microorganisms increased in the treatment cases as opposed to the control in a laboratory study, whereas Senevirathna et al. (2022) observed a decline in soil bacterial community population and diversity in soils collected from contaminated sites versus uncontaminated environmental samples. PFOS (a C8 molecule) had a greater effect than PFOA (a molecule with a 7-carbon backbone), suggesting the type of PFAS may influence impacts on soil microbial communities. Cai et al. (2019) also found that the type of PFAS influenced its effect on microbial diversity and richness, with longer chains having greater toxicity than shorter chains.
Additionally, PFAS may negatively affect plant growth. Xu et al. (2022) found that PFOA and PFOS inhibited the abundance of Azospirillum, a plant growth-promoting rhizobacteria. PFOS also enriched Hydrogenophaga, which are resistant to organic pollutants, whileMethyloversatilis was more abundant under PFOA exposure. The abundance of Methyloversatilis suggests that it may be able to degrade PFOA. Wu et al. (2023) also determined that PFAS contamination affected the fungal community in soils. The authors noted that mycotoxin concentrations were higher downstream of the Teflon plant.
Human Exposure Pathways
People are exposed to PFAS through many pathways, including via occupational exposures, consumer and household products, environmental contamination, and ingestion. Occupational exposures are myriad and may include occur where PFAS-containing products are manufactured, in the construction industry, among food workers that handle PFAS-containing food packaging, and among firefighters. PFAS are used in thousands of consumer products ranging from personal care products like sunscreen, makeup, and dental floss to textiles, artificial turf, and paint. A recent review found that exposure from contaminated household dust could account for up to 25 percent of blood concentrations but that there were many methodologic flaws in the existing studies and that more research on household exposure pathways is needed (DeLuca et al. 2022).
Environmental contamination of soils and groundwater is also thought to be an important source of PFAS exposure. Most research in nonoccupational settings has focused on ingestion of PFAS, which can occur through drinking contaminated water or eating contaminated seafood, vegetables, game, or dairy products (Domingo and Nadal 2017; Death et al. 2021). In April 2024, EPA established a regulation for maximum contaminant levels for six PFAS compounds for public water systems in the United States (EPA 2024a). It estimated that 6–10 percent of the country’s 66,000 public drinking water systems would have to take action to reduce PFAS to meet these levels (EPA 2024b). PFAS may also be ingested through food contaminated by PFAS-containing materials including food packaging, microwave popcorn bags, and cookware. PFAS can be transferred intergenerationally through the mother’s body to a developing fetus and through breastfeeding (Manzano-Salgado et al. 2015; Gao et al. 2019; Zheng et al. 2021). Exposure pathways may also include inhalation of aerosolized or volatile PFAS, which have been detected indoors and near factory emissions. The impact of inhaled and transdermal exposures, for example through bathing in contaminated water, have been less well studied (Sunderland et al. 2019).
Impacts on Human Health
PFAS are known to affect human health in multiple ways (Sunderland et al. 2019; Brase et al. 2021; Chambers et al. 2021). A recent review by the National Academies summarized the health effects of PFAS and found that cancers, endocrine effects, dysregulation of immune function, and impacts on fertility were the health effects most frequently mentioned by speakers at that committee’s information-gathering meetings (NASEM 2022a). After a systematic synthesis of the evidence, that report concluded that there was sufficient evidence to support an association with decreased antibody response (in adults and children), dyslipidemia (in adults and children), decreased infant and fetal growth, and increased risk of kidney cancer (in adults). The report also found suggestive evidence for the following diseases and health outcomes: increased risk of breast cancer (in adults), liver enzyme alterations (in adults and children), increased risk of pregnancy-induced hypertension (gestational hypertension and pre-eclampsia), increased risk of testicular cancer (in adults), increased risk of thyroid disease and dysfunction (in adults), and increased risk of ulcerative colitis (in adults).
Mitigation and Remediation
As PFAS have been found in drinking water, foods and food packaging, and the bodies of people and animals around the world, awareness and concern become increasingly palpable. This increased awareness has led to pressure for companies to stop making these compounds. After half a century of production, companies are beginning to phase out production of certain PFAS compounds. 3M began phasing out production of PFOS in 2000 (EPA 2000) and recently paid $10.3 billion to settle a multidistrict lawsuit (Kluger 2023). The company stated in 2022 that it would work to cease manufacturing PFAS by the end of 2025 (3M 2022). Reduced production has led to some decrease in the human body burden in the United States and Australian populations (Gomis et al. 2017). Although some PFAS forms are being phased out, there is evidence that companies are making replacement compounds with likely hazardous properties (Brase et al. 2021).
EPA recently published a roadmap related to PFAS contamination (EPA n.d.). As part of the roadmap, EPA is conducting a risk assessment for two specific PFAS compounds, PFOA and PFOS, in biosolids. The risk assessment is scheduled to be completed by December 2024.3 Given the large number of PFAS molecules, their persistence and potential to accumulate in the environment, their individual and collective hazards, and the difficulties of regulating them individually, some authors argue that they should be regulated as a class (e.g., Kwiatkowski et al. 2020). Others suggest that, because of the differences in toxicity among PFAS species, each PFAS subgroup and compounds within should be evaluated (Singh and Papanastasiou 2021). Cousins et al. (2020) argued that a rational approach to phasing out PFAS production would eliminate most applications but would recognize certain “essential uses.” Eliminating most uses but allowing exceptions for the most essential, such as certain medical applications, would likely be the most feasible approach.
Because of the extreme strength of the carbon–fluorine bonds that are the defining feature of PFAS, it is notably difficult to fully mineralize PFAS (that is, to break PFAS down to their elemental components rather than to smaller species of PFAS) (Shahsavari et al. 2021). Sorption of PFAS can block the movement of contaminants such as PFAS in the ecosystem. Porous, high-carbon materials such as activated carbon and biochar can be used to remove PFAS from drinking water (Box 6-1; Xiao et al. 2017). Granulated activated carbon is the most widely used sorbent for purification of air and water.Fecal biochar (e.g., made from biosolids) has been shown to be an effective sorbent for PFAS (Krahn et al. 2023).
BOX 6-1
Biochar as Decontamination Possibility.
That said, it is possible to degrade PFAS in soil and in amendments that might reach soil. A few alternatives are emerging, each with potential to address the issue in certain niches. Mechanochemical treatment by ball milling can be used to eliminate PFAS (Turner et al. 2021); this might be useful for extremely contaminated soils, though the committee finds it is difficult to envisage this being conducted at large scale in an economically viable way. Although bioremediation is challenging and generally not considered as a practical approach to PFAS remediation, there is hope that microbes can contribute to breaking down PFAS (LaFond et al. 2023). Another new biological approach involves use of enzyme-catalyzed oxidative humification reactions carried out by fungal extracellular enzymes (e.g., peroxidases and laccases). These processes can potentially lead to break down of certain PFAS molecules (Grgas et al. 2023; Kumar et al. 2023). Thermochemical transformation is an approach that has shown promise under certain conditions (Weber et al. 2023). Incineration can destroy PFAS but can lead to the emission of fluorinated byproducts (Stoiber et al. 2020).
There is evidence that pyrolysis at high temperatures (over 600°C) can be effective for removing PFAS from biosolids. Thoma et al. (2022) showed that 21 PFAS compounds detected in biosolid samples (ranging from 2 μg/kg–85 μg/kg) were undetectable in the biochar resulting from pyrolysis at 650°C. Kundu et al. (2021) demonstrated greater than 90-percent removal of PFOA and PFOS from sewage sludge after pyrolysis at temperatures between 500 and 600°C. McNamara et al. (2023b) showed more than 99-percent removal of target PFAS and PFAS precursors at pyrolysis temperatures up to 800°C. However, PFAS may be present in byproducts of pyrolysis (McNamara et al. 2023a,b). Further research and development is needed to assess the potential of pyrolysis as a general solution to the problem of PFAS in biosolids (Wallace et al. 2023). Other high-temperature treatments, plasma, and combustion under specific conditions can also be effective at breaking down PFAS (Singh et al. 2021; McNamara et al. 2023a,b; Weber et al. 2023). High-energy electron beam technology is also being tested to break down PFAS in soils and groundwater (Lassalle et al. 2021).
Integrated strategies for dealing with sources and flows of contamination are needed. These are likely to bring together elements of the strategies mentioned above, as well as selective mobilization of PFAS (Bolan et al. 2021). Figure 6-9 presents an overview of elements of remediation strategies that can be integrated.

FIGURE 6-9
Elements of a remediation strategy for PFAS. SOURCE: Reprinted from Journal of Hazardous Materials, 401, Bolan et al., “Remediation of Poly- and Perfluoroalkyl Substances (PFAS) Contaminated Soils–To Mobilize or to Immobilize or to Degrade?” (more...)
CONCLUSIONS
The properties of soil facilitate the capture and remediation of many contaminants. In some cases, the soil microbiome can mitigate the risk contaminants pose, such as through the attenuation of heavy metals via sequestration, ion efflux, and extracellular chelation (Hou et al. 2020). In other cases, the physical and chemical properties of soil can trap contaminants, making them unavailable to volatilize, leach into water, or be taken up by a plant. As alluded to in Chapter 3, the degradation of contaminants is one of many soil-derived Nature’s Contributions to People.
Soil health is key to this contribution. As reviewed in the case studies, soil organic amendments can increase the absorption of metals and organic chemical contaminants. Changes to pH can reduce the bioavailability of some contaminants. Conversely, contamination can overwhelm the capacity of soil to mitigate risk. High levels of lead and cadmium in soils reduce microbial activity and plant biomass. Microplastics can change soil structure and thereby affect water-holding capacity. The enrichment of pathogens and antibiotic resistance genes in soil microbial communities influenced by microplastics may have implications for human health, but this possibility has not yet been explored.
It is important to note that the contaminants in the case studies presented above were discussed in isolation from one another. Each contaminant class is diverse with heterogeneous impacts based on the nature and quantity of the material and the characteristics of the soil in question. The reality of soil contamination is even more complex because of the potential for co-contamination with multiple compounds. It is likely that the examples of contaminants provided here, along with contaminants found in agricultural inputs such as manure and synthetic fertilizers and pesticides, interact with one another in ways that compound the adverse effects on soil health.
Furthermore, these interacting contaminants are affected by global change factors, such as water stress and high temperatures (Rillig et al. 2019c). There is little available evidence about the effects of many combinations of soil contaminants, in part because it is prohibitively difficult to systematically test the combinatorial effects of large numbers of anything, including contaminants and stressors affecting soils. Given the practical impossibility of testing large numbers of specific combinations, Yang et al. (2022) tested a range of numbers of soil stressors under experimental reactor conditions, drawing at random from a roster of stressors that included various soil contaminants. They found that the positive ecosystem functions of soil microbial diversity were systematically compromised by the action of multiple environmental stressors, including heavy metals, pesticides, plastic film residues, and surfactants as well as nitrogen deposition, heat, drought, salinity, and compaction.
Soil plays a vital role in the cycling of nutrients, where products that are waste to humans, such as manure and biosolids, can be used as sources of energy and nutrients for soil biota and plants. However, soil can no longer be a receptacle for the untreated waste products of agricultural and industrial processes. To incorporate considerations about soil health into decisions about material processing and disposal, the committee suggests that action be taken in the following areas.
Source Identification and Targeted Surveillance
Soil contaminants have not been strategically mapped in the United States. As part of its North American Soil Geochemical Landscapes Project, the U.S. Geological Survey (USGS) mapped the spatial distribution of lead, arsenic, and cadmium (among other chemical elements) in the conterminous United States (Smith et al. 2014), but this low-density effort (one sample site per 1,600 km2) did not target agricultural lands. USGS has also recently conducted a survey of PFAS in the United States, but collectionwas from tap water and soils were not included in the survey (Smalling et al. 2023). To the committee’s knowledge, no comprehensive sampling effort of microplastics in U.S. soils has taken place.
Thus, there is a lack of comprehensive knowledge regarding the geographic distribution of these contaminants in U.S. soils and the specifics of their co-occurrence in mixed forms. This gap in understanding underscores the need for more detailed research and mapping of soil contaminants to better address soil and environmental health challenges.
Recommendation 6-1: Federal agencies should work collaboratively to support surveys of soil chemical contaminants informed by systematic risk assessments to identify where contaminant levels in soil may be particularly high (e.g., locations around, downwind, or downstream of PFAS point sources). These surveys can be used to build contaminant maps (e.g., of lead, arsenic, persistent organic pollutants) that can be viewed individually or overlaid to assess the status of contamination, identify locations of concern, and, over time, evaluate the effectiveness of interventions.
Exposure Science
The effects of heavy metals on the health of soil organisms, plants, and humans have been studied for decades. Research on the same effects of novel entities such as PFAS and microplastics is just ramping up. There is still a great deal to learn about the effects of new and old chemical contaminants on soil organisms, plants, and humans, including thresholds of exposure and compounding effects of more than one contaminant. The degree to which exposure routes from soil (e.g., through inhalation, dermal contact, or direct or food consumption) affect bioavailability and how they compare to exposure routes from other sources (e.g., water or personal care products) are also unknown.
Recommendation 6-2: Federal agencies should support interdisciplinary research to reduce gaps in knowledge about exposure pathways from soil and the compounding health effects on soil biota, plants, and people from exposure to multiple chemical contaminants.
Mitigation
The emergence of PFAS and microplastics as contaminants of concern this century and their interplay with contaminants from the last century that have yet to be addressed indicate that contaminant issues will continue to mount unless explicitly curtailed. A recent report by the National Academies on the U.S. role in ocean plastic waste recommended that “the United States should substantially reduce solid waste generation (absolute and per person) to reduce plastic waste in the environment and the environmental, economic, aesthetic, and health costs of managing waste and litter” (NASEM 2022b, 6). The committee of this report on soil health and human health wholeheartedly endorses this recommendation for reduced plastic use; this includes in agricultural production systems, as has been called for by the FAO. Use of plastic in agriculture needs to be substantially reduced by finding alternatives as well as reusing it when possible, removing it after use if persistent, stopping use of forms with toxic byproducts, and developing biodegradable plastics.
Reducing the production and use of other contaminants, such as PFAS, for all but the most essential applications, is also in order. The committee recognizes that soil is not the only exposure pathway for these contaminants to humans, but the extent to which production of these products is reduced mitigates all exposure pathways to humans and decreases their potential to enter soil.
Recommendation 6-3: The United States should mitigate the entry of plastic and PFAS contaminants into soil by reducing their overall production and use.
Biosolids can be an important source of organic matter to agricultural land and a means of recycling rather than disposing of waste. However, they can also be a route of soil contamination because water treatment processes leave heavy metals, PFAS, microplastics, and other contaminants in biosolids. The entry of contaminants into soil could also be mitigated by improvements to the processing of landfill leachate and the treatment of wastewater. Landfill leachate contains PFAS, microplastics, and many other contaminants from discarded materials, which includes municipal biosolids. Wastewater treatment plants vary in their ability to remove these contaminants during treatment. Processing landfill leachate before it enters wastewater treatment plants would be a first line of contaminant reduction.
Some PFAS precursors may be broken down in the wastewater treatment process, but the strength of the carbon–fluorine bond often means that treated water has more, shorter-chain PFAS molecules than the influent. Technology to remove PFAS during treatment needs to be advanced. Limited research has reported pyrolysis of biosolids reduces PFAS content and possibly bioavailability. The U.S. Department of Agriculture–Natural Resources Conservation Services’ recent soil carbon amendment standard (code 336) does not support application of non-gasified or non-pyrolyzed biosolids, while the standard does allow land application of biosolids biochar. Research is needed to provide information on manufacture of biochar from biosolids and the ability of pyrolysis to reduce the content and bioavailability of PFAS.
The technology exists to effectively remove microplastics from wastewater. Unfortunately, most of the plastic ends up in the biosolids (Carr et al. 2016). Pyrolysis of biosolids can result in the elimination of plastics as well as PFAS and other organic pollutants because of the high temperature attained in the process. Converting biosolids into biochar would be a means of continuing to use waste as a soil amendment while mitigating the contamination of the soil with more plastic. Biochar can also immobilize other contaminants, including PFAS and heavy metals. The carbon in biochar is stable over long periods of time, so it also has the benefit of sequestering carbon.
Recommendation 6-4: EPA should continue pursuing research and technology to remove PFAS from wastewater and biosolids.
Recommendation 6-5: EPA should pursue research to establish a threshold for plastics in land-applied soil amendments. Revisiting heavy metal thresholds would also be in order.
Remediation
Soils, especially those rich in organic matter and with healthy microbial communities, have the capacity to absorb and eliminate (e.g., through bioremediation) some, but not all, contaminants. Enhancing soil’s ability to remediate contaminants is crucial for protecting human health. Using soil organic amendments to increase organic matter as well as recycle waste can move agricultural production to a more circular approach to nutrient management. Wastes that can serve as sources of soil organic matter include food waste, agroindustrial byproducts, and human and animal excreta. The use of source-separating, container-based sanitation can enable the recycling of carbon and nutrients in excreta without the contamination risks that arise in conventional wastewater handling.
Organic amendments can be designed to be more targeted. Modified biochars with functionalized surface chemistry (“designer biochars”) can reduce contaminant bioavailability. For example, including iron in biochar production can enable greater binding of heavy metals for soil remediation while improving water holding and nutrient cycling. Research is needed to identify or produce functionalized designed biochars for soil remediation.
Recommendation 6-6: Public sector investment should be made to develop affordable technologies for converting biosolids into biochar that can be applied to agricultural land and/or used for wastewater treatment.
Recommendation 6-7: Producers and other land managers should adopt practices that increase the organic matter content, biodiversity, and other health parameters of their soils.
Recommendation 6-8: Public and private entities should invest in Green and Sustainable Remediation techniques, including the application of designer biochars and biosolids biochar, to manage soil contamination effectively.
Footnotes
- 1
The 2022 Substance Priority List of the U.S. Department of Health and Human Services’ Agency for Toxic Substances and Disease Registry. Accessed January 22, 2024. https://www
.atsdr.cdc .gov/spl/index.html#2022spl. - 2
U.S. Environmental Protection Agency. “PFAS Structure Dashboard.” EPA. Accessed April 27, 2024. https://comptox
.epa.gov /dashboard/chemical-lists/PFASSTRUCT and “PFAS Developmental Dashboard.” EPA. Accessed April 27, 2024. https://comptox .epa.gov /dashboard/chemical-lists/PFASDEV1. - 3
U.S. Environmental Protection Agency. “Risk Assessment for Pollutants in Biosolids.” EPA. Accessed April 27, 2024. https://www
.epa.gov/biosolids /risk-assessment-pollutants-biosolids#pfas.
REFERENCES
- 3M. December 20, 2022. [April 29, 2024]. “3M to Exit PFAS Manufacturing by the End of 2025,” https://news
.3m.com/2022-12-20-3M-to-Exit-PFAS-Manufacturing-by-the-End-of-2025 . - Albertsson AC. “The Shape of the Biodegradation Curve for Low and High Density Polyethenes in Prolonged Series of Experiments” European Polymer Journal. 1980;16(7):623–630. https://doi
.org/10.1016 /0014-3057(80)90100-7 . - Alengebawy Ahmed, Taha Abdelkhalek Sara, Rana Qureshi Sundas, Wang Man-Qun. “Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications” Toxics. 2021;9(3):42. https://doi
.org/10.3390%2Ftoxics9030042 . [PMC free article: PMC7996329] [PubMed: 33668829] - Ali MuhammadIshtiaq, Ahmed Safia, Robson Geoff, Javed Imran, Ali Naeem, Atiq Naima, Hameed Abdul. “Isolation and Molecular Characterization of Polyvinyl Chloride (PVC) Plastic Degrading Fungal Isolates” Journal of Basic Microbiology. 2014;54(1):18–27. https://doi
.org/10.1002/jobm.201200496 . [PubMed: 23686796] - Al-Mamun Abdullah. “Pesticide Degradations, Residues and Environmental Concerns” In: Samad Khan Mohidus, Rahman Mohammad Shafiur, editors. Pesticides Residue in Foods: Sources, Management, and Control. Cham, Switzerland: Springer; 2017. pp. 87–102.
- Andrady AnthonyL, Neal MikeA. “Applications and Societal Benefits of Plastics” Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1526):1977–1984. https://doi
.org/10.1098/rstb.2008.0304 . [PMC free article: PMC2873019] [PubMed: 19528050] - Arkatkar Ambika, Arutchelvi J, Bhaduri Sumit, Veera Uppara Parasu, Doble Mukesh. “Degradation of Unpretreated and Thermally Pretreated Polypropylene by Soil Consortia” International Biodeterioration & Biodegradation. 2009;63(1):106–111. https://doi
.org/10.1016/j .ibiod.2008.06.005 . - ATSDR (Agency for Toxic Substances and Disease Registry). 2007. [May 2, 2024]. “ Toxicological Profile for Arsenic” https://www
.atsdr.cdc .gov/ToxProfiles/tp2.pdf . [PubMed: 37184170] - ATSDR. 2012. [May 2, 2024]. “ Toxicological Profile for Cadmium” https://www
.atsdr.cdc .gov/toxprofiles/tp5.pdf . [PubMed: 24049863] - ATSDR. 2020. [May 2, 2024]. “ Toxicological Profile for Lead” https://www
.atsdr.cdc .gov/toxprofiles/tp13.pdf . - Attanayake ChammiP, Hettiarachchi GangaM, Harms Ashley, Presley DeAnn, Martin Sabine, Pierzynski GaryM. “Field Evaluations on Soil Plant Transfer of Lead from an Urban Garden Soil” Journal of Environmental Quality. 2014;43(2):475–487. https://doi
.org/10.2134/jeq2013.07.0273 . [PubMed: 25602649] - Attanayake Chammi, Hettiarachchi Ganga, Martin Sabine, Pierzynski Gary. “Potential Bioavailability of Lead, Arsenic, and Polycyclic Aromatic Hydrocarbons in Compost-Amended Urban Soils” Journal of Environmental Quality. 2015;44:930–944. https://doi
.org/10.2134/jeq2014.09.0400 . [PubMed: 26024273] - Attanayake ChammiP, Hettiarachchi GangaM, Palomo Monica, Pierzynski GaryM, Calderon Blanca. “Phytoavailability of Lead for Vegetables in Urban Garden Soils” ACS Agricultural Science & Technology. 2021;1(3):173–181. https://doi
.org/10.1021/acsagscitech .0c00068 . - Basta NicholasT, Juhasz Albert. “Using In Vivo Bioavailability and/or In Vitro Gastrointestinal Bioaccessibility Testing to Adjust Human Exposure to Arsenic from Soil Ingestion” Reviews in Mineralogy and Geochemistry. 2014;79(1):451–472. https://doi
.org/10.2138/rmg.2014.79.9 . - Basta NT, Ryan JA, Chaney RL. “Trace Element Chemistry in Residual-Treated Soil: Key Concepts and Metal Bioavailability” Journal of Environmental Quality. 2005;34(1):49–63. https://doi
.org/10.2134/jeq2005.0049dup . [PubMed: 15647534] - Basta NT, Raun WR, Gavi F. “Wheat Grain Cadmium Under Long-Term Fertilization and Continuous Winter Wheat Production” Better Crops. 1998;82(2)
- Bellinger DavidC. “Teratogen Update: Lead and Pregnancy” Birth Defects Research Part A: Clinical and Molecular Teratology. 2005;73(6):409–420. https://doi
.org/10.1002/bdra.20127 . [PubMed: 15880700] - Bihari Enikoe, Grewal Garrett, Richter DanielD. “Legacies of Pre-1960s Municipal Waste Incineration in the Pb of City Soils” Environmental Science & Technology Letters. 2023;10(10):897–902. https://doi
.org/10.1021/acs .estlett.3c00488 . [PMC free article: PMC10569037] [PubMed: 37840814] - Blackburn Kirsty, Green Dannielle. “The Potential Effects of Microplastics on Human Health: What Is Known and What Is Unknown” Ambio. 2022;51(3):518–530. https://doi
.org/10.1007 /s13280-021-01589-9 . [PMC free article: PMC8800959] [PubMed: 34185251] - Bläsing Melanie, Amelung Wulf. “Plastics in Soil: Analytical Methods and Possible Sources” Science of the Total Environment. 2018;612:422–435. https://doi
.org/10.1016/j .scitotenv.2017.08.086 . [PubMed: 28863373] - Blaylock MichaelJ, Salt DavidE, Dushenkov Slavik, Zakharova Olga, Gussman Christopher, Kapulnik Yoram, Ensley BurtD, Raskin Ilya. “Enhanced Accumulation of Pb in Indian Mustard by Soil-Applied Chelating Agents” Environmental Science & Technology. 1997;31(3):860–865. https://doi
.org/10.1021/es960552a . - Bolan Nanthi, Kirkham MB, Halsband Claudia, Nugegoda Dayanthi, Sik Ok Yong. Particulate Plastics in Terrestrial and Aquatic Environments. Boca Raton, FL: CRC Press; 2020.
- Bolan Nanthi, Kunhikrishnan Anitha, Thangarajan Ramya, Kumpiene Jurate, Park Jinhee, Makino Tomoyuki, Beth Kirkham Mary, Scheckel Kirk. “Remediation of Heavy Metal(loid)s Contaminated Soils – To Mobilize or to Immobilize?” Journal of Hazardous Materials. 2014;266:141–166. https://doi
.org/10.1016/j .jhazmat.2013.12.018 . [PubMed: 24394669] - Bolan Nanthi, Sarkar Binoy, Yan Yubo, Li Qiao, Wijesekara Hasintha, Kannan Kurunthachalam, Tsang DanielCW, Schauerte Marina, Bosch Julian, Noll Hendrik, Sik Ok Yong, Scheckel Kirk, Kumpiene Jurate, Gobindlal Kapish, Kah Melanie, Sperry Jonathan, Kirkham MB, Wang Hailong, Fai Tsang Yiu, Hou Deyi, Rinklebe Jörg. “Remediation of Poly- and Perfluoroalkyl Substances (PFAS) Contaminated Soils – To Mobilize or to Immobilize or to Degrade?” Journal of Hazardous Materials. 2021;401:123892. https://doi
.org/10.1016/j .jhazmat.2020.123892 . [PMC free article: PMC8025151] [PubMed: 33113753] - Boots Bas, William Russell Connor, Senga Green Dannielle. “Effects of Microplastics in Soil Ecosystems: Above and Below Ground” Environmental Science & Technology. 2019;53(19):11496–11506. https://doi
.org/10.1021/acs.est.9b03304 . [PubMed: 31509704] - Brase RichardA, Mullin ElizabethJ, Spink DavidC. “Legacy and Emerging Per- and Polyfluoroalkyl Substances: Analytical Techniques, Environmental Fate, and Health Effects” International Journal of Molecular Sciences. 2021;22(3):995. https://doi
.org/10.3390/ijms22030995 . [PMC free article: PMC7863963] [PubMed: 33498193] - Brown Sally, Chaney Rufus, Hallfrisch Judith, Ryan JamesA, Berti WilliamR. “In Situ Soil Treatments to Reduce the Phyto- and Bioavailability of Lead, Zinc, and Cadmium” Journal of Environmental Quality. 2004;33(2):522–531. https://doi
.org/10.2134/jeq2004.5220 . [PubMed: 15074803] - Brunn Hubertus, Arnold Gottfried, Körner Wolfgang, Rippen Gerd, Günter Steinhäuser Klaus, Valentin Ingo. “PFAS: Forever Chemicals—Persistent, Bioaccumulative and Mobile. Reviewing the Status and the Need for Their Phase Out and Remediation of Contaminated Sites” Environmental Sciences Europe. 2023;35(1):20. https://doi
.org/10.1186 /s12302-023-00721-8 . - Brusseau MarkL, Hunter Anderson R, Guo Bo. “PFAS Concentrations in Soils: Background Levels Versus Contaminated Sites” Science of the Total Environment. 2020;740:140017. https://doi
.org/10.1016/j .scitotenv.2020.140017 . [PMC free article: PMC7654437] [PubMed: 32927568] - Buck RobertC, Franklin James, Berger Urs, Conder JasonM, Cousins IanT, Voogt Pimde, Astrup Jensen Allan, Kannan Kurunthachalam, Mabury ScottA, van Leeuwen StefanPJ. “Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins” Integrated Environmental Assessment and Management. 2011;7(4):513–541. https://doi
.org/10.1002/ieam.258 . [PMC free article: PMC3214619] [PubMed: 21793199] - Büks Frederick, Kaupenjohann Martin. “Global Concentrations of Microplastics in Soils – A Review” SOIL. 2020;6(2):649–662. https://doi
.org/10.5194/soil-6-649-2020 . - Cai Yanping, Chen Huilun, Yuan Rongfang, Wang Fei, Chen Zhongbing, Zhou Beihai. “Toxicity of Perfluorinated Compounds to Soil Microbial Activity: Effect of Carbon Chain Length, Functional Group and Soil Properties” Science of the Total Environment. 2019;690:1162–1169. https://doi
.org/10.1016/j .scitotenv.2019.06.440 . [PubMed: 31470479] - Capozzi StaciL, Leang AmyL, Rodenburg LisaA, Chandramouli Bharat, Delistraty DamonA, Carter ColeH. “PFAS in Municipal Landfill Leachate: Occurrence, Transformation, and Sources” Chemosphere. 2023;334:138924. https://doi
.org/10.1016/j .chemosphere.2023.138924 . [PubMed: 37209854] - Carr SteveA, Liu Jin, Tesoro ArnoldG. “Transport and Fate of Microplastic Particles in Wastewater Treatment Plants” Water Research. 2016;91:174–182. https://doi
.org/10.1016/j .watres.2016.01.002 . [PubMed: 26795302] - Carrijo DanielaR, LaHue GabrielT, Parikh SanjaiJ, Chaney RufusL, Linquist BruceA. “Mitigating the Accumulation of Arsenic and Cadmium in Rice Grain: A Quantitative Review of the Role of Water Management” Science of the Total Environment. 2022;839:156245. https://doi
.org/10.1016/j .scitotenv.2022.156245 . [PubMed: 35644407] - Chamas Ali, Moon Hyunjin, Zheng Jiajia, Qiu Yang, Tabassum Tarnuma, Hee Jang Jun, Abu-Omar Mahdi, Scott SusannahL, Suh Sangwon. “Degradation Rates of Plastics in the Environment” ACS Sustainable Chemistry & Engineering. 2020;8(9):3494–3511. https://doi
.org/10.1021/acssuschemeng .9b06635 . - Chambers WestonS, Hopkins JaidaG, Richards SeanM. “A Review of Per- and Polyfluorinated Alkyl Substance Impairment of Reproduction” Frontiers in Toxicology. 2021;3 https://doi
.org/10.3389/ftox.2021.732436 . [PMC free article: PMC8915888] [PubMed: 35295153] - Chaney RufusL, Reeves RogerD, Baklanov IlyaA, Tiziana Centofanti CLeighBroadhurst, Baker AlanJM, Van der Ent Antony, Roseberg RichardJ. “Phytoremediation and Phytomining: Using Plants to Remediate Contaminated or Mineralized Environments” In: Rajakaruna R, Boyd RS, Harris T, editors. Plant Ecology and Evolution in Harsh Environments. Hauppauge, NY: Nova Science Publishers; 2014. pp. 365–392.
- Chaney RufusL, Ryan JamesA. DECHEMA; Risk Based Standards for Arsenic, Lead and Cadmium in Urban Soils: Summary of Information and Methods Developed to Estimate Standards for Cd, Pb and As in Urban Soils. 1994
- Chen Shan, Yang Jin-Lei, Zhang Yao-Sheng, Wang Hong-Yu, Lin Xin-Ying, Xue Rong-Yue, Li Meng-Ya, Li Shi-Wei, Juhasz AlbertL, Ma LenaQ, Zhou Dong-Mei, Li Hong-Bo. “Microplastics Affect Arsenic Bioavailability by Altering Gut Microbiota and Metabolites in a Mouse Model” Environmental Pollution. 2023;324:121376. https://doi
.org/10.1016/j .envpol.2023.121376 . [PubMed: 36863442] - Coggan TimothyL, Moodie Damien, Kolobaric Adam, Szabo Drew, Shimeta Jeff, Crosbie NicholasD, Lee Elliot, Fernandes Milena, Clarke BradleyO. “An Investigation into Per- and Polyfluoroalkyl Substances (PFAS) in Nineteen Australian Wastewater Treatment Plants (WWTPs)” Heliyon. 2019;5(8):e02316. https://doi
.org/10.1016/j .heliyon.2019.e02316 . [PMC free article: PMC6716228] [PubMed: 31485522] - Conti GeaOliveri, Ferrante Margherita, Banni Mohamed, Favara Claudia, Nicolosi Ilenia, Cristaldi Antonio, Fiore Maria, Zuccarello Pietro. “Micro- and Nano-plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population” Environmental Research. 2020;187:109677. https://doi
.org/10.1016/j .envres.2020.109677 . [PubMed: 32454310] - Costello MChristinaSchilling, Lee LindaS. Current Pollution Reports. 2020. “Sources, Fate, and Plant Uptake in Agricultural Systems of Per- and Polyfluoroalkyl Substances” https://doi
.org/10.1007 /s40726-020-00168-y . - Cousins IanT, DeWitt JamieC, Glüge Juliane, Goldenman Gretta, Herzke Dorte, Lohmann Rainer, Ng CarlaA, Scheringer Martin, Wang Zhanyun. “The High Persistence of PFAS Is Sufficient for Their Management as a Chemical Class” Environmental Science: Processes & Impacts. 2020;22(12):2307–2312. https://doi
.org/10.1039/D0EM00355G . [PMC free article: PMC7784706] [PubMed: 33230514] - Crossman Jill, Hurley RachelR, Futter Martyn, Nizzetto Luca. “Transfer and Transport of Microplastics from Biosolids to Agricultural Soils and the Wider Environment” Science of the Total Environment. 2020;724:138334. https://doi
.org/10.1016/j .scitotenv.2020.138334 . [PubMed: 32408466] - Daghighi Elaheh, Shah Tufail, Chia RW, Lee Jin-Yong, Shang Jianying, RodríguezSeijo Andrés. “The Forgotten Impacts of Plastic Contamination on Terrestrial Micro- and Mesofauna: A Call for Research” Environmental Research. 2023;231:116227. https://doi
.org/10.1016/j .envres.2023.116227 . [PubMed: 37244494] - de Souza Machado AndersonAbel, Lau ChungW, Kloas Werner, Bergmann Joana, Bachelier JulienB, Faltin Erik, Becker Roland, Görlich AnnaS, Rillig MatthiasC. “Microplastics Can Change Soil Properties and Affect Plant Performance” Environmental Science & Technology. 2019;53(10):6044–6052. https://doi
.org/10.1021/acs.est.9b01339 . [PubMed: 31021077] - Death Clare, Bell Cameron, Champness David, Milne Charles, Reichman Suzie, Hagen Tarah. “Per- and Polyfluoroalkyl Substances (PFAS) in Livestock and Game Species: A Review” Science of the Total Environment. 2021;774:144795. https://doi
.org/10.1016/j .scitotenv.2020.144795 . [PubMed: 33609849] - DeLuca NicoleM, Minucci JeffreyM, Mullikin Ashley, Slover Rachel, Cohen Hubal ElaineA. “Human Exposure Pathways to Poly- and Perfluoroalkyl Substances (PFAS) from Indoor Media: A Systematic Review” Environment International. 2022;162:107149. https://doi
.org/10.1016/j .envint.2022.107149 . [PMC free article: PMC11577573] [PubMed: 35240384] - Diamond GaryL, Bradham KarenD, Brattin WilliamJ, Burgess Michele, Griffin Susan, Hawkins CherylA, Juhasz AlbertL, Klotzbach JulieM, Nelson Clay, Lowney YvetteW, Scheckel KirkG, Thomas DavidJ. “Predicting Oral Relative Bioavailability of Arsenic in Soil from In Vitro Bioaccessibility” Journal of Toxicology and Environmental Health, Part A. 2016;79(4):165–173. https://doi
.org/10.1080/15287394 .2015.1134038 . [PubMed: 27029599] - Domingo JoséL, Nadal Martí. “Per- and Polyfluoroalkyl Substances (PFASs) in Food and Human Dietary Intake: A Review of the Recent Scientific Literature” Journal of Agricultural and Food Chemistry. 2017;65(3):533–543. https://doi
.org/10.1021/acs.jafc.6b04683 . [PubMed: 28052194] - Dong Xiaoxi, Shulzhenko Natalia, Lemaitre Julien, Greer ReneeL, Peremyslova Kate, Quamruzzaman Quazi, Rahman Mahmudar, Sharif Ibn Hasan Omar, Afroz Joya Sakila, Golam Mostofa, Christiani DavidC, Morgun Andriy, Kile MollyL. “Arsenic Exposure and Intestinal Microbiota in Children from Sirajdikhan, Bangladesh” PLOS ONE. 2017;12(12):e0188487. https://doi
.org/10.1371/journal .pone.0188487 . [PMC free article: PMC5718612] [PubMed: 29211769] - Drexler JW, Brattin WJ. “An In Vitro Procedure for Estimation of Lead Relative Bioavailability: With Validation” Human and Ecological Risk Assessment: An International Journal. 2007;13(2):383–401. https://doi
.org/10.1080 /10807030701226350 . - Ellis LyndaBM, Wackett LawrenceP. “Use of the University of Minnesota Biocatalysis/Biodegradation Database for Study of Microbial Degradation” Microbial Informatics and Experimentation. 2012;2(1):1. https://doi
.org/10.1186/2042-5783-2-1 . [PMC free article: PMC3351732] [PubMed: 22587916] - EPA (U.S. Environmental Protection Agency). n.d. [May 2, 2024]. “PFAS Strategic Roadmap: EPA’s Commitments to Action 2021-2024.” https://www
.epa.gov/system /files/documents /2021-10/pfas-roadmap_final-508.pdf . - EPA. May 16, 2000. “EPA and 3M Announce Phase Out of PFOS,” https://www
.epa.gov/archive /epapages/newsroom_archive /newsreleases /33aa946e6cb11f35852568e1005246b4 .html . - EPA. “ PFAS National Primary Drinking Water Regulation Rulemaking” Federal Register. 2024a;89(82):32532–32757.
- EPA. 2024b. [April 24, 2024]. “ Questions & Answers: PFAS National Primary Drinking Water Regulation” https://www
.epa.gov/system /files/documents /2024-04/pfasnpdwr_qa_general_4 .9.24v1.pdf . - Evich MarinaG, Davis MaryJB, McCord JamesP, Acrey Brad, Awkerman JillA, Knappe DetlefRU, Lindstrom AndrewB, Speth ThomasF, Tebes-Stevens Caroline, Strynar MarkJ, Wang Zhanyun, Weber EricJ, Matthew Henderson W, Washington JohnW. “Per- and Polyfluoroalkyl Substances in the Environment” Science. 2022;375(6580):eabg9065. [PMC free article: PMC8902460] [PubMed: 35113710]
- Eyles JE, Bramwell VW, Williamson ED, Alpar HO. “Microsphere Translocation and Immunopotentiation in Systemic Tissues Following Intranasal Administration” Vaccine. 2001;19(32):4732–4742. https://doi
.org/10.1016 /s0264-410x(01)00220-1 . [PubMed: 11535324] - Fackelmann Gloria, Sommer Simone. “Microplastics and the Gut Microbiome: How Chronically Exposed Species May Suffer from Gut Dysbiosis” Marine Pollution Bulletin. 2019;143:193–203. https://doi
.org/10.1016/j .marpolbul.2019.04.030 . [PubMed: 31789155] - FAO (Food and Agriculture Organization of the United Nations). Paper presented at International Year of Soils, 2015. Rome, Italy: 2015. [April 15, 2024]. “ Impacts of Soil Contaminants on Key Soil Functions” https://www
.fao.org/global-soil-partnership /resources/communication-material/en/ - FAO. Assessment of Agricultural Plastics and Their Sustainability – A Call for Action. Rome: 2021.
- FAO and UNEP (Food and Agriculture Organization of the United Nations and United Nations Environment Programme). Global Assessment of Soil Pollution: Report. Rome: 2021. https://doi
.org/10.4060/cb4894en . - Farhat YasmineA, Kim Soo-Hyung, Seyfferth AngeliaL, Zhang Long, Neumann RebeccaB. “Altered Arsenic Availability, Uptake, and Allocation in Rice Under Elevated Temperature” Science of the Total Environment. 2021;763:143049. https://doi
.org/10.1016/j .scitotenv.2020.143049 . [PubMed: 33153749] - FDA (U.S. Food and Drug Administration). 2020. [April 17, 2024]. “ Inorganic Arsenic in Rice Cereals for Infants: Action Level Guidance for Industry” https://www
.fda.gov/media /97234/download?attachment . - FDA. 2023. [April 17, 2024]. “ Guidance for Industry: Action Level for Inorganic Arsenic in Apple Juice” https://www
.fda.gov/regulatory-information /search-fda-guidance-documents /guidance-industry-action-level-inorganic-arsenic-apple-juice . - Forsberg CW. “Some Effects of Arsenic on the Rumen Microflora; An In Vitro Study” Canadian Journal of Microbiology. 1978;24(1):36–44. https://doi
.org/10.1139/m78-007 . [PubMed: 754875] - Galati Antonino, Scalenghe Riccardo. “Plastic End-of-Life Alternatives, with a Focus on the Agricultural Sector” Current Opinion in Chemical Engineering. 2021;32:100681. https://doi
.org/10.1016/j .coche.2021.100681 . - Gao Junfeng, Ellis LyndaBM, Wackett LawrenceP. “The University of Minnesota Biocatalysis/Biodegradation Database: Improving Public Access” Nucleic Acids Research. 2010;38(Suppl 1):D488–D491. https://doi
.org/10.1093/nar/gkp771 . [PMC free article: PMC2808978] [PubMed: 19767608] - Gao Ke, Zhuang Taifeng, Liu Xian, Fu Jianjie, Zhang Jingxing, Fu Jie, Wang Liguo, Zhang Aiqian, Liang Yong, Song Maoyong, Jiang Guibin. “Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFASs) and Association between the Placental Transfer Efficiencies and Dissociation Constant of Serum Proteins–PFAS Complexes” Environmental Science & Technology. 2019;53(11):6529–6538. https://doi
.org/10.1021/acs.est.9b00715 . [PubMed: 31099564] - Gebbink WouterA, van Leeuwen StefanPJ. “Environmental Contamination and Human Exposure to PFASs near a Fluorochemical Production Plant: Review of Historic and Current PFOA and GenX Contamination in the Netherlands” Environment International. 2020;137:105583. https://doi
.org/10.1016/j .envint.2020.105583 . [PubMed: 32106048] - Glorennec P, Peyr C, Poupon J, Oulhote Y, Le Bot B. “Identifying Sources of Lead Exposure for Children, with Lead Concentrations and Isotope Ratios” JournalofOccupational and Environmental Hygiene. 2010;7(5):253–260. https://doi
.org/10.1080 /15459621003648281 . [PubMed: 20182944] - Gokulan Kuppan, Arnold MatthewG, Jensen Jake, Vanlandingham Michelle, Twaddle NathanC, Doerge DanielR, Cerniglia CarlE, Khare Sangeeta. “Exposure to Arsenite in CD-1 Mice during Juvenile and Adult Stages: Effects on Intestinal Microbiota and Gut-Associated Immune Status” mBio. 2018;9(4) https://doi
.org/10.1128/mbio.01418-18 . [PMC free article: PMC6094480] [PubMed: 30108172] - Gomis MelissaI, Vestergren Robin, MacLeod Matthew, Mueller JochenF, Cousins IanT. “Historical Human Exposure to Perfluoroalkyl Acids in the United States and Australia Reconstructed from Biomonitoring Data Using Population-Based Pharmacokinetic Modelling” Environment International. 2017;108:92–102. https://doi
.org/10.1016/j .envint.2017.08.002 . [PubMed: 28818713] - Gong Yanyan, Zhao Dongye, Wang Qilin. “An Overview of Field-Scale Studies on Remediation of Soil Contaminated with Heavy Metals and Metalloids: Technical Progress over the Last Decade” Water Research. 2018;147:440–460. https://doi
.org/10.1016/j .watres.2018.10.024 . [PubMed: 30343201] - Grgas Dijana, Petrina Ana, Štefanac Tea, Bešlo Drago, Dragičević Tibela Landeka. “A Review: Per- and Polyfluoroalkyl Substances—Biological Degradation.” Toxics. 2023;11(5):446. https://www
.mdpi.com/2305-6304/11/5/446 . [PMC free article: PMC10220634] [PubMed: 37235260] - Griggs JenniferL, Chi Liang, Hanley NancyM, Kohan Michael, Herbin-Davis Karen, Thomas DavidJ, Lu Kun, Fry RebeccaC, Bradham KarenD. “Bioaccessibility of Arsenic from Contaminated Soils and Alteration of the Gut Microbiome in an In Vitro Gastrointestinal Model” Environmental Pollution. 2022;309:119753. https://doi
.org/10.1016/j .envpol.2022.119753 . [PMC free article: PMC9667710] [PubMed: 35835276] - Gutiérrez Carmen, Fernández Carlos, Escuer Miguel, Campos-Herrera Raquel, Eulalia Beltrán Rodríguez Ma, Carbonell Gregoria, Antonio Rodríguez Martín Jose. “Effect of Soil Properties, Heavy Metals and Emerging Contaminants in the Soil Nematodes Diversity” Environmental Pollution. 2016;213:184–194. https://doi
.org/10.1016/j .envpol.2016.02.012 . [PubMed: 26895540] - He Defu, Luo Yongming, Lu Shibo, Liu Mengting, Song Yang, Lei Lili. “Microplastics in Soils: Analytical Methods, Pollution Characteristics and Ecological Risks” TrAC Trends in Analytical Chemistry. 2018;109:163–172. https://doi
.org/10.1016/j .trac.2018.10.006 . - Helmberger MaxwellS, Tiemann LisaK, Grieshop MatthewJ. “Towards an Ecology of Soil Microplastics” Functional Ecology. 2020;34(3):550–560. https://doi
.org/10.1111/13652435.13495 . - Helmer RossW, Reeves DonaldM, Cassidy DanielP. “Per- and Polyfluorinated Alkyl Substances (PFAS) Cycling within Michigan: Contaminated Sites, Landfills and Wastewater Treatment Plants” Water Research. 2022;210:117983. https://doi
.org/10.1016/j .watres.2021.117983 . [PubMed: 34954365] - Henry Heather, Naujokas MarisaF, Attanayake Chammi, Basta NicholasT, Cheng Zhongqi, Hettiarachchi GangaM, Maddaloni Mark, Schadt Christopher, Scheckel KirkG. “Bioavailability-Based In Situ Remediation to Meet Future Lead (Pb) Standards in Urban Soils and Gardens” Environmental Science & Technology. 2015;49(15):8948–8958. https://doi
.org/10.1021/acs.est.5b01693 . [PubMed: 26140328] - Hettiarachchi GangaM, Betts AaronR, Chandima Wekumbura WG, Lake Loryssa, Mayer ManfredM, Scheckel KirkG, Basta NicholasT. “Chapter 6 - Lead: The Most Extensively Spread Toxic Environmental Contaminant” In: Naidu Ravi, editor. Inorganic Contaminants and Radionuclides. Amsterdam: Elsevier; 2023. pp. 113–150.
- Hoen AnneG, Madan JulietteC, Li Zhigang, Coker Modupe, Lundgren SaraN, Morrison HilaryG, Palys Thomas, Jackson BrianP, Sogin MitchellL, Cottingham KathrynL, Karagas MargaretR. “Sex-Specific Associations of Infants’ Gut Microbiome with Arsenic Exposure in a US Population” Scientific Reports. 2018;8(1):12627. https://doi
.org/10.1038 /s41598-018-30581-9 . [PMC free article: PMC6105615] [PubMed: 30135504] - Hoffman TC, Zitomer DH, McNamara PJ. “Pyrolysis of Wastewater Biosolids Significantly Reduces Estrogenicity” Journal of Hazardous Materials. 2016;317:579–584. https://doi
.org/10.1016/j .jhazmat.2016.05.088 . [PubMed: 27344259] - Hofmann Thilo, Ghoshal Subhasis, Tufenkji Nathalie, Franklin Adamowski Jan, Bayen Stéphane, Chen Qiqing, Demokritou Philip, Flury Markus, Hüffer Thorsten, Ivleva NataliaP, Ji Rong, Leask RichardL, Maric Milan, Mitrano DeniseM, Sander Michael, Pahl Sabine, Rillig MatthiasC, Walker TonyR, White JasonC, Wilkinson KevinJ. “Plastics Can Be Used More Sustainably in Agriculture” Communications Earth & Environment. 2023;4(1):332. https://doi
.org/10.1038 /s43247-023-00982-4 . - Hooge Asta, Hauggaard-Nielsen Henrik, Heinze WiebkeM, Lyngsie Gry, Ramos TiffanyM, Sandgaard MonicaH, Vollertsen Jes, Syberg Kristian. “Fate of Microplastics in Sewage Sludge and in Agricultural Soils” TrAC Trends in Analytical Chemistry. 2023;166:117184. https://doi
.org/10.1016/j .trac.2023.117184 . - Hou Deyi, O’Connor David, Igalavithana AvanthiD, Alessi DanielS, Luo Jie, Tsang DanielCW, Sparks DonaldL, Yamauchi Yusuke, Rinklebe Jörg, Sik Ok Yong. “Metal Contamination and Bioremediation of Agricultural Soils for Food Safety and Sustainability” Nature Reviews Earth & Environment. 2020;1(7):366–381. https://doi
.org/10.1038 /s43017-020-0061-y . - Huang Yi, Liu Qin, Jia Weiqian, Yan Changrong, Wang Jie. “Agricultural Plastic Mulching as a Source of Microplastics in the Terrestrial Environment” Environmental Pollution. 2020;260:114096. https://doi
.org/10.1016/j .envpol.2020.114096 . [PubMed: 32041035] - Hurley RachelR, Nizzetto Luca. “Fate and Occurrence of Micro(nano)plastics in Soils: Knowledge Gaps and Possible Risks” Current Opinion in Environmental Science & Health. 2018;1:6–11. https://doi
.org/10.1016/j .coesh.2017.10.006 . - Jin Yuanxiang, Lu Liang, Tu Wenqing, Luo Ting, Fu Zhengwei. “Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice” Science of the Total Environment. 2019;649:308–317. https://doi
.org/10.1016/j .scitotenv.2018.08.353 . [PubMed: 30176444] - Jury WilliamA, Ghodrati Masoud. “Overview of Organic Chemical Environmental Fate and Transport Modeling Approaches” In: Sawhney BL, Brown K, editors. Reactions and Movement of Organic Chemicals in Soils. Madison, WI: Soil Science Society of America, Inc.; 1989. pp. 271–304. https://doi
.org/10.2136/sssaspecpub22 .c11 . - Karbalaei Samaneh, Hanachi Parichehr, Walker TonyR, Cole Matthew. “Occurrence, Sources, Human Health Impacts and Mitigation of Microplastic Pollution” Environmental Science and Pollution Research. 2018;25(36):36046–36063. https://doi
.org/10.1007/s11356-0183508-7 . [PubMed: 30382517] - Kästner Matthias, Miltner Anja. “Application of Compost for Effective Bioremediation of Organic Contaminants and Pollutants in Soil” Applied Microbiology and Biotechnology. 2016;100 https://doi
.org/10.1007 /s00253-016-7378-y . [PubMed: 26921182] - Kavehei Armin, Hose GrantC, Gore DamianB. “Effects of Red Earthworms (Eisenia fetida) on Leachability of Lead Minerals in Soil” Environmental Pollution. 2018;237:851–857. https://doi
.org/10.1016/j .envpol.2017.11.021 . [PubMed: 29150255] - Khan Sardar, El-Latif Hesham Abd, Qiao Min, Rehman Shafiqur, He Ji-Zheng. “Effects of Cd and Pb on Soil Microbial Community Structure and Activities” Environmental Science and Pollution Research. 2010;17(2):288–296. https://doi
.org/10.1007 /s11356-009-0134-4 . [PubMed: 19333640] - Kluger Jeffrey. Time. 2023. [June 23, 2023]. “3M’s Historic $10 Billion ‘Forever Chemical’ Payout Is Just the Tip of the PFAS Iceberg” https://time
.com/6289893 /3m-foreverchemical-pfas-settlement/ - Kolvenbach BorisA, Helbling DamianE, Kohler Hans-PeterE, Corvini PhilippeFX. “Emerging Chemicals and the Evolution of Biodegradation Capacities and Pathways in Bacteria.” Current Opinion in Biotechnology. 2014;27:8–14. https://doi
.org/10.1016/j .copbio.2013.08.017 . [PubMed: 24863891] - Konopka A, Zakharova T, Bischoff M, Oliver L, Nakatsu C, Turco RF. “Microbial Biomass and Activity in Lead-Contaminated Soil” Applied and Environmental Microbiology. 1999;65(5):2256–2259. https://doi
.org/10.1128/AEM .65.5.2256-2259.1999 . [PMC free article: PMC91329] [PubMed: 10224032] - Krahn KatinkaM, Cornelissen Gerard, Castro Gabriela, Arp Hans Peter H, Asimakopoulos AlexandrosG, Wolf Raoul, Holmstad Rune, Zimmerman AndrewR, Sørmo Erlend. “Sewage Sludge Biochars as Effective PFAS-Sorbents.” Journal of Hazardous Materials. 2023;445:130449. https://doi
.org/10.1016/j .jhazmat.2022.130449 . [PubMed: 36459882] - Krounbi Leilah, Enders Akio, Es Haroldvan, Woolf Dominic, Herzen Brianvon, Lehmann Johannes. “Biological and Thermochemical Conversion of Human Solid Waste to Soil Amendments” Waste Management. 2019;89:366–378. https://doi
.org/10.1016/j .wasman.2019.04.010 . [PMC free article: PMC6538828] [PubMed: 31079750] - Krueger Martin, Harms Hauke, Schlosser Dietmar. “Prospects for Microbiological Solutions to Environmental Pollution with Plastics” Applied Microbiology and Biotechnology. 2015;99:8857–8874. https://doi
.org/10.1007 /s00253-015-6879-4 . [PubMed: 26318446] - Kumar Amit, Kumar Amit, Cabral-Pinto MarinaMS, Chaturvedi AshishK, Shabnam AftabA, Subrahmanyam Gangavarapu, Mondal Raju, Gupta Dipak Kumar, Malyan SandeepK, Kumar SmitaS, Khan ShakeelA, Yadav KrishnaK. “Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches.” International Journal of Environmental Research and Public Health. 2020;17(7):2179. https://doi
.org/10.3390/ijerph17072179 . [PMC free article: PMC7177270] [PubMed: 32218253] - Kumar Ravinder, Kassa Dada Tewodros, Whelan Anna, Cannon Patrick, Sheehan Madoc, Reeves Louise, Antunes Elsa. “Microbial and Thermal Treatment Techniques for Degradation of PFAS in Biosolids: A Focus on Degradation Mechanisms and Pathways” Journal of Hazardous Materials. 2023;452:131212. https://doi
.org/10.1016/j .jhazmat.2023.131212 . [PubMed: 36934630] - Kumpiene Jurate, Lagerkvist Anders, Maurice Christian. “Stabilization of As, Cr, Cu, Pb and Zn in Soil Using Amendments – A Review” Waste Management. 2008;28(1):215–225. https://doi
.org/10.1016/j .wasman.2006.12.012 . [PubMed: 17320367] - Kundu Sazal, Patel Savankumar, Halder Pobitra, Patel Tejas, Hedayati Marzbali Mojtaba, Kumar Pramanik Biplob, Paz-Ferreiro Jorge, Célio de Figueiredo Cícero, Bergmann David, Surapaneni Aravind, Megharaj Mallavarapu, Shah Kalpit. “Removal of PFASs from Biosolids Using a Semi-pilot Scale Pyrolysis Reactor and the Application of Biosolids Derived Biochar for the Removal of PFASs from Contaminated Water” Environmental Science: Water Research & Technology. 2021;7(3):638–649. https://doi
.org/10.1039/D0EW00763C . - Kwiatkowski CarolF, Andrews DavidQ, Birnbaum LindaS, Bruton ThomasA, DeWitt JamieC, Knappe DetlefRU, Maffini MaricelV, Miller MarkF, Pelch KatherineE, Reade Anna, Soehl Anna, Trier Xenia, Venier Marta, Wagner CharlotteC, Wang Zhanyun, Blum Arlene. “Scientific Basis for Managing PFAS as a Chemical Class” Environmental Science & Technology Letters. 2020;7(8):532–543. https://doi
.org/10.1021/acs .estlett.0c00255 . [PMC free article: PMC8297807] [PubMed: 34307722] - LaFond JessicaA, Hatzinger PaulB, Guelfo JenniferL, Millerick Kayleigh, Andrew Jackson W. “Bacterial Transformation of Per- and Poly-fluoroalkyl Substances: A Review for the Field of Bioremediation” Environmental Science: Advances. 2023;2(8):1019–1041. https://doi
.org/10.1039/D3VA00031A . - Laidlaw MarkAS, Filippelli GabrielM, Brown Sally, Paz-Ferreiro Jorge, Reichman SuzieM, Netherway Pacian, Truskewycz Adam, Ball AndrewS, Mielke HowardW. “Case Studies and Evidence-Based Approaches to Addressing Urban Soil Lead Contamination” Applied Geochemistry. 2017;83:14–30. https://doi
.org/10.1016/j .apgeochem.2017.02.015 . - Laird BrianD, Van de Wiele TomR, Corriveau MadeleineC, Jamieson HeatherE, Parsons MichaelB, Verstraete Willy, Siciliano StevenD. “Gastrointestinal Microbes Increase Arsenic Bioaccessibility of Ingested Mine Tailings Using the Simulator of the Human Intestinal Microbial Ecosystem” Environmental Science & Technology. 2007;41(15):5542–5547. https://doi
.org/10.1021/es062410e . [PubMed: 17822130] - Lake LoryssaM, Basta NicholasT, Barker DavidJ. “Modifying Effect of Soil Properties on Bio-Accessibility of As and Pb from Human Ingestion of Contaminated Soil” Geosciences. 2021;11(3):126. https://www
.mdpi.com/2076-3263/11/3/126 . - Lang JohnsieR, McKay Allred B, Field JenniferA, Levis JamesW, Barlaz MortonA. “National Estimate of Per- and Polyfluoroalkyl Substance (PFAS) Release to U.S. Municipal Landfill Leachate” Environmental Science & Technology. 2017;51(4):2197–2205. https://doi
.org/10.1021/acs.est.6b05005 . [PubMed: 28103667] - Lassalle John, Gao Ruilian, Rodi Robert, Kowald Corinne, Feng Mingbao, Sharma VirenderK, Hoelen Thomas, Bireta Paul, Houtz ErikaF, Staack David, Pillai SureshD. “Degradation of PFOS and PFOA in Soil and Groundwater Samples by High Dose Electron Beam Technology” Radiation Physics and Chemistry. 2021;189:109705. https://doi
.org/10.1016/j .radphyschem.2021.109705 . - Lehmann Anika, Fitschen Katharina, Rillig MatthiasC. “Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation” Soil Systems. 2019;3(1):21. https://www
.mdpi.com/2571-8789/3/1/21 . - Li Zhen, Tang Lingyi, Zheng Yangfan, Tian Da, Su Mu, Zhang Fan, Ma Shuojia, Hu Shuijin. “Characterizing the Mechanisms of Lead Immobilization via Bioapatite and Various Clay Minerals” ACS Earth and Space Chemistry. 2017;1(3):152–157. https://doi
.org/10.1021 /acsearthspacechem.7b00016 . - Lian Jiapan, Liu Weitao, Meng Lingzuo, Wu Jiani, Zeb Aurang, Cheng Liping, Lian Yuhang, Sun Hongwen. “Effects of Microplastics Derived from Polymer-Coated Fertilizer on Maize Growth, Rhizosphere, and Soil Properties” Journal of Cleaner Production. 2021;318:128571. https://doi
.org/10.1016/j .jclepro.2021.128571 . - Linn DM, Carski TH, Brusseau ML, Chang FH. Sorption and Degradation of Pesticides and Organic Chemicals in Soil. Proceedings of a Symposium, Denver, October 1991. Madison, WI: Soil Science Society of America, Inc; 1993.
- Liu Lianwen, Li Wei, Song Weiping, Guo Mingxin. “Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability” Science of the Total Environment. 2018;633:206–219. https://doi
.org/10.1016/j .scitotenv.2018.03.161 . [PubMed: 29573687] - Lowman Amy, Anne McDonald Mary, Wing Steve, Muhammad Naeema. “Land Application of Treated Sewage Sludge: Community Health and Environmental Justice” Environmental Health Perspectives. 2013;121(5):537–542. https://doi
.org/10.1289/ehp.1205470 . [PMC free article: PMC3673187] [PubMed: 23562940] - Lu Kun, Phillip Abo Ryan, Ann Schlieper Katherine, Graffam MichelleE, Levine Stuart, Wishnok JohnS, Swenberg JamesA, Tannenbaum StevenR, Fox JamesG. “Arsenic Exposure Perturbs the Gut Microbiome and Its Metabolic Profile in Mice: An Integrated Metagenomics and Metabolomics Analysis” Environmental Health Perspectives. 2014;122(3):284–291. https://doi
.org/10.1289/ehp.1307429 . [PMC free article: PMC3948040] [PubMed: 24413286] - Lu Liang, Luo Ting, Zhao Yao, Cai Chunhui, Fu Zhengwei, Jin Yuanxiang. “Interaction Between Microplastics and Microorganism as Well as Gut Microbiota: A Consideration on Environmental Animal and Human Health” Science of the Total Environment. 2019;667:94–100. https://doi
.org/10.1016/j .scitotenv.2019.02.380 . [PubMed: 30826685] - Lu Qin, He ZhenliL, Stoffella PeterJ. “Land Application of Biosolids in the USA: A Review” Applied and Environmental Soil Science. 2012;2012:201462. https://doi
.org/10.1155/2012/201462 . - Madsen EL. “Methods for Determining Biodegradability” In: Hurst CJ, Knudsen GR, Mcinerney MJ, Stetzenbach LD, Walter MV, editors. Manual of Environmental Microbiology. Washington, DC: ASM Press; 1997. pp. 709–720.
- Mandal BadalKumar, Suzuki KazuoT. “Arsenic Round the World: A Review” Talanta. 2002;58(1):201–235. https://doi
.org/10.1016 /S0039-9140(02)00268-0 . [PubMed: 18968746] - Manzano-Salgado CyntiaB, Casas Maribel, Lopez-Espinosa Maria-Jose, Ballester Ferran, Basterrechea Mikel, Grimalt JoanO, Jiménez Ana-María, Kraus Thomas, Schettgen Thomas, Sunyer Jordi, Vrijheid Martine. “Transfer of Perfluoroalkyl Substances from Mother to Fetus in a Spanish Birth Cohort” Environmental Research. 2015;142:471–478. https://doi
.org/10.1016/j .envres.2015.07.020 . [PubMed: 26257032] - Martin ToddA, Ruby MichaelV. “Review of In Situ Remediation Technologies for Lead, Zinc, and Cadmium in Soil” Remediation Journal. 2004;14(3):35–53. https://doi
.org/10.1002/rem.20011 . - Masoner Jason, Kolpin Dana, Cozzarelli Isabelle, Smalling Kelly, Bolyard Stephanie, Field Jennifer, Furlong Edward, Gray James, Lozinski Duncan, Reinhart Debra, Rodowa Alix, Bradley Paul. “Landfill Leachate Contributes Per-/Poly-Fluoroalkyl Substances (PFAS) and Pharmaceuticals to Municipal Wastewater” Environmental Science: Water Research & Technology. 2020;6 https://doi
.org/10.1039/D0EW00045K . - Mason-Renton Sarah, Luginaah Isaac. “Lasting Impacts and Perceived Inequities: Community Reappraisal of the Siting of a Regional Biosolids Processing Facility in Rural Ontario” Journal of Risk Research. 2018;22:1–18. https://doi
.org/10.1080/13669877 .2018.1440413 . - Masura Julie, Baker JoelE, Foster GregoryD, Arthur Courtney, Herring Carlie. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. NOAA Technical Memorandum NOS-OR&R. 2015. https://repository
.library .noaa.gov/view/noaa/10296 . - McDermott TimothyR, Stolz JohnF, Oremland RonaldS. “Arsenic and the Gastrointestinal Tract Microbiome” Environmental Microbiology Reports. 2020;12(2):136–159. https://doi
.org/10.1111/1758-2229.12814 . [PubMed: 31773890] - McNamara Patrick, Liu Zhongzhe, Tong Yiran, Santha Hari, Moss Lynne, Zitomer Daniel. “Pyrolysis—A Tool in the Wastewater Solids Handling Portfolio, Not a Silver Bullet: Benefits, Drawbacks, and Future Directions” Water Environment Research. 2023a;95(5):e10863. https://doi
.org/10.1002/wer.10863 . [PubMed: 37021664] - McNamara Patrick, Samuel MelvinS, Sathyamoorthy Sandeep, Moss Lynne, Valtierra Danny, Cortes Lopez Hugo, Nigro Nick, Somerville Stephen, Liu Zhongzhe. “Pyrolysis Transports, and Transforms, PFAS from Biosolids to Py-liquid” Environmental Science: Water Research & Technology. 2023b;9(2):386–395. https://doi
.org/10.1039/D2EW00677D . - Meharg AndrewA, Williams PaulN, Adomako Eureka, Lawgali YoussefY, Deacon Claire, Villada Antia, Cambell RobertCJ, Sun Guoxin, Zhu Yong-Guan, Feldmann Joerg, Raab Andrea, Zhao Fang-Jie, Islam Rafiqul, Hossain Shahid, Yanai Junta. “Geographical Variation in Total and Inorganic Arsenic Content of Polished (White) Rice” Environmental Science & Technology. 2009;43(5):1612–1617. https://doi
.org/10.1021/es802612a . [PubMed: 19350943] - Mercl Filip, Košnář Zdeněk, Maršík Petr, Vojtíšek Martin, Dušek Jakub, Száková Jiřina, Tlustoš Pavel. “Pyrolysis of Biosolids as an Effective Tool to Reduce the Uptake of Pharmaceuticals by Plants” Journal of Hazardous Materials. 2021;405:124278. https://doi
.org/10.1016/j .jhazmat.2020.124278 . [PubMed: 33168310] - Mielke HW, Reagan PL. “Soil Is an Important Pathway of Human Lead Exposure” Environmental Health Perspectives. 1998;106(Suppl 1):217–229. https://doi
.org/10.1289/ehp.98106s1217 . [PMC free article: PMC1533263] [PubMed: 9539015] - Mitra Anindita, Chatterjee Soumya, Moogouei Roxana, Gupta DharmendraK. “Arsenic Accumulation in Rice and Probable Mitigation Approaches: A Review” Agronomy. 2017;7(4):67. https://www
.mdpi.com/2073-4395/7/4/67 . - NASEM (National Academies of Sciences, Engineering, and Medicine). Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington, DC: The National Academies Press; 2022a. [PubMed: 35939564]
- NASEM. Reckoning with the U.S. Role in Global Ocean Plastic Waste. Washington, DC: The National Academies Press; 2022b.
- Noerpel Matthew, Pribil Michael, Rutherford Danny, Law Preston, Bradham Karen, Nelson Clay, Weber Rob, Gunn Gene, Scheckel Kirk. “Lead Speciation, Bioaccessibility and Source Attribution in Missouri’s Big River Watershed” Applied Geochemistry. 2020;123:104757. https://doi
.org/10.1016/j .apgeochem.2020.104757 . [PMC free article: PMC7787989] [PubMed: 33424107] - Nowack Bernd, Schulin Rainer, Robinson BrettH. “Critical Assessment of ChelantEnhanced Metal Phytoextraction” Environmental Science & Technology. 2006;40(17):5225–5232. https://doi
.org/10.1021/es0604919 . [PubMed: 16999093] - NRC (National Research Council). Frontiers in Assessing Human Exposures to Environmental Toxicants: Report of a Symposium. Washington, DC: National Academy Press; 1991. https://doi
.org/10.17226/21344 . - NRC. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications. Washington, DC: National Academy Press; 2003. https://doi
.org/10.17226/10523 . - Otake Yoshito, Kobayashi Tomoko, Asabe Hitoshi, Murakami Nobunao, Ono Katsumichi. “Biodegradation of Low-Density Polyethylene, Polystyrene, Polyvinyl Chloride, and Urea Formaldehyde Resin Buried Under Soil for over 32 years” Journal of Applied Polymer Science. 1995;56(13):1789–1796. https://doi
.org/10.1002/app .1995.070561309 . - Oulhote Youssef, Le Bot Barbara, Poupon Joel, Lucas Jean-Paul, Mandin Corinne, Etchevers Anne, Zmirou-Navier Denis, Glorennec Philippe. “Identification of Sources of Lead Exposure in French Children by Lead Isotope Analysis: A Cross-Sectional Study” Environmental Health. 2011;10(1):75. https://doi
.org/10.1186/1476-069X-10-75 . [PMC free article: PMC3176150] [PubMed: 21871122] - Paço Ana, Duarte Kátia, da Costa JoãoP, Santos PatríciaSM, Pereira R, Pereira ME, Freitas AnaC, Duarte ArmandoC, Rocha-Santos TeresaAP. “Biodegradation of Polyethylene Microplastics by the Marine Fungus Zalerion maritimum.” Science of the Total Environment. 2017;586:10–15. https://doi
.org/10.1016/j .scitotenv.2017.02.017 . [PubMed: 28199874] - Park JinHee, Kumar Choppala Girish, Sirangie Bolan Nanthi, Woo Chung Jae, Chuasavathi Thammared. “Biochar Reduces the Bioavailability and Phytotoxicity of Heavy Metals” Plant and Soil. 2011;348(1):439–451. https://doi
.org/10.1007 /s11104-011-0948-y . - Pepper IanL, Brusseau MarkL, Prevatt FrankJ, Escobar BarbaraA. “Incidence of PFAS in Soil Following Long-Term Application of Class B Biosolids” Science of the Total Environment. 2021;793:148449. https://doi
.org/10.1016/j .scitotenv.2021.148449 . [PubMed: 34174610] - Perkins Tom. The Guardian May 12, 2022. 2022. [April 29, 2024]. “Maine Bans Use of Sewage Sludge on Farms to Reduce Risk of PFAS Poisoning” https://www
.theguardian .com/environment/2022 /may/12/maine-bans-sewage-sludge-fertilizer-farms-pfas-poisoning . - Persson Linn, Carney Almroth BethanieM, Collins ChristopherD, Cornell Sarah, de Wit CynthiaA, Diamond MiriamL, Fantke Peter, Hassellöv Martin, MacLeod Matthew, Ryberg MortenW, Søgaard Jørgensen Peter, Villarrubia-Gómez Patricia, Wang Zhanyun, Zwicky Hauschild Michael. “Outside the Safe Operating Space of the Planetary Boundary for Novel Entities” Environmental Science & Technology. 2022;56(3):1510–1521. https://doi
.org/10.1021/acs.est.1c04158 . [PMC free article: PMC8811958] [PubMed: 35038861] - Petersen Fritz, Hubbart JasonA. “The Occurrence and Transport of Microplastics: The State of the Science” Science of the Total Environment. 2021;758:143936. https://doi
.org/10.1016/j .scitotenv.2020.143936 . [PubMed: 33333307] - Pfotenhauer David, Sellers Emily, Olson Mark, Praedel Katie, Shafer Martin. “PFAS Concentrations and Deposition in Precipitation: An Intensive 5-month study at National Atmospheric Deposition Program – National Trends Sites (NADP-NTN) Across Wisconsin, USA” Atmospheric Environment. 2022;291:119368. https://doi
.org/10.1016/j .atmosenv.2022.119368 . - Prata Joana, Patricio Silva AL, Da Costa Joao, Mouneyrac Catherine, Walker Tony, Duarte Armando, Rocha-Santos Teresa. “Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution” International Journal of Environmental Research and Public Health. 2019;16:2411. https://doi
.org/10.3390/ijerph16132411 . [PMC free article: PMC6651478] [PubMed: 31284627] - Restrepo-Flórez Juan-Manuel, Bassi Amarjeet, Thompson MichaelR. “Microbial Degradation and Deterioration of Polyethylene – A Review” International Biodeterioration & Biodegradation. 2014;88:83–90. https://doi
.org/10.1016/j .ibiod.2013.12.014 . - Rieuwerts JS, Thornton I, Farago ME, Ashmore MR. “Factors Influencing Metal Bioavailability in Soils: Preliminary Investigations for the Development of a Critical Loads Approach for Metals” Chemical Speciation & Bioavailability. 1998;10(2):61–75. https://doi
.org/10.3184 /095422998782775835 . - Rillig MatthiasC, Lehmann Anika. “Microplastic in Terrestrial Ecosystems” Science. 2020;368(6498):1430–1431. https://doi
.org/10.1126/science.abb5979 . [PMC free article: PMC7115994] [PubMed: 32587009] - Rillig Matthias, Lehmann Anika, Ryo Masahiro, Bergmann Joana. “Shaping Up: Toward Considering the Shape and Form of Pollutants” Environmental Science & Technology. 2019a;53 https://doi
.org/10.1021/acs.est.9b03520 . [PubMed: 31267727] - Rillig MatthiasC, Lehmann Anika, Abel de Souza Machado A, Yang Gaowen. “Microplastic Effects on Plants” New Phytologist. 2019b;223(3):1066–1070. https://doi
.org/10.1111/nph.15794 . [PubMed: 30883812] - Rillig MatthiasC, Ryo Masahiro, Lehmann Anika, Aguilar-Trigueros CarlosA, Buchert Sabine, Wulf Anja, Iwasaki Aiko, Roy Julien, Yang Gaowen. “The Role of Multiple Global Change Factors in Driving Soil Functions and Microbial Biodiversity” Science. 2019c;366(6467):886–890. https://doi
.org/10.1126/science.aay2832 . [PMC free article: PMC6941939] [PubMed: 31727838] - Rillig MatthiasC, Hoffmann Mathias, Lehmann Anika, Liang Yun, Lück Matthias, Augustin Jürgen. “Microplastic Fibers Affect Dynamics and Intensity of CO2 and N2O Fluxes from Soil Differently” Microplastics and Nanoplastics. 2021a;1(1):3. https://doi
.org/10.1186 /s43591-021-00004-0 . - Rillig MatthiasC, Leifheit Eva, Lehmann Johannes. “Microplastic Effects on Carbon Cycling Processes in Soils” PLOS Biology. 2021b;19(3):e3001130. https://doi
.org/10.1371/journal .pbio.3001130 . [PMC free article: PMC8009438] [PubMed: 33784293] - Rillig MatthiasC, Woong Kim Shin, Zhu Yong-Guan. “The Soil Plastisphere” Nature Reviews Microbiology. 2024;22(2):64–74. https://doi
.org/10.1038 /s41579-023-00967-2 . [PMC free article: PMC7615554] [PubMed: 37697003] - Rockström Johan, Steffen Will, Noone Kevin, Stuart Chapin Åsa Persson F, Lambin EricF, Lenton TimothyM, Scheffer Marten, Folke Carl, Joachim Schellnhuber Hans, Nykvist Björn, de Wit CynthiaA, Hughes Terry, Leeuw Sandervander, Rodhe Henning, Sörlin Sverker, Snyder PeterK, Costanza Robert, Svedin Uno, Falkenmark Malin, Karlberg Louise, Corell RobertW, Fabry VictoriaJ, Hansen James, Walker Brian, Liverman Diana, Richardson Katherine, Crutzen Paul, Foley JonathanA. “A Safe Operating Space for Humanity” Nature. 2009;461(7263):472–475. https://doi
.org/10.1038/461472a . [PubMed: 19779433] - Rodríguez Martín JoséAntonio, Gutiérrez Carmen, Miguel Escuer MTeresaGarcía-González, Campos-Herrera Raquel, Águila Nancy. “Effect of Mine Tailing on the Spatial Variability of Soil Nematodes from Lead Pollution in La Union (Spain)” Science of the Total Environment. 2014;473−474:518–529. https://doi
.org/10.1016/j .scitotenv.2013.12.075 . [PubMed: 24394364] - Sajjad Muhammad, Huang Qing, Khan Sardar, Amjad Khan Muhammad, Liu Yin, Wang Junfeng, Lian Faqin, Wang Qingqing, Guo Genmao. “Microplastics in the Soil Environment: A Critical Review” Environmental Technology & Innovation. 2022;27:102408. https://doi
.org/10.1016/j .eti.2022.102408 . - Santana VT, Gonçalves SPC, Agnelli JAM, Martins-Franchetti SM. “Biodegradation of a Polylactic Acid/Polyvinyl Chloride Blend in Soil” Journal of Applied Polymer Science. 2012;125(1):536–540. https://doi
.org/10.1002/app.35685 . - Santos AndressaLiberal, Carvalho Rodrigues Cândido, Oliveira Miguel, Lopes Rocha Thiago. “Microbiome: A Forgotten Target of Environmental Micro(nano)plastics?” Science of the Total Environment. 2022;822:153628. https://doi
.org/10.1016/j .scitotenv.2022.153628 . [PubMed: 35124041] - Schaefer HeatherR, Dennis Sherri, Fitzpatrick Suzanne. “Cadmium: Mitigation Strategies to Reduce Dietary Exposure” Journal of Food Science. 2020;85(2):260–267. https://doi
.org/10.1111/1750-3841.14997 . [PMC free article: PMC7027482] [PubMed: 31957884] - Scheckel KirkG, Chaney RufusL, Basta NicholasT, Ryan JamesA. “Chapter 1 Advances in Assessing Bioavailability of Metal(Loid)s in Contaminated Soils” Advances in Agronomy. 2009;104:1–52. https://doi
.org/10.1016 /S0065-2113(09)04001-2 . - Scheckel KirkG, Diamond GaryL, Burgess MicheleF, Klotzbach JulieM, Maddaloni Mark, Miller BradleyW, Partridge CharlesR, Serda SophiaM. “Amending Soils with Phosphate as Means to Mitigate Soil Lead Hazard: A Critical Review of the State of the Science” Journal of Toxicology and Environmental Health, Part B. 2013;16(6):337–380. https://doi
.org/10.1080/10937404 .2013.825216 . [PubMed: 24151967] - Schnoor JeraldL. Environmental Modeling: Fate and Transport of Pollutants in Water, Air, and Soil. New York: Wiley; 1996.
- Schooley Therese, Weaver MichaelJ, Mullins Donald, Eick Matthew. “The History of Lead Arsenate Use in Apple Production: Comparison of its Impact in Virginia with Other States” Journal of Pesticide Safety Education. 2008;10
- Scow Kate, Hicks Kristin. “Natural Attenuation and Enhanced Bioremediation of Organic Contaminants in Groundwater” Current Opinion in Biotechnology. 2005;16:246–253. https://doi
.org/10.1016/j .copbio.2005.03.009 . [PubMed: 15961025] - Scow KM, Fan S, Johnson C, Ma GM. “Biodegradation of Sorbed Chemicals in Soil” Environmental Health Perspectives. 1995;103(Suppl 5):93–95. https://doi
.org/10.1289/ehp.95103s493 . [PMC free article: PMC1519290] [PubMed: 8565921] - Scow KateM, Johnson CarolR. “Effect of Sorption on Biodegradation of Soil Pollutants” Sparks DonaldL, editor. Academic Press; Advances in Agronomy. 1996:1–56.
- Seagren EricA. “Bioremediation” In: Wexler P, editor. Encyclopedia of Toxicology Volume 2. Oxford: Academic Press; 2024. pp. 145–159.
- Senevirathna STMLD, Bal Krishna KC, Mahinroosta Reza, Sathasivan Arumugam. “Comparative Characterization of Microbial Communities that Inhabit PFAS-rich Contaminated Sites: A Case-Control Study” Journal of Hazardous Materials. 2022;423:126941. https://doi
.org/10.1016/j .jhazmat.2021.126941 . [PubMed: 34474371] - Shahsavari Esmaeil, Rouch Duncan, Khudur LeadinS, Thomas Duncan, Aburto-Medina Arturo, Ball AndrewS. “Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils” Frontiers in Bioengineering and Biotechnology. 2021;8 https://doi
.org/10.3389/fbioe .2020.602040 . [PMC free article: PMC7817812] [PubMed: 33490051] - Shao Mengmeng, Zhu Yi. “Long-Term Metal Exposure Changes Gut Microbiota of Residents Surrounding a Mining and Smelting Area” Scientific Reports. 2020;10(1):4453. https://doi
.org/10.1038 /s41598-020-61143-7 . [PMC free article: PMC7064573] [PubMed: 32157109] - Siebecker MatthewG, Li Wei, Sparks DonaldL. “Chapter One - The Important Role of Layered Double Hydroxides in Soil Chemical Processes and Remediation: What We Have Learned Over the Past 20 Years” Advances in Agronomy. 2018;147:1–59. https://doi
.org/10.1016/bs .agron.2017.10.001 . - Singh RajKamal, Brown Elizabeth, Mededovic Thagard Selma, Holsen ThomasM. “Treatment of PFAS-Containing Landfill Leachate Using an Enhanced Contact Plasma Reactor” Journal of Hazardous Materials. 2021;408:124452. https://doi
.org/10.1016/j .jhazmat.2020.124452 . [PubMed: 33243646] - Singh RajivR, Papanastasiou DimitriosK. “Comment on ‘Scientific Basis for Managing PFAS as a Chemical Class’” Environmental Science & Technology Letters. 2021;8(2):192–194. https://doi
.org/10.1021/acs .estlett.0c00765 . [PMC free article: PMC8297807] [PubMed: 34307722] - Sivan Alex. “New Perspectives in Plastic Biodegradation” Current Opinion in Biotechnology. 2011;22(3):422–426. https://doi
.org/10.1016/j .copbio.2011.01.013 . [PubMed: 21356588] - Smalling KellyL, Romanok KristinM, Bradley PaulM, Morriss MathewC, Gray JamesL, Kanagy LeslieK, Gordon StephanieE, Williams BriannaM, Breitmeyer SaraE, Jones DanielK, DeCicco LauraA, Eagles-Smith CollinA, Wagner Tyler. “Per- and Polyfluoroalkyl Substances (PFAS) in United States Tapwater: Comparison of Under-served Private-Well and Public-Supply Exposures and Associated Health Implications” Environment International. 2023;178:108033. https://doi
.org/10.1016/j .envint.2023.108033 . [PubMed: 37356308] - Smith DavidB, Cannon WilliamF, Woodruff LaurelG, Solano Federico, Ellefsen KarlJ. Geochemical and Mineralogical Maps for Soils of the Conterminous United States. Reston, VA: U. S. Geological Survey; 2014. https://pubs
.usgs.gov /publication/ofr20141082 . - Sowers TylerD, Blackmon MatthewD, Betts AaronR, Jerden MarissaL, Scheckel KirkG, Bradham KarenD. “Potassium Jarosite Seeding of Soils Decreases Lead and Arsenic Bioaccessibility: A Path Toward Concomitant Remediation” Proceedings of the National Academy of Sciences. 2023;120(50):e2311564120. https://doi
.org/10.1073/pnas.2311564120 . [PMC free article: PMC10723135] [PubMed: 38048468] - Sparks DonaldL. “Advances in the Use of Synchrotron Radiation to Elucidate Environmental Interfacial Reaction Processes and Mechanisms in the Earth’s Critical Zone” In: Xu Jianming, Sparks DonaldL, editors. Molecular Environmental Soil Science. Dordrecht: Springer Netherlands; 2013. pp. 93–114.
- Steffen Will, Richardson Katherine, Rockström Johan, Cornell SarahE, Fetzer Ingo, Bennett ElenaM, Biggs Reinette, Carpenter StephenR, Vries Wimde, de Wit CynthiaA, Folke Carl, Gerten Dieter, Heinke Jens, Mace GeorginaM, Persson LinnM, Ramanathan Veerabhadran, Reyers Belinda, Sörlin Sverker. “Planetary Boundaries: Guiding Human Development on a Changing Planet” Science. 2015;347(6223):1259855. https://doi
.org/10.1126/science.1259855 . [PubMed: 25592418] - Stevens BrookeN, Betts AaronR, Miller BradleyW, Scheckel KirkG, Anderson RichardH, Bradham KarenD, Casteel StanW, Thomas DavidJ, Basta NicholasT. “Arsenic Speciation of Contaminated Soils/Solid Wastes and Relative Oral Bioavailability in Swine and Mice” Soil Systems. 2018;2(2):27. https://doi
.org/10.3390 /soilsystems2020027 . [PMC free article: PMC6605063] [PubMed: 31276103] - Stoiber Tasha, Evans Sydney, Naidenko OlgaV. “Disposal of Products and Materials Containing Per- and Polyfluoroalkyl Substances (PFAS): A Cyclical Problem” Chemosphere. 2020;260:127659. https://doi
.org/10.1016/j .chemosphere.2020.127659 . [PubMed: 32698118] - Strynar MarkJ, Lindstrom AndrewB, Nakayama ShojiF, Egeghy PeterP, Helfant LaurenceJ. “Pilot Scale Application of a Method for the Analysis of Perfluorinated Compounds in Surface Soils” Chemosphere. 2012;86(3):252–257. https://doi
.org/10.1016/j .chemosphere.2011.09.036 . [PubMed: 22130124] - Sun Yuanze, Duan Chongxue, Cao Na, Li Xinfei, Li Xiaomin, Chen Yumei, Huang Yi, Wang Jie. “Effects of Microplastics on Soil Microbiome: The Impacts of Polymer Type, Shape, and Concentration” Science of the Total Environment. 2022;806(Pt 2):150516. https://doi
.org/10.1016/j .scitotenv.2021.150516 . [PubMed: 34592287] - Sunderland ElsieM, Hu XindiC, Dassuncao Clifton, Tokranov AndreaK, Wagner CharlotteC, Allen JosephG. “A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects” Journal of Exposure Science & Environmental Epidemiology. 2019;29(2):131–147. https://doi
.org/10.1038 /s41370-018-0094-1 . [PMC free article: PMC6380916] [PubMed: 30470793] - Swartjes FA. “Human Health Risk Assessment Related to Contaminated Land: State of the Art” Environmental Geochemistry and Health. 2015;37(4):651–673. https://doi
.org/10.1007 /s10653-015-9693-0 . [PubMed: 25809961] - Tchounwou PaulB, Yedjou ClementG, Patlolla AnitaK, Sutton DwayneJ. “Heavy Metal Toxicity and the Environment” In: Luch Andreas, editor. Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology. Basel: Springer Basel; 2012. pp. 133–164. [PMC free article: PMC4144270] [PubMed: 22945569]
- Thoma Eben, Wright Robert, George Ingrid, Krause Max, Presezzi Dario, Villa Valentino, Preston William, Deshmukh Parik, Kauppi Phil, Zemek Peter. “Pyrolysis Processing of PFAS-Impacted Biosolids, a Pilot Study” Journal of the Air & Waste Management Association. 2022;72 https://doi
.org/10.1080/10962247 .2021.2009935 . [PMC free article: PMC9128340] [PubMed: 34870569] - Thompson JakeT, Robey NicoleM, Tolaymat ThabetM, Bowden JohnA, Solo-Gabriele HelenaM, Townsend TimothyG. “Underestimation of Per- and Polyfluoroalkyl Substances in Biosolids: Precursor Transformation During Conventional Treatment” Environmental Science and Technology. 2023;57:3825–3832. https://doi
.org/10.1021/acs.est.2c06189 . [PMC free article: PMC10500628] [PubMed: 36749308] - Turner LaurenP, Kueper BernardH, Jaansalu KevinM, Patch DavidJ, Battye Nick, El-Sharnouby Omneya, Mumford KevinG, Weber KelaP. “Mechanochemical Remediation of Perfluorooctanesulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA) Amended Sand and Aqueous Film-Forming Foam (AFFF) Impacted Soil by Planetary Ball Milling” Science of the Total Environment. 2021;765:142722. https://doi
.org/10.1016/j .scitotenv.2020.142722 . [PubMed: 33268250] - Van de Wiele Tom, Gallawa ChristinaM, Kubachk KevinM, Creed JohnT, Basta Nicholas, Dayton ElizabethA, Whitacre Shane, Du Laing Gijs, Bradham Karen. “Arsenic Metabolism by Human Gut Microbiota upon In Vitro Digestion of Contaminated Soils” Environmental Health Perspectives. 2010;118(7):1004–1009. https://doi
.org/10.1289/ehp.0901794 . [PMC free article: PMC2920899] [PubMed: 20603239] - Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M. “Mobility and Bioavailability of Heavy Metals and Metalloids in Soil Environments” Journal of Soil Science and Plant Nutrition. 2010;10(3):268–292. https://doi
.org/10.4067 /S0718-95162010000100005 . - Wallace JoshuaS, Edirisinghe Dulan, Seyedi Saba, Noteboom Haley, Blate Micah, Dursun Balci Derya, Abu-Orf Mohammad, Sharp Robert, Brown Jeanette, Aga DianaS. “Burning Questions: Current Practices and Critical Gaps in Evaluating Removal of Per- and Polyfluoroalkyl Substances (PFAS) During Pyrolysis Treatments of Biosolids” Journal of Hazardous Materials Letters. 2023;4:100079. https://doi
.org/10.1016/j .hazl.2023.100079 . [PMC free article: PMC10545407] [PubMed: 37790729] - Wang Fayuan, Zhang Xiaoqing, Zhang Shuqi, Zhang Shuwu, Sun Yuhuan. “Interactions of Microplastics and Cadmium on Plant Growth and Arbuscular Mycorrhizal Fungal Communities in an Agricultural Soil” Chemosphere. 2020;254:126791. https://doi
.org/10.1016/j .chemosphere.2020.126791 . [PubMed: 32320834] - Wang Liuwei, Rinklebe Jörg, Tack FilipMG, Hou Deyi. “A Review of Green Remediation Strategies for Heavy Metal Contaminated Soil” Soil Use and Management. 2021;37(4):936–963. https://doi
.org/10.1111/sum.12717 . - Wang Quanlong, Adams CatharineA, Wang Fayuan, Sun Yuhuan, Zhang Shuwu. “Interactions Between Microplastics and Soil Fauna: A Critical Review” Critical Reviews in Environmental Science and Technology. 2022;52:3211–3243. https://doi
.org/10.1080/10643389 .2021.1915035 . - Wani AbLatif, Ara Anjum, Ahmad Usmani Jawed. “Lead Toxicity: A Review” Interdisciplinary Toxicology. 2016;8(2):55–64. https://doi
.org/10.1515/intox-2015-0009 . [PMC free article: PMC4961898] [PubMed: 27486361] - Weber NathanH, Delva CameronS, Stockenhuber SebastianP, Grimison CharlesC, Lucas JohnA, Mackie JohnC, Stockenhuber Michael, Kennedy EricM. “Thermal Mineralization of Perfluorooctanesulfonic Acid (PFOS) to HF, CO2, and SO2” Industrial & Engineering Chemistry Research. 2023;62(2):881–892. https://doi
.org/10.1021/acs.iecr.2c03197 . - Whitacre Shane, Basta Nicholas, Stevens Brooke, Hanley Valerie, Anderson Richard, Scheckel Kirk. “Modification of an Existing In Vitro Method to Predict Relative Bioavailable Arsenic in Soils” Chemosphere. 2017;180:545–552. https://doi
.org/10.1016/j .chemosphere.2017.03.134 . [PMC free article: PMC6121222] [PubMed: 28432891] - Wu Changcai, Ma Yajie, Wang Dan, Shan Yongpan, Song Xianpeng, Hu Hongyan, Ren Xiangliang, Ma Xiaoyan, Cui Jinjie, Ma Yan. “Integrated Microbiology and Metabolomics Analysis Reveal Plastic Mulch Film Residue Affects Soil Microorganisms and Their Metabolic Functions” Journal of Hazardous Materials. 2022;423:127258. https://doi
.org/10.1016/j .jhazmat.2021.127258 . [PubMed: 34844367] - Wu Enhui, Wang Kun, Liu Zhengzheng, Wang Jing, Yan Huicong, Zhu Xiangyu, Zhu Xiaomin, Chen Baoliang. “Metabolic and Microbial Profiling of Soil Microbial Community under Per- and Polyfluoroalkyl Substance (PFAS) Stress” Environmental Science & Technology. 2023;57(51):21855–21865. https://doi
.org/10.1021/acs.est.3c07020 . [PubMed: 38086098] - Xiao Xin, Ulrich BridgetA, Chen Baoliang, Higgins ChristopherP. “Sorption of Poly- and Perfluoroalkyl Substances (PFASs) Relevant to Aqueous Film-Forming Foam (AFFF)-Impacted Groundwater by Biochars and Activated Carbon” Environmental Science & Technology. 2017;51(11):6342–6351. https://doi
.org/10.1021/acs.est.7b00970 . [PubMed: 28582977] - Xu Baile, Yang Gaowen, Lehmann Anika, Riedel Sebastian, Rillig MatthiasC. “Effects of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) on Soil Structure and Function” Soil Ecology Letters. 2023;5(1):108–117. https://doi
.org/10.1007 /s42832-022-0143-5 . - Xu Rui, Tao Wan, Lin Hanzhi, Huang Duanyi, Su Pingzhou, Gao Pin, Sun Xiaoxu, Yang Zhaohui, Sun Weimin. “Effects of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) on Soil Microbial Community” Microbial Ecology. 2022;83:929–941. https://doi
.org/10.1007 /s00248-021-01808-6 . [PubMed: 34283261] - Yadav Shweta, Gupta Ekta, Patel Anju, Srivastava Suchi, Kumar Mishra Virendra, Singh PoonamC, Kumar Srivastava Pankaj, Kanta Barik Saroj. “Unravelling the Emerging Threats of Microplastics to Agroecosystems” Reviews in Environmental Science and Bio/ Technology. 2022;21(3):771–798. https://doi
.org/10.1007 /s11157-022-09621-4 . - Yan Peng-Fei, Dong Sheng, Pennell KurtD, Cápiro NatalieL. “A Review of the Occurrence and Microbial Transformation of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous Film-Forming Foam (AFFF)-impacted Environments” Science of the Total Environment. 2024;927:171883. https://doi
.org/10.1016/j .scitotenv.2024.171883 . [PubMed: 38531439] - Yang Gaowen, Ryo Masahiro, Roy Julien, Lammel Daniel, Ballhausen Max-Bernhard, Jing Xin, Zhu Xuefeng, Rillig Matthias. “Multiple Anthropogenic Pressures Eliminate the Effects of Soil Microbial Diversity on Ecosystem Functions in Experimental Microcosms” Nature Communications. 2022;13:4260. https://doi
.org/10.1038 /s41467-022-31936-7 . [PMC free article: PMC9308766] [PubMed: 35871070] - Yang Xing, Pan He, Shaheen SabryM, Wang Hailong, Rinklebe Jörg. “Immobilization of Cadmium and Lead Using Phosphorus-Rich Animal-Derived and Iron-Modified Plant-Derived Biochars Under Dynamic Redox Conditions in a Paddy Soil” Environment International. 2021;156:106628. https://doi
.org/10.1016/j .envint.2021.106628 . [PubMed: 33991874] - Yang Xue, Zhang Shiqiu, Ju Meiting, Liu Le. “Preparation and Modification of Biochar Materials and Their Application in Soil Remediation” Applied Sciences. 2019;9(7):1365. https://doi
.org/10.3390/app9071365 . - Yin Naiyi, Cai Xiaolin, Du Huili, Zhang Zhennan, Li Zejiao, Chen Xiaochen, Sun Guoxin, Cui Yanshan. “In Vitro Study of Soil Arsenic Release by Human Gut Microbiota and Its Intestinal Absorption by Caco-2 Cells” Chemosphere. 2017;168:358–364. https://doi
.org/10.1016/j .chemosphere.2016.10.091 . [PubMed: 27810535] - Yoshikawa Miho, Zhang Ming, Toyota Koki. “Biodegradation of Volatile Organic Compounds and Their Effects on Biodegradability under Co-Existing Conditions” Microbes and Environments. 2017;32(3):188–200. https://doi
.org/10.1264/jsme2.ME16188 . [PMC free article: PMC5606688] [PubMed: 28904262] - Zahran Sammy, Laidlaw MarkAS, McElmurry ShawnP, Filippelli GabrielM, Taylor Mark. “Linking Source and Effect: Resuspended Soil Lead, Air Lead, and Children’s Blood Lead Levels in Detroit, Michigan” Environmental Science & Technology. 2013;47(6):2839–2845. https://doi
.org/10.1021/es303854c . [PubMed: 23428083] - Zahran Sammy, Magzamen Sheryl, Breunig IanM, Mielke HowardW. “Maternal Exposure to Neighborhood Soil Pb and Eclampsia Risk in New Orleans, Louisiana (USA): Evidence from a Natural Experiment in Flooding” Environmental Research. 2014;133:274–281. https://doi
.org/10.1016/j .envres.2014.06.007 . [PubMed: 24981826] - Žaltauskaitė Jūratė, Sodienė Inga. “Effects of Total Cadmium and Lead Concentrations in Soil on the Growth, Reproduction and Survival of Earthworm Eisenia fetida.” Ekologija. 2010;56:10–16. https://doi
.org/10.2478 /v10055-010-0002-z . - Zavala YamilyJ, Duxbury JohnM. “Arsenic in Rice: I. Estimating Normal Levels of Total Arsenic in Rice Grain” Environmental Science & Technology. 2008;42(10):3856–3860. https://doi
.org/10.1021/es702747y . [PubMed: 18546734] - Zeng Guangming, Wan Jia, Huang Danlian, Hu Liang, Huang Chao, Cheng Min, Xue Wenjing, Gong Xiaomin, Wang Rongzhong, Jiang Danni. “Precipitation, Adsorption and Rhizosphere Effect: The Mechanisms for Phosphate-induced Pb Immobilization in Soils—A Review” Journal of Hazardous Materials. 2017;339:354–367. https://doi
.org/10.1016/j .jhazmat.2017.05.038 . [PubMed: 28668753] - Zhang Xiaokai, Wang Hailong, He Lizhi, Lu Kouping, Sarmah Ajit, Li Jianwu, Bolan NanthiS, Pei Jianchuan, Huang Huagang. “Using Biochar for Remediation of Soils Contaminated with Heavy Metals and Organic Pollutants” Environmental Science and Pollution Research. 2013;20(12):8472–8483. https://doi
.org/10.1007 /s11356-013-1659-0 . [PubMed: 23589248] - Zhang Xiyu, Wang Heting, Peng Sihan, Kang Jian, Xie Ziyan, Tang Ruobing, Xing Yiqian, He Yuchi, Yuan Haipo, Xie Chunguang, Liu Ya. “Effect of Microplastics on Nasal and Intestinal Microbiota of the High-Exposure Population” Frontiers in Public Health. 2022;10 https://doi
.org/10.3389/fpubh .2022.1005535 . [PMC free article: PMC9650105] [PubMed: 36388272] - Zheng Guomao, Schreder Erika, Dempsey JenniferC, Uding Nancy, Chu Valerie, Andres Gabriel, Sathyanarayana Sheela, Salamova Amina. “Per- and Polyfluoroalkyl Substances (PFAS) in Breast Milk: Concerning Trends for Current-Use PFAS” Environmental Science & Technology. 2021;55(11):7510–7520. https://doi
.org/10.1021/acs.est.0c06978 . [PubMed: 33982557] - Zhou Ziruo, Peng Chi, Liu Xu, Jiang Zhichao, Guo Zhaohui, Xiao Xiyuan. “Pollution and Risk Assessments of Heavy Metal(loid)s in the Soil around Lead-Zinc Smelteries via Data Integration Analysis” International Journal of Environmental Research and Public Health. 2022;19(15):9698. https://doi
.org/10.3390/ijerph19159698 . [PMC free article: PMC9368718] [PubMed: 35955055] - Zhu Dong, Ma Jun, Li Gang, Rillig MatthiasC, Zhu Yong-Guan. “Soil Plastispheres as Hotspots of Antibiotic Resistance Genes and Potential Pathogens” The ISME Journal. 2022;16(2):521–532. https://doi
.org/10.1038 /s41396-021-01103-9 . [PMC free article: PMC8776808] [PubMed: 34455424] - Zuccarello P, Ferrante M, Cristaldi A, Copat C, Grasso A, Sangregorio D, Fiore M, Oliveri Conti G. “Exposure to Microplastics (<10 μm) Associated to Plastic Bottles Mineral Water Consumption: The First Quantitative Study” Water Research. 2019;157:365–371. https://doi
.org/10.1016/j .watres.2019.03.091 . [PubMed: 30974285]
- Interactions of Soil Chemical Contaminants, Soil Health, and Human Health - Expl...Interactions of Soil Chemical Contaminants, Soil Health, and Human Health - Exploring Linkages Between Soil Health and Human Health
Your browsing activity is empty.
Activity recording is turned off.
See more...