NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Food and Nutrition Board; Board on Agriculture and Natural Resources; Committee on Exploring Linkages Between Soil Health and Human Health. Exploring Linkages Between Soil Health and Human Health. Washington (DC): National Academies Press (US); 2024 Sep 19.

Exploring Linkages Between Soil Health and Human Health.
Show detailsGiven their well-established importance in maintaining the biochemistry inherent to health, there is strong motivation to include soil microorganisms, and other biota, in assessments of health from soils and humans. In fact, in soils today nearly a third of tests recommend microbial measurements of soil respiration, biomass, or nitrogen mineralization to characterize biological properties in assessment of health (Lehmann et al. 2020). Similarly, there is mounting evidence that the human microbiome plays an important role in human health (Turnbaugh et al. 2006; Lynch and Pedersen 2016). Given the higher resolution information on microbial content and processes provided by high-throughput sequencing technologies and mass spectrometric methods, microbiome-derived content could be untapped sentinels of soil and human health.
Microbes are likely to be among the key factors explaining how the environment affects human health. While there has been substantial work on understanding how the microbiome of the built environment can affect health (NASEM 2017; Gilbert et al. 2018), far less work has specifically linked soils and their microbiomes to human health outcomes. Most of the evidence linking soil health and human health by way of the microbiome focuses on direct ingestion of soil or contaminated food, aerosolized dust, and percutaneous transfer. Areas of interest in this research include the role of diet as modulated by the gut microbiome, toxins and pollutants derived from microbial metabolisms, and soil-derived pathogens or toxins on human health (see Chapters 3, 5, and 6). There is also indirect evidence that soil health may be tied to human health via the beneficial role of exposure to microbes in human development and the maintenance of health, notably on the immune, metabolic, and central nervous systems. Both exposure to environmental microbes and colonization of the human gut, oral cavity, lung, skin, and urogenital tract are essential for normal function of these organ systems (Thompson et al. 2017). The link between the soil microbiome and human health is particularly compelling because it offers the potential as a modifiable factor and health indicator that can reduce health inequities; this is in contrast to environmental and social determinants of health such as access to greenspace and biodiversity that require long-term investment to change.
This chapter focuses on the microbiomes of soils and humans and their relationship to health in each compartment (soils and humans) and across compartments. The focus is on the coordinated invisible ecosystem of microorganisms and less on specific microorganisms, as these are covered in other areas of this report (Figure 7-1). For instance, Chapter 2 introduces how the microbiome can be incorporated into a One Health framework, provides linkages between soil, plant, and animal systems, and includes a cross-system taxonomic analysis that highlights the shared and unique members (see Figure 2-9). Chapter 5 discusses the direct linkage between soil microbes and human health in their role as soil-borne pathogens (such as Escherichia coli,Clostridium tetani, and Coccidioides) and mycotoxins. Additionally, in Chapter 6, the effects of microbial transformations of soil chemical contaminants on health are discussed. Additional human health benefits conferred by soil microbiota, such as their ability to produce antibiotic and therapeutic agents (Chapter 3) or their roles as inoculants for soil management (Chapters 4 and 5) are also detailed in other chapters of the report.

FIGURE 7-1
Discussions of specific microorganisms or microbial processes that relate to human health in other chapters of the report.
Instead, this chapter offers a forward-looking perspective of the role for the microbiome in the soil–health continuum (Figure 7-2). The approach taken in this chapter underscores the identification of two primary knowledge gaps. Firstly, there is a pressing need to determine which microbial features, if any, contribute to quantifying or fortifying health in both human and soil systems. The ultimate goal for this knowledge is to leverage this understanding for the development of improved and rapid diagnostics or for new biotic-inspired therapeutics such as inoculants or probiotics. Secondly, there is a necessity to comprehend the direct and indirect roles of soil, alongside other environmental factors, in influencing human microbial colonization and subsequenthealth outcomes. Such investigation involves delving into the relatively sparse or disconnected research regarding the microbiome continuum that links soil and human systems.
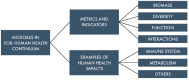
FIGURE 7-2
Organization of Chapter 7.
This chapter addresses two overarching research needs (Figure 7-2). The first section explores the current utilization of microbiome content to assess health status both in human and soil systems. This segment concludes with an examination of emerging technologies and analyses showing promise in identifying new microbiome-derived indicators for health or disease status in humans, crops, and soil systems. The second part of the chapter assesses the existing evidence regarding the microbiome continuum linking soil systems and human systems. It focuses on areas with the most compelling research evidence today: (1) the connection of environmental microorganisms to immune development and allergic disease and (2) the role of gut microbes in modifying or extracting nutrients from food for human health. In summary, by exploring the microbiome continuum from soils to human systems, the valuable insights that will inform diagnostics and interventions and promote health across both ecosystems can be unlocked.
MICROBIOME FEATURES AS INDICATORS OF HEALTH STATUS
Microbiomes as Modulators of Soil and Human Health
Structurally speaking, host systems such as the human gut microbiome and the plant rhizosphere (soils attached to or impacted by plant roots) exhibit numerous physical and chemical similarities. For example, both communities are partially shaped by host activity. In the human gut, microbial composition can be altered by dietary shifts, medication usage, host health status, and environmental factors, while the host also generates compounds such as mucin to nourish and facilitate microbial colonization (Fassarella et al. 2021). Similarly, microorganisms in the rhizosphere respond toroot exudation, where plants secrete metabolites, influencing microbial activity and community dynamics (Seitz et al. 2022). However, there are critical differences between these two habitats, as outlined below. Together these shared principles and unique drivers influence the way microbiomes are analyzed and collected and how they are interpreted in a health context.
Broadly, both gut and soil microbiomes provide benefits to their hosts through decomposition (e.g., fiber, proteins), nutrient extraction, and hormone production. As detailed later in this chapter, the human gut microbiota metabolizes proteins and fiber from the diet, generating short-chain fatty acids for the host energy. Similarly, soil microbiomes decompose litter and process metabolites from root exudation, shaping carbon and nitrogen pools crucial for maintaining soil biotic and abiotic structure as well as for plant health. In the gut microbiome, microbiota synthesize vitamins (e.g., vitamins B and K) and produce metabolic products that nurture human host cells (Nicholson et al. 2012). Similarly, soil microbiomes, particularly bacteria and fungi in the rhizosphere, play critical roles in extracting nutrients from soil and transferring them to host plants. Bacteria excrete compounds or enzymes that mobilize iron and other metals, acquire nutrients such as phosphorus and nitrogen, and produce phytohormones with regulatory properties for host plants (Chepsergon and Moleleki 2023). Additionally, mycorrhizal fungi can extend nutrient uptake beyond the plant roots by a factor of 1,000 (Larcher 1995) and alter phytohormone concentrations to promote drought tolerance (Bahadur et al. 2019). In return, the plants allocate a substantial amount of carbon to the underground fungal network, roughly equivalent to one-third of the annual carbon dioxide emissions from fossil fuels (Hawkins et al. 2023). Thus, gut and soil microbiomes play vital roles in hormone regulation and nutrient acquisition.
In both humans and rhizosphere soils, beyond providing nutritional benefits, the microbiome acts as a formidable barrier against the invasion and proliferation of pathogenic microorganisms (Nakatsuki et al. 2017; Zheng et al. 2020; Gu et al. 2022). In humans, microbiomes confer this resistance by induction of an immune response, competitive exclusion to make colonization inaccessible to other pathogens, production of antimicrobial substances, maintenance of intestinal barrier function, and immune training in early life (Zheng et al. 2020). In plants, commensal microbes can serve as a passive barrier by occupying niches that become inaccessible to pathogens, but current evidence also suggests that the plant immune response may be partially suppressed to allow for commensal colonization (Durán et al. 2018). Taken together, these findings highlight the complexity of microbial interactions in promoting host health and defense mechanisms, offering valuable insights for both human health and agricultural practices.
This report commonly refers to microbiomes associated with their broad habitat categories such as the gut and rhizosphere, but it is important to acknowledge that each habitat comprises vast microheterogeneity. Therefore, generalizing shared features may oversimplify the diverse metabolic regimes that exist in each compartment and span small physical distances or fluctuate over daily temporal scales. For instance, within the intestinal lumen, microbial density increases from the stomach to the colon, and there are distinct gradients of microbiota and metabolic lifestyles between the lumen and the adjacent mucus layer (Li et al. 2015). Similar nutrient gradients are observed between the bulk soil and rhizosphere, and even within the rhizosphere, where specific taxonomic compositions and functionalities are associated with different microenvironments (Ling et al. 2022; Fitzpatrick et al. 2023).
The committee also recognizes that there are differences across gut and rhizosphere systems, which provide unique challenges in the microbiome methodologies and their analyses, a feature highlighted below in Table 7-1. For example, rhizosphere systems are external to their host and embedded within the environment, which means these microbiomes experience diurnal and seasonal fluctuations in temperature, nutrients, and water availability and are open to microbial dispersal. In contrast, the gut system is internal and is well modulated by the host in temperature and pH, with dispersals only happening via ingestion. These differences contribute to differences in the active and dormant members in each system, with proportionally more dormant cells in the soil and rhizosphere than in the gut (Lennon and Jones 2011), a finding that has ramifications for how the communities are composed, structured, and buffer perturbations. In summary, the committee has attempted to provide threads of comparison and context for identifying areas of synergy and perhaps knowledge transferability, while still recognizing the domain specifics that contribute to these unique microbiota, their derived processes, and host relationships across soil and human habitats.
TABLE 7-1
Microbiota-Based Content and the Use of This Information for Establishing Health in Human, Soil, and Plant Systems.
Microbiomes as Diagnostic Agents for Health
Given the shared roles as modulators of chemical transformations and host-interactions in human and soil systems, the potential exists to use microbiome content as health indicators in soil, crop, and human systems. Because microbial catalysis is responsible (either directly or a few steps removed) for many of the chemical indices used today as health indicators, it is also believable that the microbiome functional content underpinning these transformations could serve as an even earlier indicator of health changes prior to measured chemical differences. In this scenario, microbiomes themselves may act as canaries in the coal mine, providing early warning signals for other desirable or undesirable outcomes (Gu et al. 2022). While the soil and human microbiomes have considerable potential to serve in this capacity, the ability to define and interpret microbial health indicators is limited today by sparse understanding of the ecology and function of microorganisms in both soil and human systems. Bridging this gap, along with experimental design and methodological advances for sampling and analyzing microbiome content along health states, will improve the incorporation of microbiome data in health monitoring (Wilhelm et al. 2023).
This section provides an inventory of microbiome features that are used to infer health in both human and soil systems and is organized based on different microbial units that have been used, or proposed to be used, as health indicators. The committee categorized the microbiome into four constituent classes that can be used to inform health status: (1) biomass, (2) compositional, (3) functional, and (4) interaction. Measurements derived from these framing categories can be used as indicators themselves (e.g., biomass) or used to calculate new indices (e.g., diversity metric) of health status in human, soil, and plant microbiomes. Table 7-1 summarizes a selection of these microbiome features that are used or proposed for use in diagnosing human and soil health conditions. The table outlines assumed relationships between the microbial measurement and health status and identifies some caveats with the application. Examples included in this table are not meant to be exhaustive, and they are organized first by microbiome measures that are actively used in clinical human diagnostics or soil health assessment frameworks today (as denoted by [in use]). Approaches under development today are indicated in the bottom half of the table. The interaction framework for health is nascent such that it is described in the text but not included in Table 7-1.
Discerning health metrics within and between the biological categories of biomass, composition, and function has relied on a variety of methods based on cultivation (e.g., colony forming units), molecular (e.g., quantitative PCR, amplicon sequencing), functional (e.g., enzyme and physiological assays), and -omics inspired approaches (e.g., metabolomics, metagenome, metatranscriptome, metaproteome). As such, Table 7-1 is not organized by method, as many of them measure different aspects of biomass, taxonomy, and functional components of the microbiome. For example, cultivation-based approaches can provide microbial load (biomass insights) as well as specific types of microorganisms (compositional insights). Similarly, both amplicon sequencing and metagenomics uncover the microbial membership and distribution in a sample. In the text that follows, the four microbiome constituents are described in subsections, articulating the importance of diversity metrics (composition), chemical catalysis (function), and food webs (interaction) as promising diagnostic indicators of ecosystem health and functioning, providing examples from soils to humans. This section is ultimately written to highlight microbiome indicators in use today but also highlight the promise of emerging technologies for deriving new microbiome-informed indicators. Although enthusiasm is universally shared across human and soil systems, the practice and quantifiable metrics of health using microbiome-derived information are not well defined at present.
Biomass Measurements of Health
Quantification or enumeration of biomass and its inferences to health varies across soils and human systems. In soil systems, microbial biomass, a measure of the mass of microbes both active and dormant per gram of soil, is a parameter often used to assess soil health. In fact, a third of soil health assessment tests, such as those from the European Commission or the Soil Management Assessment Framework, recommend microbial biomass measurements as a critical estimate of the biological properties of soils (Lehmann et al. 2020). This metric is estimated using a variety of methodologies, with lipid measurements or a chloroform fumigation-incubation being the most prescribed. Because microbial biomass accounts for a large proportion of total soil carbon and nitrogen, higher microbial biomass content or carbon is often considered as soil fertility constituent (Sparling 1997).
Fungal biomass is used as a biological indicator of soil health, as not only are these eukaryotic organisms vital to carbon and nutrient cycling but their hyphae and products play critical roles in improving soil structure and water retention. Typically, fungal biomass is assessed through lipid-based or ergosterol content or through microscopic quantification of hyphal biomass. While conventionally managed soils are reported to exhibit decreased fungal biomass relative to more regenerative management strategies, a response thought to be due to tillage, high rates of fertilization, and fallow, this response is not always uniform (Frąc et al. 2018). In addition, the number of fungi versus bacteria in given soil, expressed as the fungal:bacterial ratio, has been historically used as an indicator of soil health, with higher ratios indicating a more sustainable soil system with higher carbon accrual. However, recently this ratio has received criticism as it is affected by methodological constraints that particularly impact fungal assessment in soils and it does not reflect current ecological understanding of complex, multi-trophic soil food webs (Fierer et al. 2021). For instance, some fungi and bacteria have overlapped or syntrophic functionalities in soils, which are functionalities that will not be captured by this ratio.
While biomass or its derivatives is one of the most used metrics for assessing soil health, the application of microbial biomass in health assessment has confounding interpretations. Recently, it has been debated whether biomass provides a useful, or readily interpretable, assessment of soil health (Fierer et al. 2021), as there are many biotic and abiotic factors that could contribute, directly or indirectly, to changes in soil microbial biomass. For instance, biomass does not account for the types or diversity of microbes present in a sample, such that overgrowth of a pathogen or single microbial type would increase overall biomass but would not necessarily be considered healthy. Additionally, more biomass does not necessarily equate with specific “healthy” catalytic properties of microorganisms. Despite these concerns, microbial biomass or microbial biomass carbon remains one of the commonly recommended biological-based indicators of healthy soils in use for soil health assessments.
Demonstrating how microbiome indicators are often ecosystem specific, broad measurements of microbial biomass were not commonly used as an indicator in host health in past human research. However, current work is increasingly recognizing the need for assessments of absolute abundance in addition to relative abundance of microbes in a microbiome. Specifically quantifying populations of microorganisms associated with disease, such as pathogen loads, is used to diagnose health in human and plant systems. Pathogen biomass can be enumerated using cultivation-based approaches to measure the amount of a specific type of microbe in sample mass or volume (e.g., selective media or most probable numbering techniques) as well as with molecular approaches that determine the abundance or relative abundance of a taxon in sample mass or volume (e.g., quantitative PCR and 16S or internally transcribed spacer region [ITS] ribosomal amplicon sequencing). Given that some taxa are obligately pathogenic, the presence of these members can be easily ascribed to poor health conditions observed in plants or human individuals. Yet, interpreting pathogen content is not always so clear-cut, as pathogen abundance does not necessarily correlate to disease severity (Swanson et al. 2007; Genin and Denny 2012; Leggett et al. 2012; Yadav and Pandey 2022). Further, many pathogens are opportunistic, meaning they can be present and active in healthy individuals, and disease severity is controlled instead by other conditions, such as stress or host immunity (Kaper et al. 2004). Thus, the biomass of targeted taxa is not always a robust indicator of health and depends on the taxon and the ecosystem.
Measuring Diversity and Composition of the Microbiome
Amplicon sequencing is a targeted approach that uses deep sequencing of a single gene to provide information on the diversity and composition of the microbiome. The sampling of bacteria and archaea (16S rRNA gene), fungi (ITS), and eukaryotes (18S rRNA gene) has provided new dimensions on the microbial community constructs that contribute to health across ecosystems. Data from amplicon sequencing projects can provide three lines of microbial information: (1) measurements of diversity such as richness (number of types of microbes in a sample) or evenness (their distribution in a sample), (2) the microbial composition (which microbes are present in a sample), which provides insights into taxa that are indicators of disease or health status (Wilhelm et al. 2022), and (3) the relative abundance of each member in a sample, which describes the distribution of microbial members (e.g., enrichment or dominance that can occur along a health axis).
Today, because of increased affordability and streamlined data analytics, there is widespread adoption of amplicon-based analyses of microbial communities across habitats from soil to plant and human microbiomes. This reduced cost per sample, and the ability to process hundreds to thousands of samples, allows researchers to better contextualize heterogeneity with a sample site, developing more robust temporal and spatial awareness of microbial diversity and membership dynamics. For example, it is now warranted to sample microbial communities during different temporal stages of soil management or crop and human development, both in health and disease, to capture monthly and yearly scale changes in the microbiome (Lauber et al. 2013) or even hourly responses to daily routines and fluctuations (e.g., temperature and feeding). Similarly, more intensive sampling of soil compartments (e.g., rhizoplane from rhizosphere) or anatomical sites (e.g., different sections of the gastrointestinal tract) will further discern microbiota responsive signals from natural variation (Singh et al. 2020; Chinda et al. 2022). Additionally, in soils, regional differences in climate and soil type, as well as local land management differences, can make comparisons of microbiome diagnostic patterns across studies or even samples within closely related plots confounding. Similarly, human genetic backgrounds, the history of clinical treatments, and variations in diets, age, and other factors confound universal microbiome metrics for human health (Falony et al. 2016). Amplicon sequencing today is an important tool for allowing researchers to sample this heterogeneity in microbiomes across different gradients of variation.
Metrics derived from amplicon sequencing, either organismal or diversity-based indicators, are being evaluated as diagnostic tools that could be incorporated into formalized health assessments in the near future. In this chapter, the committee focuses on compositionally defined diversity as an indicator of health as it is used in both soil and human microbiomes. Yet, the committee recognizes that diversity can be measured on any level of biological organization (Whittaker et al. 2001) and that microbiome diversity calculated using gene diversity, gene expression, or metabolite profiles may become more commonplace indicators of health status in the future as these methods have greater development and adoption as well (see the section “Functional Components of the Microbiome” below).
The explanation of how compositional diversity can relate to health is best understood through the lens of the insurance hypothesis. Originally proposed by Yachi and Loreau (1999), this hypothesis suggests that increasing biodiversity can insure ecosystems against functional declines in response to environmental perturbations (see also Box 3-1). This stability is achieved because different species respond differently to environmental fluctuations, but they can have overlapping functions within an ecosystem (i.e., functional redundancy). Therefore, greater species diversity increases the likelihood of maintained functionality in response to perturbations. With advancements in measuring functional diversity directly (through gene content or expression, see the discussion in the next section), it may no longer be necessary to be inferred from compositional richness, which may or may not be directly related to functional content at the scale required to withstand environmental perturbations.
Diversity measurements based on compositional data are often touted as indicators of health (Lozupone et al. 2012). In the human gut, the presence of a higher number of microbial members (richness) is inferred to make the microbial community more resistant to stressful events, such as antibiotic treatments (Lozupone et al. 2012). Moreover, it has also been shown that richer and more diverse microbiomes can guard against pathogen proliferation (Zheng et al. 2020; Bertola et al. 2021). On the other hand, decreased compositional diversity in the microbiome has been linked with chronic conditions such as obesity and type 2 diabetes, gastrointestinal diseases, and neurodegenerative diseases, among others (Cho and Blaser 2012; Fan and Pedersen 2021). Compositional diversity metrics may be used to assess health status more broadly. For example, gingivitis (gum inflammation) is associated with a lower diversity of the oral microbiome and the dominance of the pathogen Porphyromonas gingivalis (Hajishengallis et al. 2012). The findings suggest that microbiome compositional diversity could be an indicator of health in adult humans.
Microbial communities are structured by a few more dominant members followed by thousands of rare members. These rare members are thought to act as a seedbank, preserving genetic diversity until it is needed. Although not abundant, these rare members can become provisionally enriched under specific environmental conditions, maintaining ecosystem performance in the face of changing conditions (Shade et al. 2014; Jousset et al. 2017). It is regarded that increased microbial compositional diversity or richness, often due to a large number of rare taxa, contributes to higher nutrient-cycling rates (Fierer et al. 2021). Additionally, in the rhizosphere, as in humans, increased microbial (both fungal and bacterial) diversity is associated with pathogen suppression for crop wellness (Bollmann-Giolai et al. 2022; Ling et al. 2022). Conversely, heavy metal contamination has been associated with a decrease in bacteria, fungi, and protist diversity due to both a loss of rare members and the increased dominance of a few select taxa that can resist changes in the abiotic conditions (Qi et al. 2022). In fact, the four most common threats to soil microbial diversity are cited as intensive human exploitation, land use change, soil contamination, and climate change (Jeffery and Gardi 2010; Tibbett et al. 2020). Thus, as emphasized in other chapters of this report, there is an ever-increasing interest in maintaining soil microbial diversity to maintain soil functionality and health.
Beyond microbial compositional richness and diversity, the relative abundance and taxonomic identity of members can serve as indicators of health status. For example, in the vaginal microbiome, the genus Lactobacillus play a major role in maintaining female health—such that increased lactobacilli prevent against vaginal infection and reduce the risk of acquisition of HIV and sexually transmitted pathogens (Das Purkayastha et al. 2020). Despite these clear linkages in research publications, not enough is known about the vaginal microbiome and disease to be able to add lactobacilli probiotics to reliably shift in the direction of improved health (France et al. 2022). Also, large cohort studies of healthy and unhealthy individuals combined with machine-learning approaches are paving the way for discovering new microbial classifiers of health (Lee and Rho 2022). Despite compelling results from individual studies, further investigation is needed to reveal the roles of human microbiota in all body habitats in order to support the development of microbiome-based diagnoses and therapeutics (Hou et al. 2022).
Compared to the human microbiome, specific microbiome-derived indicators of health are less defined in soil systems. There are generally regarded plant beneficial microbes that are used as inoculants (see Chapters 4 and 5), but more extensive studies assessing the presence, plant colonization, and persistence of biostimulants in the rhizosphere are warranted. It is also unknown whether taxa extant in soils can respond to land management to serve as indicators of health. To investigate this possibility, a study surveyed more than 900 agricultural soils from diverse geographic locations and management types across the continental United States, with each soil ranked with a health score based on biological, physical, and chemical measurements encompassed by the Comprehensive Assessment of Soil Health (CASH) framework (Wilhelm et al. 2022). Researchers identified microbial taxa that differentiated soils with low and high health scores, and these new organismal indicators also predicted conventional metrics of soil health. Although it is a single study and not partitioned for regional or crop differences, the results indicate that, as with the human gut, microbiome compositional information obtained through amplicon sequencing could inform measures of health status.
However, in human and soil systems, using ribosomal RNA (rRNA) and ITS-based metrics for assessing health in microbiomes is not without constraints (Ames et al. 2017; Matchado et al. 2024). Firstly, these methods provide limited taxonomic resolution and cannot differentiate well-known pathogens (e.g., Salmonella typhi from E. coli), necessitating higher resolution for accurate identification of health-associated microbial taxa. Secondly, biases in data processing and reliance on incomplete databases can skew detection and interpretation of microbial groups in the microbiome. Additionally, while taxonomic composition may offer some limited insights into potential functional roles (Nguyen et al. 2016; Douglas et al. 2020), these methods do not fully represent the overall metabolic activity or functional potential of the microbiome. Moreover, using DNA as input material fails to distinguish active from dormant microbes or extracellular DNA (Lennon and Jones 2011; Carini et al. 2016), which are prevalent in soils and the human gut and could further overrepresent inactive members. Understanding the distinction between active and dormant microbiota, along with their functional capacities, may become crucial indicators when assessing implications for soil and human health. While rRNA and ITS sequencing provide valuable insights into microbial community composition, diversity, and dynamics, their use in health assessment could be bolstered with other methods discussed below and careful consideration of their limitations.
Functional Components of the Microbiome: A Promising Indicator of Health
Assessing metabolic potential
There is broad recognition that harnessing microbiomes for improving plant, human, and soil health requires decoding the genetic underpinnings of the microbiome and how these molecular constituents are translated into chemical changes and ecosystem outputs. This is no trivial task, but newer -omics technologies, such as metagenomic sequencing, and other multi-omic technologies, such as metatranscriptomics, metaproteomics, and metabolomics, can provide advanced functional insights into the microbiome (Figure 7-3). This section defines these methods and begins to illuminate how these tools and their measurement of the functional aspects of the microbiome may be called upon to assess health status in human and soil systems in the future.
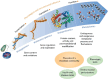
FIGURE 7-3
A schematic illustrating the information gained from each -omic technology and how their integration is essential to fully understanding the phenome. SOURCE: Used with permission of Springer Nature BV, from “A Multi-omic Future for Microbiome (more...)
It is increasingly possible to use untargeted sequencing approaches to inventory the cache of gene content in a microbial community, an approach known as metagenomics (Jansson and Baker 2016). Metagenomics approaches are best developed for bacteria and viruses (Kang et al. 2017; Emerson et al. 2018; Leleiwi et al. 2023), where results have showed greater ability to predict ecosystem outputs and health status. These methods are still in development for eukaryotic members like fungi and protists (Donovan et al. 2018). Instead of community-wide genomics (metagenomics) for eukaryotes, valuable insights into the functional attributes or traits of fungi have been gleaned from genomic approaches that sequence specific taxa individually or map environmental sequences to genomes derived from relevant isolates (Treseder and Lennon 2015; Ravn et al. 2021; Zhang et al. 2023). Microbial gene information derived from genomic sequencing not only yields taxonomic content (although not as deeply sampled as amplicon-based sequencing) but also provides critical measurements on the genes that govern metabolic or functional capabilities in a microbiome.
Specifically, metagenomics has advanced knowledge of human health, uncovering new metabolic assignments for previously uncultivated lineages (Wrighton et al. 2012; Di Rienzi et al. 2013), identifying potential for microbially derived antibiotics and secondary metabolites with health-promoting benefits (Crits-Christoph et al. 2018), and outlining the metabolic wiring underpinning the functional outputs critical for soil and human health (Table 7-1). Advances in data processing (software), decreased sequencing cost, and improvement of databases for data processing (Pasolli et al. 2019) have made it possible to apply metagenomics to larger cohort sizes and to utilize sequencing more effectively within a sample. The metagenomic-derived functional gene profiles derived from fecal microbial genomic content can predict human heart disease (Borton et al. 2023) and various types of cancers, from colon to prostate (Banerjee et al. 2015; Gao et al. 2022). Additionally, metagenomics can rapidly identify infectious disease-causing pathogens, such as bacteria, viruses, and fungi in a single test without the need for culturing (d’Humières et al. 2021), thereby playing a promising role in the clinical diagnoses of disease (Box 7-1). Yet, to date, there remain few FDA-approved microbial therapies (Jain et al. 2023).
BOX 7-1
Human Microbiome Project.
In comparison to human clinical assessments, metagenomic studies have not been used to study soil health as often (Duque Zapata et al. 2023), and larger cohort studies with paired genomic insights and robust physical, chemical, and biological soil health indicators are needed. To these ends, genome-resolved metagenomic databases from soils are on the rise both in the public (Woodcroft et al. 2018) and private sectors,1 however, developing an open, collective database infrastructure, such as the Human Microbiome Project, and targeting agricultural soils with paired soil health dataspecifically, could advance the discovery of microbial gene indicators in these soils. These efforts are buoyed by additional large-scale global soil microbiome catalogs, such as the Earth Microbiome Project (Thompson et al. 2017) and the federally funded Genomic Encyclopedia of Bacteria and Archaea project (Whitman et al. 2015). Yet, more studies are needed across geographic and agricultural landscapes with paired microbiome and soil health measurements (Wilhelm et al. 2022) to begin fortifying the predictive capabilities of the microbiome for soil health and fertility. The federal government has taken steps to develop the necessary infrastructure for sharing data across projects with a project called the National Microbiome Data Collaborative, which supports a Findable, Accessible, Interoperable, and Reusable (FAIR) microbiome data sharing network, through infrastructure, data standards, and community building (NMDC 2022).
However, reusing microbiome data collected across many different studies for machine-learning approaches (Box 7-2), especially those spanning analyses across soil and human compartments, is not trivial today. Barriers include that the data are often housed without metadata standards, hindering reprocessing of the data to create uniform analysis metrics. Public microbiome data are also not housed with health outcome data, and the latter is often linked within studies rather than housed in public repositories. Beyond data organization, while many artificial intelligence approaches may uncover genes or sets of genes linked to disease, many times these “predictors” lack accurate biochemical annotations with unknown functional outcomes. Thus, both from a data-mining point of view and a biochemical knowledge perspective, empowering gene content into health frameworks will require ongoing research. Yet, there are enough promising studies, especially on the human microbiome side, highlighting the promise that this content can be used for microbiome-inspired diagnostics and interventions.
BOX 7-2
Machine Learning and Artificial Intelligence for the Microbiome.
Activity assessment of the microbiome
While metagenomics provides insights into the gene-encoded metabolic potential of a microbial community, it may not fully capture the dynamic responses to environmental perturbations (Jansson and Baker 2016). Instead, functional assays, which measure or attempt to measure active processes in the microbiome, may be more direct indicators of health. These can include either (1) targeted approaches that use process-based assays or (2) untargeted approaches that measure expressed gene products, such as transcripts or enzymes. In soil health assessments today, enzyme assays are commonly used as biological indicators. For example, beta-glucosidase and cellobiohydrolase assays are employed to estimate carbon decomposition activity in the Soil Management Assessment Framework (Stott et al. 2010), an assessment tool to evaluate impacts of land management on soil quality. While these process-based assays provide valuable insights into targeted processes like nitrification or potentially mineralizable soil nitrogen, their interpretation may be complicated by soil matrix effects and the inability to effectively capture all relevant processes, and the need for further validation persists (see Table 7-1 for a detailed discussion).
Untargeted approaches, such as metatranscriptomics (the sequencing of the transcribed cellular content in a sample) and metaproteomics (mass spectrometric analysis of proteins), sample the expressed gene content in soil and human microbiomes (Aguiar-Pulido et al. 2016). These methods offer opportunities to identify new, more comprehensive targets beyond those represented in the handful of established enzyme assays today, but they also can be more responsive than metagenome methods (or other DNA-based approaches) because they reveal an immediate response to perturbations (e.g., land management, diet interventions, disease onset). In both human and soil systems, metatranscriptomics and metaproteomics are technological advancements that could be leveraged for measuring and monitoring health aspects, offering new directions for research and clinical diagnosis and management (Berg et al. 2020; Bertola et al. 2021). While promising and growing in their application in soil (McGivern et al. 2021; Starke et al. 2021) and human microbiomes (Long et al. 2020; Borton et al. 2023; Wang et al. 2023), these methods are often only as robust as their underlying database used to contextualize the expression data, which can be challenging in samples with high strain diversity. The data collection can be affected by the heterogeneity of the matrix, and, compared to other methods, the cost to process and analyze at scale to identify health outcomes is limiting (Issa Isaac et al. 2019). These or other methods, such as quantitative stable isotope probing (Hungate et al. 2015; Wilhelm et al. 2021), probe-based functional profiling (Whidbey and Wright 2019), or flow cytometric single cell metabolic assays (Salazar et al. 2019), may further quantify the abundance of active and dormant microbes and processes in the future, providing better descriptions of microbial traits relevant to soil and human health outcomes.
Likewise, metabolomics—the study of low-molecular-weight organic compounds—can be targeted (directed at specific chemical compounds) or untargeted (simultaneous measurement of large number of compounds in a sample). This approach offers insight into the metabolic status of a sample, measuring the chemical products of the biological community. In humans, the detection of metabolites produced by gut microbes from dietary metabolism has been linked to pathologies such as hypertension, atherosclerosis, heart failure, obesity, kidney disease, and type 2 diabetes (German et al. 2005; Tang et al. 2019). In soil systems, metabolite approaches are used but trail studies in humans (Ellenbogen et al. 2024; Song et al. 2024) and are less developed for measuring health status. However, a recent study using nine different topsoils along a land use gradient with paired chemical and physical soil health data showed that the untargeted detection of more than 400 soil metabolites had discriminatory power as a potential soil quality indicator (Withers et al. 2020). Additionally, measurements of soil metabolomes from 188 backyard soils across 14 U.S. states demonstrated that soil metabolomes reflected the effects of local factors such as temperature, light level, and human activities on the soil (Nguyen et al. 2020). However, methodological improvements, including overcoming extraction biases from matrices, enhanced annotation and identification of soil metabolites, and discerning the fate of metabolites due to transient fluxes, will be necessary to identify the close relationships between microbiota and metabolomes in a wellness context from complex microbiomes in soils and the human gut (Song et al. 2024).
Both in human and soil microbiomes, multi-omics or the complementary use of multiple methods (metabolite, proteomic, genomic, transcriptomic approaches, see Figure 7-3) can provide more definitive evidence on the microbial metabolic pathways related to health status. The next step is to establish metrics to evaluate performance aspects of metabolomic and other multi-omic methods under a wide range of health and disease conditions, as well as management regimes, so that they can be used for the quantitative assessment of human health or soil health (Withers et al. 2020).
Interactions: An Unstudied Metric of Health
Biodiversity includes not only the number and type of species and their abundances but also the complex interactions among different species. There is growing appreciation for network-based analyses that incorporate taxonomic (co-association or abundance network) or functional (co-expression network) content to estimate the interactions that occur among different species and their contributions to ecosystem functioning (Harvey et al. 2017; Wagg et al. 2019; Box 7-3). It should be noted that these networks do not provide explicit evidence for species interactions but suggest that organisms or the gene content co-occur over space and time, hinting at a possible interaction potential. Networks constructed from taxonomic or functional information can be assessed for their connectivity, network size and structure, and relationship to health or ecosystem properties (Shi et al. 2016; Banerjee et al. 2019; Dundore-Arias et al. 2023). It is hypothesized that more complex and connected networks are more robust to disease and other biotic perturbations as well as abiotic stressors (Barabási et al. 2011). In support of this, a recent study of bacterial rhizosphere networks in tobacco plants strongly affected by the pathogen Ralstonia showed that disease-suppressive soils had greater network complexity and that the bacterial abundances of highly connected keystone taxa within disease-suppressive soils were negatively correlated with pathogen density (Zheng et al. 2021). Similar network approaches have been used in human gut microbiomes to identify coordinated species and pathways associated with inflammatory bowel disease that are absent in obese and healthy population cohorts (Chen et al. 2020). While nascent, network analyses that use culturing and multi-omics approaches to capture the interactions of phenotypes could be indicators of health in the future.
BOX 7-3
Viruses.
Building on the ideas of trophic structure comprising all soil biota, not just viral, archaeal, bacterial, or fungal members, it is increasingly apparent that biodiversity maintenance is an essential constituent for soil health. Here, the idea in soils is that preserving belowground biota from microbes to protozoan to animals such as nematodes and earthworms is critical not only for soil functioning today but also for long-term biodiversity management with repercussions extending to human health. Collectively, a loss of soil diversity (microbes, fungi, protozoa, and fauna) has been associated with lower plant diversity and crop yields, leading to reduced food security, erosion that reduces air and water quality, and increased soil-borne pathogen and pest load, which all can have indirect and direct human health impacts (Wall et al. 2015). Consequently, current research initiatives across global organizations, such as the Global Soil Biodiversity Initiative, are committed to inventorying and maintaining soil biodiversity to protect soil and human health. Beyond microbial biomass, taxonomic, and functional metrics, soil biodiversity management should target the ecological complexity and food web architectures necessary to provide robustness of soil ecosystem services. In summary, an ecosystem management approach to health in soils can be extended to managing humans and plant health more holistically. As stipulated in the One Health framework, there is a recognized need to move beyond managing a single aspect of health, with biodiversity stewardship and overall ecosystem wellness being increasingly realized as a metric unto its own (FAO, UNEP, WHO, and WOAH 2022).
While promising, most of the modern -omics-based metrics are exploratory and have not been readily incorporated into human clinical or soil health assessments (Table 7-1). Similarly, interactions as a measurable unit of health is a newer concept not widely adopted today. Given the complexity of microbiomes, the reality of creating a robust, scalable health index using a single microbiome measurement (e.g., gene, organism, metabolite, interaction network) is unlikely. This situation is not entirelydifferent from applications of health assessments in humans and soils today, which use a handful of metrics derived from physical, biological, and chemical measures for a more comprehensive picture of wellness. To discover new indicators or features of the microbiome that can predict health in humans and soils (see Box 7-2), enhancing the spatial and temporal resolution of the data stored in collections, moving beyond a handful of 10–100 samples per study to thousands, will help resolve localized responsive “signals” across a background of heterogenous “noise.” Additionally, these analyses need to be performed when there is a clear health outcome or gradient to compare to, to detect capacity of microbiome-derived indicators to predict different health status. Artificial intelligence approaches will likely play a critical role in this discovery given the deluge of data produced from modern -omics methods. Rather than being derived from genes or sets of gene content, it is likely that microbiome functional estimates of health will be distilled to aggregate properties that capture more responsive indicators that may function as a final single health index (Lehmann et al. 2020).
SOILS, MICROBES, AND HUMAN HEALTH
Though microbes are often associated with disease and infection, the relationship between microbes and human health is far more complex. Research over the past few decades has unveiled the indispensable role of exposure to microbes in human development and the maintenance of health, notably on the immune, metabolic, and central nervous systems. Both exposure to environmental microbes and colonization of the human gut, oral cavity, lung, skin, and urogenital tracts appear to be important for normal function and protection against pathogenic microorganism and toxins (Thompson et al. 2017). Microbes outnumber human cells in the body, and their metabolic products make up over a third of the small molecules in the peripheral blood, many of which affect physiology (Wikoff et al. 2009).
To what extent exposure specifically to the soil microbiome influences human health is as yet unknown. Despite the growing capacity to characterize taxa and study the functions of microbiomes in humans and other systems through next-generation sequencing, the full continuum between soil health and human health by way of microbiome influence is not established in the literature. Additionally, even when skipping past the soil microbiome for which the metrics are under development, the committee found limited research that spans the investigation on how soil health more broadly may influence the nutrient density of foods in a way that would affect the gut microbiome’s metabolic capacity or human health (Chapter 5). Further, the committee found inconclusive support for the direct linkage of soil microbiomes to human gut microbiomes leading to human health outcomes. As such, this section focuses on areas where promising linkages have been made for (1) environmental microbes as immunomodulators for human health, (2) soil microbiomes and gut microbiomes in animal models, and (3) the gut microbiome as a connector between nutritional inputs and human health.
The Hygiene Hypothesis and the Importance of Balancing “Good” and “Bad” Microbial Exposures
Humans, like all eukaryotic life forms, have evolved from microbes and have co-evolved to form a symbiotic relationship with microbes (Domazet-Lošo and Tautz 2008). For example, the immune system is dependent on receiving appropriate inputs from microbial interactions, and these must be received early in life and then maintained and updated throughout life (Bach 2018; Rook 2021). It has been shown that maternal and familial transfer of microbiota is crucial for the development of an infant’s microbiota and that lifestyle factors that reduce this transfer and correlate with increased immunoregulatory disorders include caesarean deliveries, lack of breast feeding, antibiotic use, and diet (Penders and van Best 2022). Environmental exposures, including proximity to green space and farms, has also repeatedly been shown to be influential (von Mutius 2021). For example, Hanski et al. (2012) showed that environmental biodiversity based on land use data was correlated with a greater diversity of commensal skin bacteria commonly found in soil and vegetation, which translated into functional measurements of serum immune markers and lower rates of allergic disease.
Exposure to the environment and microbes is essential to the selection of a repertoire of cells that can eliminate pathogens while tolerating the microbiota and avoiding self-recognition and autoimmunity. Therefore, each individual develops an immune repertoire that is matched to the microbial world into which he or she is born and resides (Rook 2021, 2022). The individualization of this process has important implications for health. For example, purposeful infection with soil-transmitted helminths2 has shown some efficacy in early-stage trials for treating autoimmune diseases in humans, but later-stage clinical trials have not confirmed these results (Ryan et al. 2020). It may be that the elimination of helminth exposure during childhood in some parts of the world plays a role in the response (or lack thereof) to exposure to helminths later in life to treat autoimmune diseases (Rook et al. 2015).
The hygiene hypothesis, first proposed in the late 1980s based on data showing an inverse correlation between hay fever and the number of older siblings, suggests that reduced exposure to infections and microbes during childhood may contribute to the increasing prevalence of allergic and autoimmune diseases in developed countries (Strachan 1989). The hypothesis, popularized by the media, highlights the paradox that while hygiene is needed to avoid exposure to dangerous pathogens, humans also need exposure to “beneficial” microbes. Rook (2022) suggests a framework for reconciling these conflicting needs, as illustrated in Figure 7-4.
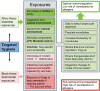
FIGURE 7-4
Essential microbial exposures and nonessential or detrimental exposures. NOTES: The essential exposures include the microbiota of mothers, family, and the natural environment. Some of the benefits derive from molecular signals rather than colonization. (more...)
A common interpretation of the hygiene hypothesis is that humans have become “too clean for our own good.” Early humans lived in shelters built from natural products that were likely to provide beneficial microbial exposures. In contrast, modern homesare largely built from synthetic products, and bacterial and fungal microbiota frequently invade damp and deteriorating modern homes and can produce infections (Rook and Bloomfield 2021). Therefore, upkeep and cleanliness are important. However, this hygiene must be balanced against the risks of exposure to cleaning agents that may have immunostimulatory properties. Exposure to antigens via sites like the gut, airways, or skin in the presence of toxins, common in cleaning products, activate TH2 immune responses and can result in allergies and autoimmune disease (Akdis 2021; Rook and Bloomfield 2021). Therefore, both building products and household cleaners are likely to influence escalating rates of allergic and autoimmune disease.
Another common misconception is that childhood infections are necessary to “strengthen” the immune system. Today’s common infections of childhood are mostly crowd infections that were not present during most of human evolution, and the risks of infection likely outweigh any potential benefits of long-term and nonspecific immunity (Rook and Bloomfield 2021). Moreover, many infections can now largely be replaced by vaccines, which have been shown to have disease-specific benefits and to improve resistance to other infectious agents (Dagenais et al. 2023).
Putative Role for Soils as Influencers of Gut Microbiomes
From animal models, there is evidence that soil biodiversity is interrelated with the mammalian gut microbiome. For example, a study assessing the impact of different lifestyles on the development of mouse gut microbiomes found that mice in contact with soils and dust harbored a gut microbiome with greater diversity and richness (Zhou et al. 2016). While this diversity was not correlated to nutritional performance or overall health, it was found that these mice had lower serum immunoglobulin. Such results indicate that contact with soils could enhance gut microbial diversity and innate immunity, in support of the hygiene hypothesis by way of the gut microbiome and soils. Supporting the stimulatory role of soil biodiversity for gut microbiome diversity, another study found that gut microbial diversity increased in mice that were in contact with non-sterile soil, while it was unaffected when the mice were in contact with sterile soil (Zhou et al. 2018). The study also showed that non-sterile soils were a key factor influencing the gut microbiota and its effect was comparable to the effect caused by diet, which is recognized to have an impact on human health (see the section “Gut Microbes, Nutrition, and Human Health” below). Given the positive health impacts illustrated by having a more rich and diverse gut microbiome, it is promising but not conclusive that contact with soil and its microbiome could be beneficial for animal health.
Supporting these findings, a study of baboons collected genetic material on 14 populations in southwest Kenya and examined 13 variables in each population’s environment (e.g., soil traits, vegetation) to understand the factors that supported gut microbiome membership and abundance across populations (Grieneisen et al. 2019). Neither host ancestry nor distance between populations were predictors of baboon gut microbial diversity. Instead, the effects from soil (namely geologic history and sodium content) were 15 times stronger than host population in predicting gut microbiome composition and diversity. It is possible that the baboons’ gut microbiomes are being colonized through geophagy via consumption of soil microbes with their food. Regardless of the route, the study lends support to soils and their biotic content playing a structuring role in mammalian gut microbiomes. However, the exact mechanism of how soil and the environment more broadly shape the human gut microbiome, and particularly how healthy and degraded soils (and their microbiomes) could differentially affect the gut microbiome, needs further evaluation (Blum et al. 2019; Banerjee and van der Heijden 2023). While poorly resolved today, the relationship between soil health, soil microbiome, gut microbiome, and human health may have relevance for preventive medicine (Blum et al. 2019).
Gut Microbes, Nutrition, and Human Health
The largest concentration of microbes in the human body can be found in the large intestine, with roughly 1011 microbes per gram of intestinal content (Sender et al. 2016). The gut microbiome is the most widely studied ecosystem in humans. This section focuses on diet as a major mediator of microbiome composition and human health. Primary aspects of nutrient processing by gut microbes involve extraction of calories and nutrients from ingested foods, and production of a vast array of metabolites that can be absorbed by the host with organismal-wide benefits far beyond the origin of absorption in the gut. Some examples of microbial products in the gut lumen include amino acids, short-chain fatty acids (SCFA), methylamines, and cometabolites that result from metabolism of human-derived compounds such as bile acids.
Dietary intake can influence gut microbiome composition both in the short and long terms. On the one hand, individual gut microbiomes respond differently to identical meals (Zeevi et al. 2015; Korem et al. 2017; Johnson et al. 2019), while commonalities in dominant taxonomic groups appear with similar dietary patterns, such as vegetarian or omnivore (Wu et al. 2011; Gorvitovskaia et al. 2016; Figure 7-5). Certain dietary components may have a high potential to shape the gut microbiome and human health. For instance, the Fiber and Fermented Foods (FeFiFo) study demonstrated that a diet rich in fermented foods substantially increased microbial richness and decreased inflammatory markers (Wastyk et al. 2021).
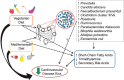
FIGURE 7-5
A schematic of known and proposed interactions between diet and cardiometabolic risk illustrates the known risk-reducing properties of vegetarian and Mediterranean diets on cardiometabolic diseases and the proposed interactions between the vegetarian (more...)
Notably, in the same study, the impact of a high-fiber diet depended on individuals’ baseline level of fiber consumption, suggesting that the metabolic capacity of the gut microbiota may hinge on its composition before significant dietary changes. The bacteria in fermented foods may have accounted for the benefits observed in the FeFiFo study, which exemplifies how, in addition to nutrients and other compounds, microbes in foods can influence the composition of the gut microbiota. Astonishingly, even a single apple contains approximately 100 million bacterial cells, and the apple’s microbial richness can be shaped by agricultural management practices (Wassermann et al. 2019). Recent research highlighted the role of fruits and vegetables as sources of human gut microbial seeding (Wicaksono et al. 2023), revealing that foods can be a key linkage between the environmental microbiome (including the soil microbiome) and the human microbiome. This research underscores the significance of agricultural practices and plant microbial diversity for human health.
Dietary patterns may promote the expansion of taxonomic groups that have the metabolic capacity to process frequently consumed nutrients by (1) making these nutrients more bioavailable, (2) extracting calories from frequently consumed macronutrients, and (3) metabolizing nutrients into compounds with either health-promoting or disease-inducing properties. The latter pathway has been covered in previous sections of this chapter, so here the focus is on the influence of dietary patterns on microbiota composition and the role of gut microbes in human energy metabolism.
The involvement of gut microbes in energy metabolism was first observed in animal studies where germ-free rodents gained less weight than their conventional counterparts when consuming the same caloric load. It was later shown that the obese phenotypecould be transferred between mice through the microbiome (Turnbaugh et al. 2006; Bäckhed et al. 2007). The same phenomenon was observed when transferring fecal microbial samples from humans with obesity to germ-free mice in a diet-dependent manner (Ridaura et al. 2013). Animal studies have shown that energy metabolism, and in particular glucose and lipid homeostasis, are affected by the gut microbiome (Bäckhed et al. 2004; Caesar et al. 2015). Further, microbes and their metabolites can regulate hunger and satiety signaling as part of the gut-brain axis (Fetissov 2017).
Understanding the mechanisms underlying the regulation of energy metabolism is important in the context of both under- and over-nutrition, which are responsible for the greatest disease burdens around the world. Evidence thus far shows that the potential primary pathways for caloric extraction from macronutrients involve the production of metabolites from nutrient precursors by gut microbes. It has been suggested that the liberation of molecules such as proteins from the constant turnover of the bacterial biomass in the gut lumen contributes to energy extraction (Fetissov 2017). In rodents, modification of dietary fatty acids by gut microbes has been shown to generate lipid intermediates that may modify host lipid profiles (Kishino et al. 2013). Most notably, fiber fermentation yields SCFA such as butyrate, which serves as an energy source for colonocytes (Canfora et al. 2015; T. Chen et al. 2017). These microbially derived SCFA can modulate metabolic and anti-inflammatory pathways in the human host (Canfora et al. 2015; Nogal et al. 2021), but these mechanisms are not yet fully understood. The connections between fiber, the gut microbiome, and human health have been extensively reviewed elsewhere (Martens 2016; Holscher 2017; Sawicki et al. 2017).
Americans are far from aligning their diets with dietary guidelines and, in particular, from reaching daily dietary fiber recommendations (King et al. 2012). Intakes are especially low among low-income households and marginalized groups (McAnulty et al. 2017). Diets low in fiber, as seen, for example, in populations migrating to the United States, have been associated with low richness and diversity of gut microbiomes (Deehan and Walter 2016). Dietary patterns rich in fiber have been connected to multiple gastrointestinal and cardiometabolic health benefits (Anderson et al. 2009; Sawicki et al. 2017; McKeown et al. 2020). Strategies to promote fiber consumption are therefore urgently needed to promote human health.
CONCLUSIONS
The evidence strongly suggests that microorganisms create a link between the health of soils and the health of humans, but the processes by which microbiomes are established and influenced across these systems are still unexplored. The similarities and dissimilarities between soil and human microbiome composition and function need to be studied in detail while considering the environmental conditions and other contextual factors that may account for the lack of research reproducibility thus far. The committee proposes that attention be paid to the following areas.
Robust Sampling
Microbiome functional or compositional information could serve as early indicators of health. However, all microbiome indicators from biomass to gene expression traits lack universal standards, as these metrics are often context specific or relative to other samples and do not have universal cut-offs for what constitutes health responses. Moreover, it is uncertain how microbiome-derived indicators maintain their signals or diagnostic capabilities over time and changing external landscapes. Fortunately, microbiome sampling is becoming more cost-effective and efficiency in data processing is improving, enabling researchers to address the microbiome heterogeneity across relevant experimentally defined spatiotemporal scales, which offers a better understanding of the variation in microbiomes in both untreated and response to different treatments and health gradients. This knowledge combined with statistical approaches and a priori data exploration (both in sequencing depth and numbers of samples) can support funding for microbiome experiments designed with appropriate rigor for indicator discovery (Calgaro et al. 2020). These higher-dimensionality sampled microbiome data, combined with better reuse of existing data across studies, are needed to build more robust artificial learning models to discover microbiome indicators of health, uncovering local drivers and microbiome-enabled responsive indicators across axes of variation.
Recommendation 7-1: Researchers must incorporate sufficient rigor in the sampling design to capture the spatial and temporal heterogeneity of the microbiome to reveal responsive indicators of health.
Recommendation 7-2: Researchers should enhance universal methodologies (sampling, documentation) for microbiome analysis across different sample materials.
Data Tools and Data Management
It is posited that the next frontier in microbiome science lies in understanding the phenotype of a microbiome, the product of the combined genetic potential of the microbiome and available resources (Jansson and Hofmockel 2018). In microbiome science, linkages between phenotype and genotype are hindered by an inability to obtain most microorganisms in pure culture. Culture-independent multi-omic sequencing technologies, like those outlined in Figure 7-3, have the capacity to infer phenotypic features that would be otherwise difficult or time-consuming to assess experimentally. Inferring phenotype is considered one of the holy grails of biological research, as this knowledge can enhance predictive outcomes and provide targeted biotechnological applications in soil and human health realms. In fact, the integration of large-scale datasets with systematic meta-analysis has revolutionized biology, particularly in areas like translational research (Winkler et al. 2014). The ability to access and share well-annotated data collections allows for more robust genome-wide association studies and the application of machine-learning techniques. These advancements underscore the importance of open data and standardized methods. Here, the collation of dozens of studies equates to the analysis of hundreds of thousands of experimental assays without lifting a pipette. Now, microbiome research is poised to leverage data sharing and reproduction for similar translational value to phenotypic exploration (Huttenhower et al. 2023).
To this end, the genomic repertoire of soil and human communities is ever more tractable today, spurred by federally enabled efforts like the HMP and the Genomic Encyclopedia of Bacteria and Archaea project, but also by research collectives like the Earth Microbiome Project and independently generated genomic compendiums (Almeida et al. 2021) that each generated hundreds of thousands of genomes from diverse sources. With the establishment of these massive sequencing catalogs, it is evident that a large portion of the species and functional diversity within human and soil microbiomes remain uncharacterized (Almeida et al. 2021). Thus, research efforts to experimentally characterize and computationally improve annotation of genomic content are urgently needed for accurate phenotypic extrapolations. Likewise, data management practices guided by FAIR principles are conduits for data reuse and mining, enabling further knowledge discovery and innovation. Incentivization by funding agencies and scholarly publishers for additional data that facilitates reuse—such as on environmental or land use properties for soil sequences or population characteristics for human sequences—will extend the collective value of these data collections beyond journal pages. As was true in 2007 when the first metagenomic report from the National Academies was published (NRC 2007), funding sources that create and enable appropriate data management resources are needed.
The committee recognizes data management in microbiology presents a multifaceted challenge, particularly when it comes to the collection and maintenance of metadata. While funding for research endeavors is crucial, it is often overlooked that explicit allocation for metadata collection is essential for comprehensive data management. However, the process of gathering robust metadata is far from simple. Balancing the need for robust metadata against the practical limitations of available resources is a persistent issue. Without adequate support, the quality and depth of metadata suffer, undermining the integrity and utility of research findings. It is a dilemma that requires careful consideration and realistic solutions, as simply expecting exhaustive metadata without appropriate funding is unrealistic and unsustainable within existing grant structures. With increasing awareness in these directions, microbiome-based data sciences and applications can transition from a discipline of description to one that delivers more novel and surprising technologies.
Recommendation 7-3: Federal funders should require resources to ensure metadata as well as data on environmental and ecosystem properties or population characteristics be included, properly stored, and reusable, as accessible data are necessary but not enough.
Diagnostics
New analytical and conceptual approaches will likely be developed that capture microbiome-based systems characteristic of health in both soils and humans. These advances will operationalize both the monitoring of health but also further understanding of how microbiomes influence the stability and functioning of ecosystems and how these underlying properties scale to provide qualities of health or wellness. More efficient measurements of microbiome content with chemical constituents, which could be enhanced by precision or digital agricultural infrastructures and more thorough assessment of human ecosystems along time and special dimensions, are avenues that will be leveraged for quantifying microbiome diagnostics of health. A goal would be to move from correlation to predictive outcomes.
Recommendation 7-4: Funding agencies should support discovery of scalable diagnostics, with the goal that affordable, rapid assays will be developed for use on soil microbiomes in the field and with diverse human populations.
Recommendation 7-5: Funding agencies should support research designed to investigate causal relationships in soil and human microbiomes, toward the development of microbial therapeutics.
Interdisciplinary Research
Microbial ecosystems are an important link in the continuum of soil health and human health. There are a variety of direct (e.g., exposure to dust and contaminants) and indirect (e.g., quality of crops and bioavailability of nutrients) pathways from soil to human health that are deeply affected by microbial composition and function. However, the evidence thus far is mostly correlational and needs validation and replication. With the ever-increasing challenges posed by human activities and climate change, it is imperative to further unveil the mechanistic links underlying microbiota composition and function, soil health, and human health. Furthermore, parallel processes whereby microbiome function influences soil, plant, and animal health should be leveraged in the development of strategies to mitigate emerging challenges to the health of these systems. As was noted in the 2007 National Academies report on metagenomics (NRC 2007), overcoming such challenges will require a systems approach across and within disciplines, supported by multiple funding agencies, to explore the existence of the microbiome continuum spanning soils to human health.
Recommendation 7-6: Funding agencies should support microbiome research within disciplines (e.g., community ecology, soil ecology, and soil biogeochemistry or microbiology and medicine) to integrate methodologies to bring together composition and functional assessments of microbiomes.
Recommendation 7-7: Funding agencies should support microbiome research among disciplines (e.g., agronomy, plant science, soil ecology, microbiology, immunology, human nutrition, medicine, engineering) to explore the connectivity of the microbiome across systems (e.g., soil, plants, and humans).
Footnotes
- 1
See, for example, “Pattern Ag Announces World’s Largest Metagenomics Database,” September 18, 2023. Accessed April 30, 2024. https://www
.pattern.ag /news/pattern-ag-announces-worlds-largestmetagenomics-database. - 2
Helminths are worm-like parasites, such as flukes, tapeworms, and roundworms. Hookworm is an example of soil-transmitted helminth for which infection was common in the southern United States until eliminated by a public health campaign in the early 20th century. Worldwide, hookworm continues to cause hundreds of millions of infections in humans each year.
REFERENCES
- Aderemi AdewaleVictor, Olabode Ayeleso Ademola, Oluokun Oyedapo Oluboade, Mukwevho Emmanuel. “Metabolomics: A Scoping Review of Its Role as a Tool for Disease Biomarker Discovery in Selected Non-Communicable Diseases” Metabolites. 2021;11(7):418. https://doi
.org/10.3390/metabo11070418 . [PMC free article: PMC8305588] [PubMed: 34201929] - Aguiar-Pulido Vanessa, Huang Wenrui, Suarez-Ulloa Victoria, Cickovski Trevor, Mathee Kalai, Narasimhan Giri. “Metagenomics, Metatranscriptomics, and Metabolomics Approaches for Microbiome Analysis: Supplementary Issue: Bioinformatics Methods and Applications for Big Metagenomics Data” Evolutionary Bioinformatics. 2016;12s1:EBO.S36436. https://doi
.org/10.4137/ebo.S36436 . [PMC free article: PMC4869604] [PubMed: 27199545] - Akdis CezmiA. “Does the Epithelial Barrier Hypothesis Explain the Increase in Allergy, Autoimmunity and Other Chronic Conditions?” Nature Reviews Immunology. 2021;21(11):739–751. https://doi
.org/10.1038 /s41577-021-00538-7 . [PubMed: 33846604] - Almeida Alexandre, Nayfach Stephen, Boland Miguel, Strozzi Francesco, Beracochea Martin, Jason Shi Zhou, Pollard KatherineS, Sakharova Ekaterina, Parks DonovanH, Hugenholtz Philip, Segata Nicola, Kyrpides NikosC, Finn RobertD. “A Unified Catalog of 204,938 Reference Genomes from the Human Gut Microbiome” Nature Biotechnology. 2021;39(1):105–114. https://doi
.org/10.1038 /s41587-020-0603-3 . [PMC free article: PMC7801254] [PubMed: 32690973] - Altheide STravis. American Society for Clinical Laboratory Science: ascls.119.001875. 2020. “Biochemical and Culture-Based Approaches to Identification in the Diagnostic Microbiology Laboratory” https://doi
.org/10.29074/ascls .119.001875 . - Ames NancyJ, Ranucci Alexandra, Moriyama Brad, Wallen GwenythR. “The Human Microbiome and Understanding the 16S rRNA Gene in Translational Nursing Science” Nursing Research. 2017;66(2) https://doi
.org/10.1097/nnr .0000000000000212 . [PMC free article: PMC5535273] [PubMed: 28252578] - Anderson JamesW, Baird Pat, Davis RichardH Jr, Ferreri Stefanie, Knudtson Mary, Koraym Ashraf, Waters Valerie, Williams ChristineL. “Health Benefits of Dietary Fiber” Nutrition Reviews. 2009;67(4):188–205. https://doi
.org/10.1111/j .1753-4887.2009.00189.x . [PubMed: 19335713] - Asnicar Francesco, Maltez Thomas Andrew, Passerini Andrea, Waldron Levi, Segata Nicola. “Machine Learning for Microbiologists” Nature Reviews Microbiology. 2024;22(4):191–205. https://doi
.org/10.1038 /s41579-023-00984-1 . [PubMed: 37968359] - Bach Jean-François. “The Hygiene Hypothesis in Autoimmunity: The Role of Pathogens and Commensals” Nature Reviews Immunology. 2018;18(2):105–120. https://doi
.org/10.1038/nri.2017.111 . [PubMed: 29034905] - Bäckhed Fredrik, Ding Hao, Wang Ting, Hooper LoraV, Young Koh Gou, Nagy Andras, Semenkovich ClayF, Gordon JeffreyI. “The Gut Microbiota as an Environmental Factor That Regulates Fat Storage” Proceedings of the National Academy of Sciences. 2004;101(44):15718–15723. https://doi
.org/10.1073/pnas.0407076101 . [PMC free article: PMC524219] [PubMed: 15505215] - Bäckhed Fredrik, Manchester JillK, Semenkovich ClayF, Gordon JeffreyI. “Mechanisms Underlying the Resistance to Diet-induced Obesity in Germ-Free Mice” Proceedings of the National Academy of Sciences. 2007;104(3):979–984. https://doi
.org/10.1073/pnas.0605374104 . [PMC free article: PMC1764762] [PubMed: 17210919] - Bahadur Ali, Batool Asfa, Nasir Fahad, Jiang Shengjin, Mingsen Qin, Zhang Qi, Pan Jianbin, Liu Yongjun, Feng Huyuan. “Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants” International Journal of Molecular Sciences. 2019;20(17):4199. https://www
.mdpi.com /1422-0067/20/17/4199 . [PMC free article: PMC6747277] [PubMed: 31461957] - Banerjee Joyita, Mishra Neetu, Dhas Yogita. “Metagenomics: A New Horizon in Cancer Research” Meta Gene. 2015;5:84–89. https://doi
.org/10.1016/j .mgene.2015.05.005 . [PMC free article: PMC4477109] [PubMed: 26110115] - Banerjee Samiran, van der Heijden MarcelGA. “Soil Microbiomes and One Health” Nature Reviews Microbiology. 2023;21(1):6–20. https://doi
.org/10.1038 /s41579-022-00779-w . [PubMed: 35999468] - Banerjee Samiran, Walder Florian, Büchi Lucie, Meyer Marcel, Held AlainY, Gattinger Andreas, Keller Thomas, Charles Raphael, van der Heijden MarcelGA. “Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots” The ISME Journal. 2019;13(7):1722–1736. https://doi
.org/10.1038 /s41396-019-0383-2 . [PMC free article: PMC6591126] [PubMed: 30850707] - Barabási Albert-László, Gulbahce Natali, Loscalzo Joseph. “Network Medicine: A Network-Based Approach to Human Disease” Nature Reviews Genetics. 2011;12(1):56–68. https://doi
.org/10.1038/nrg2918 . [PMC free article: PMC3140052] [PubMed: 21164525] - Berg Gabriele, Rybakova Daria, Fischer Doreen, Cernava Tomislav, Champomier Vergès Marie-Christine, Charles Trevor, Chen Xiaoyulong, Cocolin Luca, Eversole Kellye, Herrero Corral Gema, Kazou Maria, Kinkel Linda, Lange Lene, Lima Nelson, Loy Alexander, Macklin JamesA, Maguin Emmanuelle, Mauchline Tim, McClure Ryan, Mitter Birgit, Ryan Matthew, Sarand Inga, Smidt Hauke, Schelkle Bettina, Hugo Roume GSeghalKiran, Selvin Joseph, Soares Correa de Souza Rafael, Overbeek Leovan, Singh BrajeshK, Wagner Michael, Walsh Aaron, Sessitsch Angela, Schloter Michael. “Microbiome Definition Re-visited: Old Concepts and New Challenges” Microbiome. 2020;8(1):103. https://doi
.org/10.1186 /s40168-020-00875-0 . [PMC free article: PMC7329523] [PubMed: 32605663] - Bertola Marta, Ferrarini Andrea, Visioli Giovanna. “Improvement of Soil Microbial Diversity through Sustainable Agricultural Practices and Its Evaluation by -Omics Approaches: A Perspective for the Environment, Food Quality and Human Safety” Microorganisms. 2021;9(7):1400. https://doi
.org/10.1016/j .agee.2011.01.017 . [PMC free article: PMC8308033] [PubMed: 34203506] - Bhagchandani Tannu, Verma Nikita Anjali, Tandon Ravi. “Exploring the Human Virome: Composition, Dynamics, and Implications for Health and Disease.” Current Microbiology. 2023;81(1):16. https://doi
.org/10.1007 /s00284-023-03537-0 . [PubMed: 38006423] - Blum WinfriedEH, Zechmeister-Boltenstern Sophie, Keiblinger KatharinaM. “Does Soil Contribute to the Human Gut Microbiome?” Microorganisms. 2019;7(9):287. https://doi
.org/10.3390 %2Fmicroorganisms7090287 . [PMC free article: PMC6780873] [PubMed: 31450753] - Bollmann-Giolai Anita, Malone JacobG, Arora Sanu. “Diversity, Detection and Exploitation: Linking Soil Fungi and Plant Disease” Current Opinion in Microbiology. 2022;70:102199. https://doi
.org/10.1016/j .mib.2022.102199 . [PubMed: 36108394] - Borton MikaylaA, Shaffer Michael, Hoyt DavidW, Jiang Ruisheng, Ellenbogen JaredB, Purvine Samuel, Nicora CarrieD, Eder ElizabethK, Wong AllisonR, George Smulian A, Lipton MaryS, Krzycki JosephA, Wrighton KellyC. “Targeted Curation of the Gut Microbial Gene Content Modulating Human Cardiovascular Disease” mBio. 2023;14(5):e01511–23. https://doi
.org/10.1128/mbio.01511-23 . [PMC free article: PMC10653893] [PubMed: 37695138] - Brooks JPaul, Buck GregoryA, Chen Guanhua, Diao Liyang, Edwards DavidJ, Fettweis JenniferM, Huzurbazar Snehalata, Rakitin Alexander, Satten GlenA, Smirnova Ekaterina, Waks Zeev, Wright MichelleL, Yanover Chen, Zhou Yi-Hui. “Changes in Vaginal Community State Types Reflect Major Shifts in the Microbiome” Microbial Ecology in Health and Disease. 2017;28(1):1303265. https://doi
.org/10.1080/16512235 .2017.1303265 . [PMC free article: PMC5443090] [PubMed: 28572753] - Caesar Robert, Tremaroli Valentina, Kovatcheva-Datchary Petia, Cani PatriceD, Bäckhed Fredrik. “Crosstalk Between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling” Cell Metabolism. 2015;22(4):658–668. https://doi
.org/10.1016/j .cmet.2015.07.026 . [PMC free article: PMC4598654] [PubMed: 26321659] - Calderone Alberto, Licata Luana, Cesareni Gianni. “VirusMentha: A New Resource for Virus-Host Protein Interactions” Nucleic Acids Research. 2015;43(D1):D588–D592. https://doi
.org/10.1093/nar/gku830 . [PMC free article: PMC4384001] [PubMed: 25217587] - Calgaro Matteo, Romualdi Chiara, Waldron Levi, Risso Davide, Vitulo Nicola. “Assessment of Statistical Methods from Single Cell, Bulk RNA-seq, and Metagenomics Applied to Microbiome Data” Genome Biology. 2020;21(1):191. https://doi
.org/10.1186 /s13059020-02104-1 . [PMC free article: PMC7398076] [PubMed: 32746888] - Canfora EmanuelE, Jocken JohanW, Blaak EllenE. “Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity” Nature Reviews Endocrinology. 2015;11(10):577–591. https://doi
.org/10.1038/nrendo.2015.128 . [PubMed: 26260141] - Carini Paul, Marsden PatrickJ, Leff JonathanW, Morgan EmilyE, Strickland MichaelS, Fierer Noah. “Relic DNA Is Abundant in Soil and Obscures Estimates of Soil Microbial Diversity” Nature Microbiology. 2016;2(3):16242. https://doi
.org/10.1038/nmicrobiol .2016.242 . [PubMed: 27991881] - Chagas CésardaSilva, Carvalho Junior Waldirde, Barge Bhering Silvio, Calderano Filho Braz. “Spatial Prediction of Soil Surface Texture in a Semiarid Region Using Random Forest and Multiple Linear Regressions” CATENA. 2016;139:232–240. https://doi
.org/10.1016/j .catena.2016.01.001 . - Chen Fang, Li Shenghui, Guo Ruochun, Song Fanghua, Zhang Yue, Wang Xifan, Huo Xiaokui, Lv Qingbo, Ullah Hayan, Wang Guangyang, Ma Yufang, Yan Qiulong, Ma Xiaochi. “Meta-analysis of Fecal Viromes Demonstrates High Diagnostic Potential of the Gut Viral Signatures for Colorectal Cancer and Adenoma Risk Assessment” Journal of Advanced Research. 2023;49:103–114. https://doi
.org/10.1016/j .jare.2022.09.012 . [PMC free article: PMC10334131] [PubMed: 36198381] - Chen Lianmin, Collij Valerie, Jaeger Martin, van den Munckhof IngeCL, Vich Vila Arnau, Kurilshikov Alexander, Gacesa Ranko, Sinha Trishla, Oosting Marije, Joosten LeoAB, Rutten JoostHW, Riksen NielsP, Xavier RamnikJ, Kuipers Folkert, Wijmenga Cisca, Zhernakova Alexandra, Netea MihaiG, Weersma RinseK, Fu Jingyuan. “Gut Microbial Co-abundance Networks Show Specificity in Inflammatory Bowel Disease and Obesity” Nature Communications. 2020;11(1):4018. https://doi
.org/10.1038 /s41467-020-17840-y . [PMC free article: PMC7419557] [PubMed: 32782301] - Chen Tingting, Long Wenmin, Zhang Chenhong, Liu Shuang, Zhao Liping, Hamaker BruceR. “Fiber-Utilizing Capacity Varies in Prevotella- versus Bacteroides-Dominated Gut Microbiota” Scientific Reports. 2017;7(1):2594. https://doi
.org/10.1038 /s41598-017-02995-4 . [PMC free article: PMC5453967] [PubMed: 28572676] - Chepsergon Jane, Moleleki LucyN. “Rhizosphere Bacterial Interactions and Impact on Plant Health” Current Opinion in Microbiology. 2023;73:102297. https://doi
.org/10.1016/j .mib.2023.102297 . [PubMed: 37002974] - Chinda Daisuke, Takada Toshihiko, Mikami Tatsuya, Shimizu Kensuke, Oana Kosuke, Arai Tetsu, Akitaya Kazuki, Sakuraba Hirotake, Katto Miyuki, Nagara Yusuke, Makino Hiroshi, Fujii Daichi, Oishi Kenji, Fukuda Shinsaku. “Spatial Distribution of Live Gut Microbiota and Bile Acid Metabolism in Various Parts of Human Large Intestine” Scientific Reports. 2022;12:3593. https://doi
.org/10.1038 /s41598-022-07594-6 . [PMC free article: PMC8897406] [PubMed: 35246580] - Cho Ilseung, Blaser MartinJ. “The Human Microbiome: At the Interface of Health and Disease” Nature Reviews Genetics. 2012;13(4):260–270. https://doi
.org/10.1038/nrg3182 . [PMC free article: PMC3418802] [PubMed: 22411464] - Crits-Christoph Alexander, Diamond Spencer, Butterfield CristinaN, Thomas BrianC, Banfield JillianF. “Novel Soil Bacteria Possess Diverse Genes for Secondary Metabolite Biosynthesis” Nature. 2018;558(7710):440–444. https://doi
.org/10.1038 /s41586-018-0207-y . [PubMed: 29899444] - d’Humières Camille, Salmona Maud, Dellière Sarah, Leo Stefano, Rodriguez Christophe, Angebault Cécile, Alanio Alexandre, Fourati Slim, Lazarevic Vladimir, Woerther Paul-Louis, Schrenzel Jacques, Ruppé Etienne. “The Potential Role of Clinical Metagenomics in Infectious Diseases: Therapeutic Perspectives” Drugs. 2021;81:1453–1466. https://doi
.org/10.1007 /s40265-021-01572-4 . [PMC free article: PMC8323086] [PubMed: 34328626] - Dagenais Amy, Villalba-Guerrero Carlos, Olivier Martin. “Trained Immunity: A ‘New’ Weapon in the Fight Against Infectious Diseases” Frontiers in Immunology. 2023;14 https://doi
.org/10.3389/fimmu .2023.1147476 . [PMC free article: PMC10040606] [PubMed: 36993966] - Das Purkayastha Sumi, Bhattacharya MrinalK, Prasad HimanshuK, De Mandal Surajit. “Chapter 17 - Diversity and the Antimicrobial Activity of Vaginal Lactobacilli: Current Status and Future Prospective” De Mandal Surajit, Bhatt Pankaj, editors. Academic Press; Recent Advancements in Microbial Diversity. 2020:397–422.
- Deehan EdwardC, Walter Jens. “The Fiber Gap and the Disappearing Gut Microbiome: Implications for Human Nutrition” Trends in Endocrinology & Metabolism. 2016;27(5):239–242. https://doi
.org/10.1016/j .tem.2016.03.001 . [PubMed: 27079516] - Delgado-Baquerizo Manuel, Grinyer Jasmine, Reich PeterB, Singh BrajeshK. “Relative Importance of Soil Properties and Microbial Community for Soil Functionality: Insights from a Microbial Swap Experiment” Functional Ecology. 2016a;30(11):1862–1873. https://doi
.org/10.1111/1365-2435.12674 . - Delgado-Baquerizo Manuel, Maestre FernandoT, Reich PeterB, Jeffries ThomasC, Gaitan JuanJ, Encinar Daniel, Berdugo Miguel, Campbell ColinD, Singh BrajeshK. “Microbial Diversity Drives Multifunctionality in Terrestrial Ecosystems” Nature Communications. 2016b;7(1):10541. https://doi
.org/10.1038/ncomms10541 . [PMC free article: PMC4738359] [PubMed: 26817514] - DeLuca ThomasH, Pingree MelissaRA, Gao Si. “Chapter 16 - Assessing Soil Biological Health in Forest Soils” In: Busse Matt, Giardina ChristianP, Morris DaveM, Page-Dumroese DebbieS, editors. Global Change and Forest Soils. Amsterdam: Elsevier; 2019. pp. 397–426.
- Di Rienzi SaraC, Sharon Itai, Wrighton KellyC, Koren Omry, Hug LauraA, Thomas BrianC, Goodrich JuliaK, Bell JordanaT, Spector TimothyD, Banfield JillianF, Ley RuthE. “The Human Gut and Groundwater Harbor Non-photosynthetic Bacteria Belonging to a New Candidate Phylum Sibling to Cyanobacteria” Elife. 2013;2:e01102. https://doi
.org/10.7554/eLife.01102 . [PMC free article: PMC3787301] [PubMed: 24137540] - Domazet-Lošo Tomislav, Tautz Diethard. “An Ancient Evolutionary Origin of Genes Associated with Human Genetic Diseases” Molecular Biology and Evolution. 2008;25(12):2699–2707. https://doi
.org/10.1093/molbev/msn214 . [PMC free article: PMC2582983] [PubMed: 18820252] - Donovan PaulD, Gonzalez Gabriel, Higgins DesmondG, Butler Geraldine, Ito Kimihito. “Identification of Fungi in Shotgun Metagenomics Datasets” PLOS ONE. 2018;13(2):e0192898. https://doi
.org/10.1371/journal .pone.0192898 . [PMC free article: PMC5812651] [PubMed: 29444186] - Douglas GavinM, Maffei VincentJ, Zaneveld JesseR, Yurgel SvetlanaN, Brown JamesR, Taylor ChristopherM, Huttenhower Curtis, Langille MorganGI. “PICRUSt2 for Prediction of Metagenome Functions.” Nature Biotechnology. 2020;38(6):685–688. https://doi
.org/10.1038 /s41587-020-0548-6 . [PMC free article: PMC7365738] [PubMed: 32483366] - Dundore-Arias JP, Michalska-Smith M, Millican M, Kinkel LL. “More Than the Sum of Its Parts: Unlocking the Power of Network Structure for Understanding Organization and Function in Microbiomes” Annual Review of Phytopathology. 2023;61(1):403–423. https://doi
.org/10.1146 /annurev-phyto-021021-041457 . [PubMed: 37217203] - Duque Zapata JuanDiego, Eduardo Muñoz Florez Jaime, Lopez Alvarez Diana. “Metagenomics Approaches to Understanding Soil Health in Environmental Research - A Review” Soil Science Annual. 2023;74(1):1–11. https://doi
.org/10.37501/soilsa/163080 . - Durán Paloma, Thiergart Thorsten, Garrido-Oter Ruben, Agler Matthew, Kemen Eric, Schulze-Lefert Paul, Hacquard Stéphane. “Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival” Cell. 2018;175(4):973–983.e14. https://doi
.org/10.1016/j .cell.2018.10.020 . [PMC free article: PMC6218654] [PubMed: 30388454] - Eisenstein Michael. “The Hunt for a Healthy Microbiome” Nature. 2020;577(7792):S6–S8. https://doi
.org/10.1038 /d41586-020-00193-3 . [PubMed: 31996823] - Ellenbogen JaredB, Borton MikaylaA, McGivern BridgetB, Cronin DylanR, Hoyt DavidW, Freire-Zapata Viviana, McCalley CarmodyK, Varner RuthK, Crill PatrickM, Wehr RichardA, Chanton JeffreyP, Woodcroft BenJ, Tfaily MalakM, Tyson GeneW, Rich VirginiaI, Wrighton KellyC. “Methylotrophy in the Mire: Direct and Indirect Routes for Methane Production in Thawing Permafrost” mSystems. 2024;9(1):e00698–23. https://doi
.org/10.1128/msystems .00698-23 . [PMC free article: PMC10805028] [PubMed: 38063415] - Emerson JoanneB, Roux Simon, Brum JenniferR, Bolduc Benjamin, Woodcroft BenJ, Bin Jang Ho, Singleton CaitlinM, Solden LindseyM, Naas AdrianE, Boyd JoelA, Hodgkins SuzanneB, Wilson RachelM, Trubl Gareth, Li Changsheng, Frolking Steve, Pope PhillipB, Wrighton KellyC, Crill PatrickM, Chanton JeffreyP, Saleska ScottR, Tyson GeneW, Rich VirginiaI, Sullivan MatthewB. “Host-Linked Soil Viral Ecology Along a Permafrost Thaw Gradient” Nature Microbiology. 2018;3(8):870–880. https://doi
.org/10.1038 /s41564-018-0190-y . [PMC free article: PMC6786970] [PubMed: 30013236] - Falony Gwen, Joossens Marie, Vieira-Silva Sara, Wang Jun, Darzi Youssef, Faust Karoline, Kurilshikov Alexander, Jan Bonder Marc, Valles-Colomer Mireia, Vandeputte Doris, Tito Tadeo Raul, Chaffron Samuel, Rymenans Leen, Verspecht Chloë, Sutter Lise, Lima-Mendez Gipsi, D’hoe Kevin, Jonckheere Karl, Homola Daniel, Raes Jeroen. “Population-Level Analysis of Gut Microbiome Variation” Science. 2016;352:560–564. https://doi
.org/10.1126/science.aad3503 . [PubMed: 27126039] - Fan Yong, Pedersen Oluf. “Gut Microbiota in Human Metabolic Health and Disease” Nature Reviews Microbiology. 2021;19(1):55–71. https://doi
.org/10.1038 /s41579-020-0433-9 . [PubMed: 32887946] - FAO, UNEP, WHO, and WOAH (Food and Agriculture Organization of the United Nations, United Nations Environment Programme, World Health Organization, and World Organisation for Animal Health). One Health Joint Plan of Action (2022– 2026). Working Together for the Health of Humans, Animals, Plants and the Environment. Rome: 2022. https://doi
.org/10.4060/cc2289en . - Fassarella Marina, Blaak EllenE, Penders John, Nauta Arjen, Smidt Hauke Hauke, Zoetendal ErwinG. “Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health” Gut. 2021;70(3):595. https://doi
.org/10.1136 /gutjnl-2020-321747 . [PubMed: 33051190] - Ferrocino Ilario, Rantsiou Kalliopi, McClure Ryan, Kostic Tanja, Soares Correa de Souza Rafael, Lange Lene, FitzGerald Jamie, Kriaa Aicha, Cotter Paul, Maguin Emmanuelle, Schelkle Bettina, Schloter Michael, Berg Gabriele, Sessitsch Angela, Cocolin Luca, MicrobiomeSupport Consortium The. “The Need for an Integrated Multi-OMICs Approach in Microbiome Science in the Food System” Comprehensive Reviews in Food Science and Food Safety. 2023;22(2):1082–1103. https://doi
.org/10.1111/1541-4337.13103 . [PubMed: 36636774] - Fetissov SergueïO. “Role of the Gut Microbiota in Host Appetite Control: Bacterial Growth to Animal Feeding Behaviour” Nature Reviews Endocrinology. 2017;13(1):11–25. https://doi
.org/10.1038/nrendo.2016.150 . [PubMed: 27616451] - Fierer Noah, Wood StephenA, Bueno de Mesquita CliftonP. “How Microbes Can, and Cannot, Be Used to Assess Soil Health” Soil Biology and Biochemistry. 2021;153:108111. https://doi
.org/10.1016/j .soilbio.2020.108111 . - Fitzpatrick ConnorR, Copeland Julia, Wang PaulineW, Guttman DavidS, Kotanen PeterM, Johnson MarcTJ. “Habitats Within the Plant Root Differ in Bacterial Network Topology and Taxonomic Assortativity.” Molecular Plant-Microbe Interactions® 2023;36(3):165–175. https://doi
.org/10.1094 /mpmi-09-22-0188-r . [PubMed: 36463399] - Frąc Magdalena, Hannula SiljaE, Bełka Marta, Jędryczka Małgorzata. “Fungal Biodiversity and Their Role in Soil Health” Frontiers in Microbiology. 2018;9 https://doi
.org/10.3389/fmicb.2018.00707 . [PMC free article: PMC5932366] [PubMed: 29755421] - France Michael, Alizadeh Madeline, Brown Sarah, Ma Bing, Ravel Jacques. “Towards a Deeper Understanding of the Vaginal Microbiota” Nature Microbiology. 2022;7(3):367–378. https://doi
.org/10.1038 /s41564-022-01083-2 . [PMC free article: PMC8910585] [PubMed: 35246662] - Fujita Hiroaki, Ushio Masayuki, Suzuki Kenta, Abe MasatoS, Yamamichi Masato, Iwayama Koji, Canarini Alberto, Hayashi Ibuki, Fukushima Keitaro, Shinji Fukuda ETobyKiers, Toju Hirokazu. “Alternative Stable States, Nonlinear Behavior, and Predictability of Microbiome Dynamics” Microbiome. 2023;11(1):63. https://doi
.org/10.1186 /s40168-02301474-5 . [PMC free article: PMC10052866] [PubMed: 36978146] - Gacesa R, Kurilshikov A, Vich Vila A, Sinha T, Klaassen MAY, Bolte LA, Andreu-Sánchez S, Chen L, Collij V, Hu S, Dekens JAM, Lenters VC, Björk JR, Swarte JC, Swertz MA, Jansen BH, Gelderloos-Arends J, Jankipersadsing S, Hofker M, Vermeulen RCH, Sanna S, Harmsen HJM, Wijmenga C, Fu J, Zhernakova A, Weersma RK. “Environmental Factors Shaping the Gut Microbiome in a Dutch Population” Nature. 2022;604(7907):732–739. https://doi
.org/10.1038 /s41586-022-04567-7 . [PubMed: 35418674] - Gao Yilin, Zhu Zifan, Sun Fengzhu. “Increasing Prediction Performance of Colorectal Cancer Disease Status Using Random Forests Classification Based on Metagenomic Shotgun Sequencing Data” Synthetic and Systems Biotechnology. 2022;7(1):574–585. https://doi
.org/10.1016/j .synbio.2022.01.005 . [PMC free article: PMC8801753] [PubMed: 35155839] - Genin Stéphane, Denny TimothyP. “Pathogenomics of the Ralstonia solanacearum Species Complex” Annual Review of Phytopathology. 2012;50(1):67–89. https://doi
.org/10.1146 /annurev-phyto-081211-173000 . [PubMed: 22559068] - German JBruce, Hammock BruceD, Watkins StevenM. “Metabolomics: Building on a Century of Biochemistry to Guide Human Health” Metabolomics. 2005;1(1):3–9. https://doi
.org/10.1007 /s11306-005-1102-8 . [PMC free article: PMC1457093] [PubMed: 16680201] - Ghimire Rajan, Thapa VeshR, Acosta-Martinez Veronica, Schipanski Meagan, Slaughter LindseyC, Fonte StevenJ, Shukla ManojK, Bista Prakriti, Angadi SangameshV, Mikha MaysoonM, Adebayo Olufemi, Noble Strohm Tess. “Soil Health Assessment and Management Framework for Water-Limited Environments: Examples from the Great Plains of the USA” Soil Systems. 2023;7(1):22. https://www
.mdpi.com/2571-8789/7/1/22 . - Gilbert JackA, Blaser MartinJ, Gregory Caporaso J, Jansson JanetK, Lynch SusanV, Knight Rob. “Current Understanding of the Human Microbiome” Nature Medicine. 2018;24(4):392–400. https://doi
.org/10.1038/nm.4517 . [PMC free article: PMC7043356] [PubMed: 29634682] - Gorvitovskaia Anastassia, Holmes SusanP, Huse SusanM. “Interpreting Prevotella and Bacteroides as Biomarkers of Diet and Lifestyle” Microbiome. 2016;4(1):15. https://doi
.org/10.1186 /s40168-016-0160-7 . [PMC free article: PMC4828855] [PubMed: 27068581] - Gregory AnnC, Sullivan MatthewB, Segal LeopoldoN, Keller BrianC. “Smoking Is Associated with Quantifiable Differences in the Human Lung DNA Virome and Metabolome” Respiratory Research. 2018;19(1):174. https://doi
.org/10.1186 /s12931-018-0878-9 . [PMC free article: PMC6136173] [PubMed: 30208886] - Grieneisen LauraE, Charpentier MarieJE, Alberts SusanC, Blekhman Ran, Bradburd Gideon, Tung Jenny, Archie ElizabethA. “Genes, Geology and Germs: Gut Microbiota Across a Primate Hybrid Zone Are Explained by Site Soil Properties, Not Host Species” Proceedings of the Royal Society B: Biological Sciences. 2019;286(1901):20190431. https://doi
.org/10.1098/rspb.2019.0431 . [PMC free article: PMC6501927] [PubMed: 31014219] - Gu Yian, Banerjee Samiran, Dini-Andreote Francisco, Xu Yangchun, Shen Qirong, Jousset Alexandre, Wei Zhong. “Small Changes in Rhizosphere Microbiome Composition Predict Disease Outcomes Earlier than Pathogen Density Variations” The ISME Journal. 2022;16(10):2448–2456. https://doi
.org/10.1038 /s41396-022-01290-z . [PMC free article: PMC9478146] [PubMed: 35869387] - Hajishengallis George, Darveau RichardP, Curtis MichaelA. “The Keystone-Pathogen Hypothesis” Nature Reviews Microbiology. 2012;10(10):717–725. https://doi
.org/10.1038/nrmicro2873 . [PMC free article: PMC3498498] [PubMed: 22941505] - Halfvarson Jonas, Brislawn ColinJ, Lamendella Regina, Vázquez-Baeza Yoshiki, Walters WilliamA, Bramer LisaM, D’Amato Mauro, Bonfiglio Ferdinando, McDonald Daniel, Gonzalez Antonio, McClure ErinE, Dunklebarger MitchellF, Knight Rob, Jansson JanetK. “Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease” Nature Microbiology. 2017;2(5):17004. https://doi
.org/10.1038/nmicrobiol .2017.4 . [PMC free article: PMC5319707] [PubMed: 28191884] - Hanski Ilkka, Hertzen Leenavon, Fyhrquist Nanna, Koskinen Kaisa, Torppa Kaisa, Laatikainen Tiina, Karisola Piia, Auvinen Petri, Paulin Lars, Mäkelä MikaJ, Vartiainen Erkki, Kosunen TimoU, Alenius Harri, Haahtela Tari. “Environmental Biodiversity, Human Microbiota, and Allergy Are Interrelated” Proceedings of the National Academy of Sciences. 2012;109(21):8334–8339. https://doi
.org/10.1073/pnas.1205624109 . [PMC free article: PMC3361383] [PubMed: 22566627] - Hariharan Ganeshamoorthy, Prasannath Kandeeparoopan. “Recent Advances in Molecular Diagnostics of Fungal Plant Pathogens: A Mini Review” Frontiers in Cellular and Infection Microbiology. 2021;10 https://doi
.org/10.3389/fcimb .2020.600234 . [PMC free article: PMC7829251] [PubMed: 33505921] - Harvey Eric, Gounand Isabelle, Ward ColetteL, Altermatt Florian. “Bridging Ecology and Conservation: From Ecological Networks to Ecosystem Function” Journal of Applied Ecology. 2017;54(2):371–379. https://doi
.org/10.1111/1365-2664.12769 . - Hawkins Heidi-Jayne, Cargill RachaelIM, Van Nuland MichaelE, Hagen StephenC, Field KatieJ, Sheldrake Merlin, Soudzilovskaia NadejdaA, Toby Kiers E. “Mycorrhizal Mycelium as a Global Carbon Pool” Current Biology. 2023;33(11):R560–R573. https://doi
.org/10.1016/j .cub.2023.02.027 . [PubMed: 37279689] - Hernández Medina Ricardo, Kutuzova Svetlana, Nor Nielsen Knud, Johansen Joachim, Hestbjerg Hansen Lars, Nielsen Mads, Rasmussen Simon. “Machine Learning and Deep Learning Applications in Microbiome Research” ISME Communications. 2022;2(1):98. https://doi
.org/10.1038 /s43705-022-00182-9 . [PMC free article: PMC9723725] [PubMed: 37938690] - Heung Brandon, Bulmer ChuckE, Schmidt MargaretG. “Predictive Soil Parent Material Mapping at a Regional-Scale: A Random Forest Approach” Geoderma. 2014;214−215:141–154. https://doi
.org/10.1016/j .geoderma.2013.09.016 . - HMP (The Integrative HMP Research Network Consortium). “ The Integrative Human Microbiome Project” Nature. 2019;569(7758):641–648. https://doi
.org/10.1038/s41586-0191238-8 . [PMC free article: PMC6784865] [PubMed: 31142853] - Hodinka RichardL, Kaiser Laurent. “Point-Counterpoint: Is the Era of Viral Culture Over in the Clinical Microbiology Laboratory?” Journal of Clinical Microbiology. 2013;51(1):2–8. https://doi
.org/10.1128/jcm.02593-12 . [PubMed: 23393647] - Holscher HannahD. “Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota” Gut Microbes. 2017;8(2):172–184. https://doi
.org/10.1080/19490976 .2017.1290756 . [PMC free article: PMC5390821] [PubMed: 28165863] - Hou Kaijian, Wu Zhuo-Xun, Chen Xuan-Yu, Wang Jing-Quan, Zhang Dongya, Xiao Chuanxing, Zhu Dan, Koya JagadishB, Wei Liuya, Li Jilin, Chen Zhe-Sheng. “Microbiota in Health and Diseases” Signal Transduction and Targeted Therapy. 2022;7(1):135. https://doi
.org/10.1038 /s41392-022-00974-4 . [PMC free article: PMC9034083] [PubMed: 35461318] - Hungate BruceA, Mau RebeccaL, Egbert Schwartz JGregoryCaporaso, Dijkstra Paul, Gestel Natasjavan, Koch BenjaminJ, Liu CindyM, McHugh TheresaA, Marks JaneC, Morrissey EmberM, Price LanceB. “Quantitative Microbial Ecology through Stable Isotope Probing” Applied and Environmental Microbiology. 2015;81(21):7570–7581. https://doi
.org/10.1128/AEM.02280-15 . [PMC free article: PMC4592864] [PubMed: 26296731] - Huttenhower Curtis, Finn RobertD, Carolyn McHardy Alice. “Challenges and Opportunities in Sharing Microbiome Data and Analyses” Nature Microbiology. 2023;8(11):1960–1970. https://doi
.org/10.1038 /s41564-023-01484-x . [PubMed: 37783751] - Issa Isaac Ngom, Philippe Decloquement, Nicholas Armstrong, Raoult Didier, Eric Chabrière. “Metaproteomics of the Human Gut Microbiota: Challenges and Contributions to Other OMICS” Clinical Mass Spectrometry. 2019;14:18–30. https://doi
.org/10.1016/j .clinms.2019.06.001 . [PMC free article: PMC8669434] [PubMed: 34917758] - Jain Nityanand, Pratama Umar Tungki, Fahner Anne-Fleur, Gibietis Valdis. “Advancing Therapeutics for Recurrent Clostridioides Difficile Infections: An Overview of Vowst’s FDA Approval and Implications” Gut Microbes. 2023;15(1):2232137. https://doi
.org/10.1080/19490976 .2023.2232137 . [PMC free article: PMC10337487] [PubMed: 37431860] - Jansson JanetK, Baker ErinS. “A Multi-omic Future for Microbiome Studies” Nature Microbiology. 2016;1(5):16049. https://doi
.org/10.1038/nmicrobiol .2016.49 . [PubMed: 27572648] - Jansson JanetK, Hofmockel KirstenS. “The Soil Microbiome—From Metagenomics to Metaphenomics” Current Opinion in Microbiology. 2018;43:162–168. https://doi
.org/10.1016/j .mib.2018.01.013 . [PubMed: 29454931] - Jansson JanetK, Wu Ruonan. “Soil Viral Diversity, Ecology and Climate Change” Nature Reviews Microbiology. 2023;21(5):296–311. https://doi
.org/10.1038 /s41579-022-00811-z . [PubMed: 36352025] - Jeffery Simon, Gardi Ciro. “Soil Biodiversity Under Threat – A Review” Acta Societatis Zoologicae Bohemicae. 2010;74:7–12.
- Johnson AbigailJ, Vangay Pajau, Al-Ghalith GabrielA, Hillmann BenjaminM, Ward TonyaL, Shields-Cutler RobinR, Kim AustinD, Konstantinovna Shmagel Anna, Syed ArzangN, Walter Jens, Menon Ravi, Koecher Katie, Knights Dan. “Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans” Cell Host & Microbe. 2019;25(6):789–802.e5. https://doi
.org/10.1016/j .chom.2019.05.005 . [PubMed: 31194939] - Jousset Alexandre, Bienhold Christina, Chatzinotas Antonis, Gallien Laure, Gobet Angélique, Kurm Viola, Küsel Kirsten, Rillig MatthiasC, Rivett DamianW, Salles JoanaF, van der Heijden MarcelGA, Youssef NohaH, Zhang Xiaowei, Wei Zhong, Gera Hol WH. “Where Less May Be More: How the Rare Biosphere Pulls Ecosystems Strings” The ISME Journal. 2017;11(4):853–858. [PMC free article: PMC5364357] [PubMed: 28072420]
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R. “Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study” Microbiome. 2017;5(1):10. https://doi
.org/10.1186 /s40168-016-0225-7 . [PMC free article: PMC5264285] [PubMed: 28122648] - Kaper JamesB, Nataro JamesP, Mobley HarryLT. “Pathogenic Escherichia coli.” Nature Reviews Microbiology. 2004;2(2):123–140. https://doi
.org/10.1038/nrmicro818 . [PubMed: 15040260] - Khiyami MohammadA, Almoammar Hassan, Awad YasserM, Alghuthaymi MousaA, Abd-Elsalam KamelA. “Plant Pathogen Manodiagnostic Techniques: Forthcoming Changes?” Biotechnology & Biotechnological Equipment. 2014;28(5):775–785. https://doi
.org/10.1080/13102818 .2014.960739 . [PMC free article: PMC4684063] [PubMed: 26740775] - King DanaE, Mainous ArchG, Lambourne CarolA. “Trends in Dietary Fiber Intake in the United States, 1999-2008” Journal of the Academy of Nutrition and Dietetics. 2012;112(5):642–648. https://doi
.org/10.1016/j .jand.2012.01.019 . [PubMed: 22709768] - Kishino Shigenobu, Takeuchi Michiki, Park Si-Bum, Hirata Akiko, Kitamura Nahoko, Kunisawa Jun, Kiyono Hiroshi, Iwamoto Ryo, Isobe Yosuke, Arita Makoto, Arai Hiroyuki, Ueda Kazumitsu, Shima Jun, Takahashi Satomi, Yokozeki Kenzo, Shimizu Sakayu, Ogawa Jun. “Polyunsaturated Fatty Acid Saturation by Gut Lactic Acid Bacteria Affecting Host Lipid Composition” Proceedings of the National Academy of Sciences. 2013;110(44):17808–17813. https://doi
.org/10.1073/pnas.1312937110 . [PMC free article: PMC3816446] [PubMed: 24127592] - Korem Tal, Zeevi David, Zmora Niv, Weissbrod Omer, Bar Noam, Lotan-Pompan Maya, Avnit-Sagi Tali, Kosower Noa, Malka Gal, Rein Michal, Suez Jotham, Goldberg BenZ, Weinberger Adina, Levy AvrahamA, Elinav Eran, Segal Eran. “Bread Affects Clinical Parameters and Induces Gut Microbiome-Associated Personal Glycemic Responses” Cell Metabolism. 2017;25(6):1243–1253.e5. https://doi
.org/10.1016/j .cmet.2017.05.002 . [PubMed: 28591632] - Larcher Walter. Physiological Plant Ecology. 3rd. Berlin: Springer-Verlag; 1995.
- Lauber ChristianL, Ramirez KellyS, Aanderud Zach, Lennon Jay, Fierer Noah. “Temporal Variability in Soil Microbial Communities Across Land-Use Types” The ISME Journal. 2013;7(8):1641–1650. https://doi
.org/10.1038/ismej.2013.50 . [PMC free article: PMC3721119] [PubMed: 23552625] - Le Chatelier Emmanuelle, Nielsen Trine, Qin Junjie, Prifti Edi, Hildebrand Falk, Falony Gwen, Almeida Mathieu, Arumugam Manimozhiyan, Batto Jean-Michel, Kennedy Sean. “Richness of Human Gut Microbiome Correlates with Metabolic Markers” Nature. 2013;500(7464):541–546. https://doi
.org/10.1038/nature12506 . [PubMed: 23985870] - Lee SeungJae, Rho Mina. “Multimodal Deep Learning Applied to Classify Healthy and Disease States of Human Microbiome” Scientific Reports. 2022;12(1):824. https://doi
.org/10.1038 /s41598-022-04773-3 . [PMC free article: PMC8763943] [PubMed: 35039534] - Leggett HelenC, Cornwallis CharlieK, West StuartA. “Mechanisms of Pathogenesis, Infective Dose and Virulence in Human Parasites” PLOS Pathogens. 2012;8(2):e1002512. https://doi
.org/10.1371/journal .ppat.1002512 . [PMC free article: PMC3280976] [PubMed: 22359500] - Lehmann Johannes, Bossio DeborahA, Kögel-Knabner Ingrid, Rillig MatthiasC. “The Concept and Future Prospects of Soil Health” Nature Reviews Earth & Environment. 2020;1(10):544–553. https://doi
.org/10.1038 /s43017-020-0080-8 . [PMC free article: PMC7116140] [PubMed: 33015639] - Leleiwi Ikaia, Rodriguez-Ramos Josué, Shaffer Michael, Sabag-Daigle Anice, Kokkinias Katherine, Flynn RoryM, Daly RebeccaA, Kop LinneaFM, Solden LindseyM, Ahmer BrianMM, Borton MikaylaA, Wrighton KellyC. “Exposing New Taxonomic Variation with Inflammation — A Murine Model-Specific Genome Database for Gut Microbiome Researchers” Microbiome. 2023;11(1):114. https://doi
.org/10.1186 /s40168023-01529-7 . [PMC free article: PMC10199544] [PubMed: 37210515] - Lennon JayT, Jones StuartE. “Microbial Seed Banks: The Ecological and Evolutionary Implications of Dormancy” Nature Reviews Microbiology. 2011;9(2):119–130. https://doi
.org/10.1038/nrmicro2504 . [PubMed: 21233850] - Li Hai, Limenitakis JulienP, Fuhrer Tobias, Geuking MarkusB, Lawson MelissaA, Wyss Madeleine, Brugiroux Sandrine, Keller Irene, Macpherson JamieA, Rupp Sandra, Stolp Bettina, Stein JensV, Stecher Bärbel, Sauer Uwe, McCoy KathyD, Macpherson AndrewJ. “The Outer Mucus Layer Hosts a Distinct Intestinal Microbial Niche” Nature Communications. 2015;6(1):8292. https://doi
.org/10.1038/ncomms9292 . [PMC free article: PMC4595636] [PubMed: 26392213] - Liao Hu, Li Hu, Duan Chen-Song, Zhou Xin-Yuan, Luo Qiu-Ping, An Xin-Li, Zhu Yong-Guan, Su Jian-Qiang. “Response of Soil Viral Communities to Land Use Changes” Nature Communications. 2022;13(1):6027. https://doi
.org/10.1038 /s41467-022-33771-2 . [PMC free article: PMC9556555] [PubMed: 36224209] - Ling Ning, Wang Tingting, Kuzyakov Yakov. “Rhizosphere Bacteriome Structure and Functions” Nature Communications. 2022;13(1):836. https://doi
.org/10.1038 /s41467022-28448-9 . [PMC free article: PMC8837802] [PubMed: 35149704] - Lloyd-Price Jason, Arze Cesar, Ananthakrishnan AshwinN, Schirmer Melanie, Avila-Pacheco Julian, Poon TiffanyW, Andrews Elizabeth, Ajami NadimJ, Bonham KevinS, Brislawn ColinJ, Casero David, Courtney Holly, Gonzalez Antonio, Graeber ThomasG, Brantley Hall A, Lake Kathleen, Landers CarolJ, Mallick Himel, Plichta DamianR, Prasad Mahadev, Rahnavard Gholamali, Sauk Jenny, Shungin Dmitry, Vázquez-Baeza Yoshiki, White RichardA, Bishai Jason, Bullock Kevin, Deik Amy, Dennis Courtney, Kaplan JessL, Khalili Hamed, McIver LaurenJ, Moran ChristopherJ, Nguyen Long, Pierce KerryA, Schwager Randall, Sirota-Madi Alexandra, Stevens BetsyW, Tan William, ten Hoeve JohannaJ, Weingart George, Wilson RobinG, Yajnik Vijay, Braun Jonathan, Denson LeeA, Jansson JanetK, Knight Rob, Kugathasan Subra, McGovern DermotPB, Petrosino JosephF, Stappenbeck ThaddeusS, Winter HarlandS, Clish ClaryB, Franzosa EricA, Vlamakis Hera, Xavier RamnikJ, Huttenhower Curtis, Investigators IBDMDB. “Multiomics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases” Nature. 2019;569(7758):655–662. https://doi
.org/10.1038 /s41586-019-1237-9 . [PMC free article: PMC6650278] [PubMed: 31142855] - Long Shuping, Yang Yi, Shen Chengpin, Wang Yiwen, Deng Anmei, Qin Qin, Qiao Liang. “Metaproteomics Characterizes Human Gut Microbiome Function in Colorectal Cancer” npj Biofilms and Microbiomes. 2020;6(1):14. https://doi
.org/10.1038 /s41522-020-0123-4 . [PMC free article: PMC7093434] [PubMed: 32210237] - Lozupone CatherineA, Stombaugh JesseI, Gordon JeffreyI, Jansson JanetK, Knight Rob. “Diversity, Stability and Resilience of the Human Gut Microbiota” Nature. 2012;489(7415):220–230. https://doi
.org/10.1038/nature11550 . [PMC free article: PMC3577372] [PubMed: 22972295] - Lynch SusanV, Pedersen Oluf. “The Human Intestinal Microbiome in Health and Disease” New England Journal of Medicine. 2016;375(24):2369–2379. https://doi
.org/10.1056/NEJMra1600266 . [PubMed: 27974040] - Ma Bin, Wang Yiling, Zhao Kankan, Stirling Erinne, Lv Xiaofei, Yu Yijun, Hu Lingfei, Tang Chao, Wu Chuyi, Dong Baiyu, Xue Ran, Dahlgren RandyA, Tan Xiangfeng, Dai Hengyi, Zhu Yong-Guan, Chu Haiyan, Xu Jianming. “Biogeographic Patterns and Drivers of Soil Viromes” Nature Ecology & Evolution. 2024;8(4):717–728. https://doi
.org/10.1038 /s41559-024-02347-2 . [PubMed: 38383853] - Martens EricC. “Fibre for the Future” Nature. 2016;529(7585):158–159. https://doi
.org/10.1038/529158a . [PubMed: 26762451] - Martinez KristinaB, Leone Vanessa, Chang EugeneB. “Microbial Metabolites in Health and Disease: Navigating the Unknown in Search of Function” Journal of Biological Chemistry. 2017;292(21):8553–8559. https://doi
.org/10.1074/jbc.R116.752899 . [PMC free article: PMC5448084] [PubMed: 28389566] - Matchado MonicaSteffi, Rühlemann Malte, Reitmeier Sandra, Kacprowski Tim, Frost Fabian, Haller Dirk, Baumbach Jan, List Markus. “On the Limits of 16S rRNA Gene-Based Metagenome Prediction and Functional Profiling” Microbial Genomics. 2024;10(2) https://doi
.org/10.1099/mgen.0.001203 . [PMC free article: PMC10926695] [PubMed: 38421266] - McAnulty JoanneT, Akabas SharonR, Thuppal SowmyanarayananV, Paxson ErinE, Saklani Shilpa, Tucker KatherineL, Bailey ReganL. “Fiber Intake Varies by Poverty-Income Ratio and Race/Ethnicity in the US Adults” Nutrition Today. 2017;52(2):73–79. https://doi
.org/10.1097/nt .0000000000000207 . - McGivern BridgetB, Tfaily MalakM, Borton MikaylaA, Kosina SuzanneM, Daly RebeccaA, Nicora CarrieD, Purvine SamuelO, Wong AllisonR, Lipton MaryS, Hoyt DavidW, Northen TrentR, Hagerman AnnE, Wrighton KellyC. “Decrypting Bacterial Polyphenol Metabolism in an Anoxic Wetland Soil” Nature Communications. 2021;12(1):2466. https://doi
.org/10.1038 /s41467-021-22765-1 . [PMC free article: PMC8084988] [PubMed: 33927199] - McKeown NicolaM, Livingston KaraA, Sawicki CaleighM, Miller KevinB. “Evidence Mapping to Assess the Available Research on Fiber, Whole Grains, and Health” Nutrition Reviews. 2020;78(Suppl 1):37–42. https://doi
.org/10.1093/nutrit/nuz062 . [PMC free article: PMC7390649] [PubMed: 32728740] - Nakatsuji Teruaki, Chen TiffanyH, Narala Saisindhu, Chun KimberlyA, Two AimeeM, Yun Tong, Shafiq Faiza, Kotol PaulF, Bouslimani Amina, Melnik AlexeyV, Latif Haythem, Kim Ji-Nu, Lockhart Alexandre, Artis Keli, David Gloria, Taylor Patricia, Streib Joanne, Dorrestein PieterC, Grier Alex, Gill StevenR, Zengler Karsten, Hata TissaR, Leung DonaldYM, Gallo RichardL. “Antimicrobials from Human Skin Commensal Bacteria Protect Against Staphylococcus aureus and Are Deficient in Atopic Dermatitis” Science Translational Medicine. 2017;9(378) https://doi
.org/10.1126/scitranslmed .aah4680 . [PMC free article: PMC5600545] [PubMed: 28228596] - NASEM (National Academies of Sciences, Engineering, and Medicine). Microbiomes of the Built Environment: A Research Agenda for Indoor Microbiology, Human Health, and Buildings. Washington, DC: The National Academies Press; 2017. https://doi
.org/10.17226/23647 . [PubMed: 29035489] - Nguyen NhuH, Song Zewei, Bates ScottT, Branco Sara, Tedersoo Leho, Menke Jon, Schilling JonathanS, Kennedy PeterG. “FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild” Fungal Ecology. 2016;20:241–248. https://doi
.org/10.1016/j .funeco.2015.06.006 . - Nguyen TraD, Lesani Mahbobeh, Forrest Ines, Lan Yunpeng, Dean DanyaA, Gibaut QuentinMR, Guo Yanting, Hossain Ekram, Olvera Marcela, Panlilio Hannah, Parab AdwaitaR, Wu Chaoyi, Bernatchez JeanA, Cichewicz RobertH, McCall Laura-Isobel. “Local Phenomena Shape Backyard Soil Metabolite Composition” Metabolites. 2020;10(3):86. https://www
.mdpi.com/2218-1989/10/3/86 . [PMC free article: PMC7143036] [PubMed: 32121389] - Nicholson JeremyK, Holmes Elaine, Kinross James, Burcelin Remy, Gibson Glenn, Jia Wei, Pettersson Sven. “Host-Gut Microbiota Metabolic Interactions” Science. 2012;336(6086):1262–1267. https://doi
.org/10.1126/science.1223813 . [PubMed: 22674330] - NMDC (National Microbiome Data Collaborative). National Microbiome Data Collaborative: Progress Report 2022. U.S. Department of Energy; 2022. https:
//microbiomedata .org/wp-content/uploads /sites/2/2023/03 /NMDC-2022-Annual-Report.pdf . - Nogal Ana, Valdes AnaM, Menni Cristina. “The Role of Short-Chain Fatty Acids in the Interplay Between Gut Microbiota and Diet in Cardio-Metabolic Health” Gut Microbes. 2021;13(1):1897212. https://doi
.org/10.1080/19490976 .2021.1897212 . [PMC free article: PMC8007165] [PubMed: 33764858] - NRC (National Research Council). The New Science of Metagenomics: Revealing the Secrets of Our Microbial Planet. Washington, DC: The National Academies Press; 2007. https://doi
.org/10.17226/11902 . [PubMed: 21678629] - Pasolli Edoardo, Asnicar Francesco, Manara Serena, Zolfo Moreno, Karcher Nicolai, Armanini Federica, Beghini Francesco, Manghi Paolo, Tett Adrian, Ghensi Paolo, Carmen Collado Maria, Rice BenjaminL, DuLong Casey, Morgan XochitlC, Golden ChristopherD, Quince Christopher, Huttenhower Curtis, Segata Nicola. “Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle” Cell. 2019;176(3):649–662.e20. https://doi
.org/10.1016/j .cell.2019.01.001 . [PMC free article: PMC6349461] [PubMed: 30661755] - Penders John, Best Nielsvan. “The Development of the Gut Microbiota in Childhood and Its Distortion by Lifestyle Changes” In: Rook GrahamAW, Lowry ChristopherA, editors. Evolution, Biodiversity and a Reassessment of the Hygiene Hypothesis. Cham: Springer International Publishing; 2022. pp. 197–219.
- Põlme Sergei, Abarenkov Kessy, Henrik Nilsson R, Lindahl BjörnD, Engelbrecht Clemmensen Karina, Kauserud Havard, Nguyen Nhu, Kjøller Rasmus, Bates ScottT, Baldrian Petr. “FungalTraits: A User-Friendly Traits Database of Fungi and Fungus-like Stramenopiles” Fungal Diversity. 2020;105(1):1–16. https://doi
.org/10.1007 /s13225-02000466-2 . - Qi Qian, Hu Caixia, Lin Jiahui, Wang Xuehua, Tang Caixian, Dai Zhongmin, Xu Jianming. “Contamination with Multiple Heavy Metals Decreases Microbial Diversity and Favors Generalists as the Keystones in Microbial Occurrence Networks” Environmental Pollution. 2022;306:119406. https://doi
.org/10.1016/j .envpol.2022.119406 . [PubMed: 35561794] - Ramirez KellyS, Knight ChristopherG, Hollander Mattiasde, Brearley FrancisQ, Constantinides Bede, Cotton Anne, Creer Si, Crowther ThomasW, Davison John, Delgado-Baquerizo Manuel, Dorrepaal Ellen, Elliott DavidR, Fox Graeme, Griffiths RobertI, Hale Chris, Hartman Kyle, Houlden Ashley, Jones DavidL, Krab EvelineJ, Maestre FernandoT, McGuire KristaL, Monteux Sylvain, Orr CarolineH, van der Putten WimH, Roberts IanS, Robinson DavidA, Rocca JenniferD, Rowntree Jennifer, Schlaeppi Klaus, Shepherd Matthew, Singh BrajeshK, Straathof AngelaL, Bhatnagar JenniferM, Thion Cécile, van der Heijden MarcelGA, Franciska TdeVries. “Detecting Macroecological Patterns in Bacterial Communities Across Independent Studies of Global Soils” Nature Microbiology. 2018;3(2):189–196. https://doi
.org/10.1038 /s41564-017-0062-x . [PubMed: 29158606] - Ravn JonasL, Engqvist MartinKM, Larsbrink Johan, Geijer Cecilia. “CAZyme Prediction in Ascomycetous Yeast Genomes Guides Discovery of Novel Xylanolytic Species with Diverse Capacities for Hemicellulose Hydrolysis” Biotechnology for Biofuels. 2021;14(1):150. https://doi
.org/10.1186 /s13068-021-01995-x . [PMC free article: PMC8254220] [PubMed: 34215291] - Ridaura VanessaK, Faith JeremiahJ, Rey FedericoE, Cheng Jiye, Duncan AlexisE, Kau AndrewL, Griffin NicholasW, Lombard Vincent, Henrissat Bernard, Bain JamesR, Muehlbauer MichaelJ, Ilkayeva Olga, Semenkovich ClayF, Funai Katsuhiko, Hayashi DavidK, Lyle BarbaraJ, Martini MargaretC, Ursell LukeK, Clemente JoseC, Van Treuren William, Walters WilliamA, Knight Rob, Newgard ChristopherB, Heath AndrewC, Gordon JeffreyI. “Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice” Science. 2013;341(6150):1241214. https://doi
.org/10.1126/science.1241214 . [PMC free article: PMC3829625] [PubMed: 24009397] - Rodríguez-Ramos JosuéA, Borton MikaylaA, McGivern BridgetB, Smith GarrettJ, Solden LindseyM, Shaffer Michael, Daly RebeccaA, Purvine SamuelO, Nicora CarrieD, Eder ElizabethK, Lipton Mary, Hoyt DavidW, Stegen JamesC, Wrighton KellyC. “Genome-Resolved Metaproteomics Decodes the Microbial and Viral Contributions to Coupled Carbon and Nitrogen Cycling in River Sediments” mSystems. 2022;7(4):e00516–22. https://doi
.org/10.1128/msystems .00516-22 . [PMC free article: PMC9426555] [PubMed: 35861508] - Rook GrahamAW. “Human Evolution, Microorganisms, Socioeconomic Status and Reconciling Necessary Microbial Exposures with Essential Hygiene” In: Rook GrahamAW, Lowry ChristopherA, editors. Evolution, Biodiversity and a Reassessment of the Hygiene Hypothesis. Cham: Springer International Publishing; 2022. pp. 27–66.
- Rook GrahamAW. “Darwinian Medicine: We Evolved to Require Continuing Contact with the Microbiota of the Natural Environment. Evolution Turns the Inevitable into a Necessity” In: Hurst ChristonJ, editor. Microbes: The Foundation Stone of the Biosphere. Cham: Springer International Publishing; 2021. pp. 327–364.
- Rook GrahamAW, Bloomfield SallyF. “Microbial Exposures That Establish Immunoregulation Are Compatible with Targeted Hygiene” Journal of Allergy and Clinical Immunology. 2021;148(1):33–39. https://doi
.org/10.1016/j .jaci.2021.05.008 . [PubMed: 34033844] - Rook GrahamAW, Lowry ChristopherA, Raison CharlesL. “Hygiene and Other Early Childhood Influences on the Subsequent Function of the Immune System” Brain Research. 2015;1617:47–62. https://doi
.org/10.1016/j .brainres.2014.04.004 . [PubMed: 24732404] - Roux Simon, Emerson JoanneB. “Diversity in the Soil Virosphere: To Infinity and Beyond?” Trends in Microbiology. 2022;30(11):1025–1035. https://doi
.org/10.1016/j .tim.2022.05.003 . [PubMed: 35644779] - Ryan StephanieM, Eichenberger RamonM, Ruscher Roland, Giacomin PaulR, Loukas Alex. “Harnessing Helminth-Driven Immunoregulation in the Search for Novel Therapeutic Modalities” PLOS Pathogens. 2020;16(5):e1008508. https://doi
.org/10.1371/journal .ppat.1008508 . [PMC free article: PMC7224462] [PubMed: 32407385] - Salazar Alejandro, Lennon JT, Dukes JS. “Microbial Dormancy Improves Predictability of Soil Respiration at the Seasonal Time Scale” Biogeochemistry. 2019;144(1):103–116. https://doi
.org/10.1007 /s10533-019-00574-5 . - Santos-Medellin Christian, Zinke LauraA, ter Horst AnneliekM, Gelardi DanielleL, Parikh SanjaiJ, Emerson JoanneB. “Viromes Outperform Total Metagenomes in Revealing the Spatiotemporal Patterns of Agricultural Soil Viral Communities” The ISME Journal. 2021;15(7):1956–1970. https://doi
.org/10.1038 /s41396-021-00897-y . [PMC free article: PMC8245658] [PubMed: 33612831] - Sawicki CaleighM, Livingston KaraA, Obin Martin, Roberts SusanB, Chung Mei, McKeown NicolaM. “Dietary Fiber and the Human Gut Microbiota: Application of Evidence Mapping Methodology” Nutrients. 2017;9(2):125. https://doi
.org/10.3390%2Fnu9020125 . [PMC free article: PMC5331556] [PubMed: 28208609] - Seitz ValerieA, McGivern BridgetB, Daly RebeccaA, Chaparro JacquelineM, Borton MikaylaA, Sheflin AmyM, Kresovich Stephen, Shields Lindsay, Schipanski MeaganE, Wrighton KellyC, Prenni JessicaE. “Variation in Root Exudate Composition Influences Soil Microbiome Membership and Function” Applied and Environmental Microbiology. 2022;88(11):e00226–22. https://doi
.org/10.1128/aem.00226-22 . [PMC free article: PMC9195941] [PubMed: 35536051] - Sender Ron, Fuchs Shai, Milo Ron. “Revised Estimates for the Number of Human and Bacteria Cells in the Body” PLOS Biology. 2016;14(8):e1002533. https://doi
.org/10.1371/journal .pbio.1002533 . [PMC free article: PMC4991899] [PubMed: 27541692] - Shade Ashley, Jones StuartE, Gregory Caporaso J, Handelsman Jo, Knight Rob, Fierer Noah, Gilbert JackA. “Conditionally Rare Taxa Disproportionately Contribute to Temporal Changes in Microbial Diversity” mBio. 2014;5(4) https://doi
.org/10.1128/mbio.01371-14 . [PMC free article: PMC4161262] [PubMed: 25028427] - Sharma Shikha, Lal Kashyap Prem, Sharma Abhishek. “3 - Plant Virome: Current Understanding, Mechanisms, and Role in Phytobiome” In: Solanki Manoj Kumar, Kashyap Prem Lal, Ansari Rizwan Ali, Kumari Baby, editors. Microbiomes and Plant Health. London: Academic Press; 2021. pp. 53–81.
- Shi Shengjing, Nuccio ErinE, Shi ZhouJ, He Zhili, Zhou Jizhong, Firestone MaryK. “The Interconnected Rhizosphere: High Network Complexity Dominates Rhizosphere Assemblages” Ecology Letters. 2016;19(8):926–936. https://doi
.org/10.1111/ele.12630 . [PubMed: 27264635] - Šimura Jan, Antoniadi Ioanna, Široká Jitka, Tarkowská Danu¡e, Strnad Miroslav, Ljung Karin, Novák Ondřej. “Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics.” Plant Physiology. 2018;177(2):476–489. https://doi
.org/10.1104/pp.18.00293 . [PMC free article: PMC6001343] [PubMed: 29703867] - Singh BrajeshK, Liu Hongwei, Trivedi Pankaj. “Eco-holobiont: A New Concept to Identify Drivers of Host-associated Microorganisms” Environmental Microbiology. 2020;22(2):564–567. https://doi
.org/10.1111/1462-2920.14900 . [PubMed: 31849163] - Song Yang, Yao Shi, Li Xiaona, Wang Tao, Jiang Xin, Bolan Nanthi, Warren CharlesR, Northen TrentR, Chang ScottX. “Soil Metabolomics: Deciphering Underground Metabolic Webs in Terrestrial Ecosystems” Eco-Environment & Health. 2024;3(2):227–237. https://doi
.org/10.1016/j .eehl.2024.03.001 . [PMC free article: PMC11047296] [PubMed: 38680731] - Sparling GP. “Soil Microbial Biomass, Activity and Nutrient Cycling as Indicators of Soil Health” In: Pankhurst CE, Doube BM, Gupta VVSR, editors. Biological Indicators of Soil Health. Wallingford: CAB International; 1997. pp. 97–119.
- Starke Robert, López Mondéjar Ruebén, Rainer Human Zander, Navrátilová Diana, Martina Štursová, Větrovský Tomáš, Olson HeatherM, Orton DanielJ, Callister StephenJ, Lipton MaryS, Howe Adina, Ann McCue Lee, Pennacchio Christa, Grigoriev Igor, Baldrian Petr. “Niche Differentiation of Bacteria and Fungi in Carbon and Nitrogen Cycling of Different Habitats in a Temperate Coniferous Forest: A Metaproteomic Approach” Soil Biology and Biochemistry. 2021;155:108170. https://doi
.org/10.1016/j .soilbio.2021.108170 . - Stott DE, Andrews SS, Liebig MA, Wienhold BJ, Karlen DL. “Evaluation of β-Glucosidase Activity as a Soil Quality Indicator for the Soil Management Assessment Framework” Soil Science Society of America Journal. 2010;74(1):107–119. https://doi
.org/10.2136/sssaj2009.0029 . - Strachan DavidP. “Hay Fever, Hygiene, and Household Size” British Medical Journal. 1989;299(6710):1259–1260. https://doi
.org/10.1136/bmj .299.6710.1259 . [PMC free article: PMC1838109] [PubMed: 2513902] - Swanson JillK, Montes Luis, Mejia Luis, Allen Caitilyn. “Detection of Latent Infections of Ralstonia solanacearum Race 3 Biovar 2 in Geranium” Plant Disease. 2007;91(7):828–834. https://doi
.org/10.1094/pdis-91-7-0828 . [PubMed: 30780392] - Tang WHWilson, Li DanielY, Hazen StanleyL. “Dietary Metabolism, the Gut Microbiome, and Heart Failure” Nature Reviews Cardiology. 2019;16(3):137–154. https://doi
.org/10.1038 /s41569-018-0108-7 . [PMC free article: PMC6377322] [PubMed: 30410105] - Thomas AndrewMaltez, Manghi Paolo, Asnicar Francesco, Pasolli Edoardo, Armanini Federica, Zolfo Moreno, Beghini Francesco, Manara Serena, Karcher Nicolai, Pozzi Chiara, Gandini Sara, Serrano Davide, Tarallo Sonia, Francavilla Antonio, Gallo Gaetano, Trompetto Mario, Ferrero Giulio, Mizutani Sayaka, Shiroma Hirotsugu, Shiba Satoshi, Shibata Tatsuhiro, Yachida Shinichi, Yamada Takuji, Wirbel Jakob, Schrotz-King Petra, Ulrich CorneliaM, Brenner Hermann, Arumugam Manimozhiyan, Bork Peer, Zeller Georg, Cordero Francesca, Dias-Neto Emmanuel, Carlos Setubal João, Tett Adrian, Pardini Barbara, Rescigno Maria, Waldron Levi, Naccarati Alessio, Segata Nicola. “Metagenomic Analysis of Colorectal Cancer Datasets Identifies Cross-cohort Microbial Diagnostic Signatures and a Link with Choline Degradation” Nature Medicine. 2019;25(4):667–678. https://doi
.org/10.1038 /s41591-019-0405-7 . [PMC free article: PMC9533319] [PubMed: 30936548] - Thompson LukeR, Sanders JonG, McDonald Daniel, Amir Amnon, Ladau Joshua, Locey KennethJ, Prill RobertJ, Tripathi Anupriya, Gibbons SeanM, Ackermann Gail. “A Communal Catalogue Reveals Earth’s Multiscale Microbial Diversity” Nature. 2017;551(7681):457–463. https://doi
.org/10.1038/nature24621 . [PMC free article: PMC6192678] [PubMed: 29088705] - Tibbett Mark, Fraser TandraD, Duddigan Sarah. “Identifying Potential Threats to Soil Biodiversity” PeerJ. 2020;8:e9271. https://doi
.org/10.7717/peerj.9271 . [PMC free article: PMC7295018] [PubMed: 32566399] - Tindall AlyssaM, Petersen KristinaS, Kris-Etherton PennyM. “Dietary Patterns Affect the Gut Microbiome—The Link to Risk of Cardiometabolic Diseases” Journal of Nutrition. 2018;148(9):1402–1407. https://doi
.org/10.1093/jn/nxy141 . [PMC free article: PMC7263841] [PubMed: 30184227] - Treseder KathleenK, Lennon JayT. “Fungal Traits That Drive Ecosystem Dynamics on Land” Microbiology and Molecular Biology Reviews. 2015;79(2):243–262. https://doi
.org/10.1128/mmbr.00001-15 . [PMC free article: PMC4429240] [PubMed: 25971588] - Trivedi P, Delgado-Baquerizo M, Trivedi C, Hamonts K, Anderson IC, Singh BK. “Keystone Microbial Taxa Regulate the Invasion of a Fungal Pathogen in Agro-ecosystems” Soil Biology and Biochemistry. 2017;111:10–14. https://doi
.org/10.1016/j .soilbio.2017.03.013 . - Turnbaugh PeterJ, Ley RuthE, Mahowald MichaelA, Magrini Vincent, Mardis ElaineR, Gordon JeffreyI. “An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest” Nature. 2006;444(7122):1027–1031. https://doi
.org/10.1038/nature05414 . [PubMed: 17183312] - von Mutius Erika. “The ‘Hygiene Hypothesis’ and the Lessons Learnt from Farm Studies” Frontiers in Immunology. 2021;12 https://doi
.org/10.3389/fimmu .2021.635522 . [PMC free article: PMC8044987] [PubMed: 33868259] - Wagg Cameron, Schlaeppi Klaus, Banerjee Samiran, Kuramae EikoE, van der Heijden MarcelGA. “Fungal-Bacterial Diversity and Microbiome Complexity Predict Ecosystem Functioning” Nature Communications. 2019;10(1):4841. https://doi
.org/10.1038 /s41467-019-12798-y . [PMC free article: PMC6813331] [PubMed: 31649246] - Wall DianaH, Nielsen UffeN, Six Johan. “Soil Biodiversity and Human Health” Nature. 2015;528(7580):69–76. https://doi
.org/10.1038/nature15744 . [PubMed: 26595276] - Walsh CorinneM, Becker-Uncapher Isadore, Carlson Madeline, Fierer Noah. “Variable Influences of Soil and Seed-Associated Bacterial Communities on the Assembly of Seedling Microbiomes” The ISME Journal. 2021;15(9):2748–2762. https://doi
.org/10.1038 /s41396-021-00967-1 . [PMC free article: PMC8397733] [PubMed: 33782567] - Wang Lin, Cao Jia-Bao, Xia Bin-Bin, Li Yue-Juan, Zhang Xuan, Mo Guo-Xin, Wang Rui-Juan, Guo Si-Qi, Zhang Yu-Qing, Xiao Kun, Zhu Guang-Fa, Liu Peng-Fei, Song Li-Cheng, Ma Xi-Hui, Xiang Ping-Chao, Wang Jiang, Liu Yu-Hong, Xie Fei, Zhang Xu-Dong, Li Xiang-Xin, Sun Wan-Lu, Cao Yan, Wang Kai-Fei, Zhang Wen-Hui, Zhao Wei-Chao, Yan Peng, Chen Ji-Chao, Yang Yu-Wei, Yu Zhong-Kuo, Tang Jing-Si, Xiao Li, Zhou Jie-Min, Xie Li-Xin, Wang Jun. “Metatranscriptome of Human Lung Microbial Communities in a Cohort of Mechanically Ventilated COVID-19 Omicron Patients” Signal Transduction and Targeted Therapy. 2023;8(1):432. https://doi
.org/10.1038 /s41392-023-01684-1 . [PMC free article: PMC10638395] [PubMed: 37949875] - Washington JohnA. “Chapter 10 - Principles of Diagnosis” In: Baron Samuel, editor. Medical Microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch at Galveston; 1996. [PubMed: 21413287]
- Wassermann Birgit, Müller Henry, Berg Gabriele. “An Apple a Day: Which Bacteria Do We Eat with Organic and Conventional Apples?” Frontiers in Microbiology. 2019;10 https://doi
.org/10.3389/fmicb.2019.01629 . [PMC free article: PMC6667679] [PubMed: 31396172] - Wastyk HannahC, Fragiadakis GabrielaK, Perelman Dalia, Dahan Dylan, Merrill BryanD, Yu FeiqiaoB, Topf Madeline, Gonzalez CarlosG, Van Treuren William, Han Shuo, Robinson JenniferL, Elias JoshuaE, Sonnenburg EricaD, Gardner ChristopherD, Sonnenburg JustinL. “Gut-Microbiota-Targeted Diets Modulate Human Immune Status” Cell. 2021;184(16):4137–4153.e14. https://doi
.org/10.1016/j .cell.2021.06.019 . [PMC free article: PMC9020749] [PubMed: 34256014] - Weis Caroline, Cuénod Aline, Rieck Bastian, Dubuis Olivier, Graf Susanne, Lang Claudia, Oberle Michael, Brackmann Maximilian, Søgaard KirstineK, Osthoff Michael, Borgwardt Karsten, Egli Adrian. “Direct Antimicrobial Resistance Prediction from Clinical MALDI-TOF Mass Spectra Using Machine Learning” Nature Medicine. 2022;28(1):164–174. https://doi
.org/10.1038 /s41591-021-01619-9 . [PubMed: 35013613] - Whidbey Christopher, Wright AaronT. “Activity-Based Protein Profiling—Enabling Multimodal Functional Studies of Microbial Communities” In: Cravatt BenjaminF, Hsu Ku-Lung, Weerapana Eranthie, editors. Activity-Based Protein Profiling. Cham: Springer International Publishing; 2019. pp. 1–21. [PMC free article: PMC6561099] [PubMed: 30406866]
- Whitman WilliamB, Woyke Tanja, Klenk Hans-Peter, Zhou Yuguang, Lilburn TimothyG, Beck BrianJ, De Vos Paul, Vandamme Peter, Eisen JonathanA, Garrity George, Hugenholtz Philip, Kyrpides NikosC. “Genomic Encyclopedia of Bacterial and Archaeal Type Strains, Phase III: The Genomes of Soil and Plant-Associated and Newly Described Type Strains” Standards in Genomic Sciences. 2015;10(1):26. https://doi
.org/10.1186 /s40793-015-0017-x . [PMC free article: PMC4511459] [PubMed: 26203337] - Whittaker RobertJ, Willis KatherineJ, Field Richard. “Scale and Species Richness: Towards a General, Hierarchical Theory of Species Diversity” Journal of Biogeography. 2001;28(4):453–470. https://doi
.org/10.1046/j .1365-2699.2001.00563.x . - Wicaksono WisnuAdi, Cernava Tomislav, Wassermann Birgit, Abdelfattah Ahmed, Soto-Giron MariaJ, Toledo GerardoV, Virtanen SuviM, Knip Mikael, Hyöty Heikki, Berg Gabriele. “The Edible Plant Microbiome: Evidence for the Occurrence of Fruit and Vegetable Bacteria in the Human Gut” Gut Microbes. 2023;15(2):2258565. https://doi
.org/10.1080/19490976 .2023.2258565 . [PMC free article: PMC10519362] [PubMed: 37741805] - Wiesmeier Martin, Barthold Frauke, Blank Benjamin, Kögel-Knabner Ingrid. “Digital Mapping of Soil Organic Matter Stocks Using Random Forest Modeling in a Semi-arid Steppe Ecosystem” Plant and Soil. 2011;340(1):7–24. https://doi
.org/10.1007 /s11104-010-0425-z . - Wikoff WilliamR, Anfora AndrewT, Liu Jun, Schultz PeterG, Lesley ScottA, Peters EricC, Siuzdak Gary. “Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites” Proceedings of the National Academy of Sciences. 2009;106(10):3698–3703. https://doi
.org/10.1073/pnas.0812874106 . [PMC free article: PMC2656143] [PubMed: 19234110] - Wilhelm RolandC, Pepe-Ranney Charles, Weisenhorn Pamela, Lipton Mary, Buckley DanielH. “Competitive Exclusion and Metabolic Dependency among Microorganisms Structure the Cellulose Economy of an Agricultural Soil” mBio. 2021;12(1):10.1128/mbio.03099–20. https://doi
.org/10.1128/mbio.03099-20 . [PMC free article: PMC8545098] [PubMed: 33402535] - Wilhelm RolandC, van Es HaroldM, Buckley DanielH. “Predicting Measures of Soil Health Using the Microbiome and Supervised Machine Learning” Soil Biology and Biochemistry. 2022;164:108472. https://doi
.org/10.1016/j .soilbio.2021.108472 . - Wilhelm RolandC, Amsili JosephP, Kurtz KirstenSM, van Es HaroldM, Buckley DanielH. “Ecological Insights into Soil Health According to the Genomic Traits and Environment-wide Associations of Bacteria in Agricultural Soils” ISME Communications. 2023;3(1):1. https://doi
.org/10.1038 /s43705-022-00209-1 . [PMC free article: PMC9829723] [PubMed: 37081121] - Winkler ThomasW, Day FelixR, Croteau-Chonka DamienC, Wood AndrewR, Locke AdamE, Mägi Reedik, Ferreira Teresa, Fall Tove, Graff Mariaelisa, Justice AnneE, Luan Jian’an, Gustafsson Stefan, Randall JoshuaC, Vedantam Sailaja, Workalemahu Tsegaselassie, Kilpeläinen TuomasO, Scherag André, Esko Tonu, Kutalik Zoltán, Heid IrisM, Loos RuthJF. the Genetic Investigation of Anthropometric Traits Consortium. “Quality Control and Conduct of Genome-wide Association Meta-analyses.” Nature Protocols. 2014;9(5):1192–1212. https://doi
.org/10.1038/nprot.2014.071 . [PMC free article: PMC4083217] [PubMed: 24762786] - Withers Emma, Hill PaulW, Chadwick DavidR, Jones DaveyL. “Use of Untargeted Metabolomics for Assessing Soil Quality and Microbial Function” Soil Biology and Biochemistry. 2020;143:107758. https://doi
.org/10.1016/j .soilbio.2020.107758 . - Woodcroft BenJ, Singleton CaitlinM, Boyd JoelA, Evans PaulN, Emerson JoanneB, Zayed AhmedAF, Hoelzle RobertD, Lamberton TimothyO, McCalley CarmodyK, Hodgkins SuzanneB, Wilson RachelM, Purvine SamuelO, Nicora CarrieD, Li Changsheng, Frolking Steve, Chanton JeffreyP, Crill PatrickM, Saleska ScottR, Rich VirginiaI, Tyson GeneW. “Genome-Centric View of Carbon Processing in Thawing Permafrost” Nature. 2018;560(7716):49–54. https://doi
.org/10.1038 /s41586-018-0338-1 . [PubMed: 30013118] - Wrighton KellyC, Thomas BrianC, Sharon Itai, Miller ChristopherS, Castelle CindyJ, VerBerkmoes NathanC, Wilkins MichaelJ, Hettich RobertL, Lipton MaryS, Williams KennethH, Long PhilipE, Banfield JillianF. “Fermentation, Hydrogen, and Sulfur Metabolism in Multiple Uncultivated Bacterial Phyla” Science. 2012;337(6102):1661–1665. https://doi
.org/10.1126/science.1224041 . [PubMed: 23019650] - Wu GaryD, Chen Jun, Hoffmann Christian, Bittinger Kyle, Chen Ying-Yu, Keilbaugh SueA, Bewtra Meenakshi, Knights Dan, Walters WilliamA, Knight Rob, Sinha Rohini, Gilroy Erin, Gupta Kernika, Baldassano Robert, Nessel Lisa, Li Hongzhe, Bushman FredericD, Lewis JamesD. “Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes” Science. 2011;334(6052):105–108. https://doi
.org/10.1126/science.1208344 . [PMC free article: PMC3368382] [PubMed: 21885731] - Yachi Shigeo, Loreau Michel. “Biodiversity and Ecosystem Productivity in a Fluctuating Environment: The Insurance Hypothesis” Proceedings of the National Academy of Sciences. 1999;96(4):1463–1468. https://doi
.org/10.1073/pnas.96.4.1463 . [PMC free article: PMC15485] [PubMed: 9990046] - Yadav Aanchal, Pandey Rajesh. “Viral Infectious Diseases Severity: Co-presence of Transcriptionally Active Microbes (TAMs) Can Play an Integral Role for Disease Severity” Frontiers in Immunology. 2022;13 https://doi
.org/10.3389/fimmu .2022.1056036 . [PMC free article: PMC9755851] [PubMed: 36532032] - Yang Yi, Chai Yabo, Xie Hanjie, Zhang Lu, Zhang Zhiming, Yang Xue, Hao Shenglei, Gai Jingping, Chen Yongliang. “Responses of Soil Microbial Diversity, Network Complexity and Multifunctionality to Three Land-Use Changes” Science of the Total Environment. 2023;859:160255. https://doi
.org/10.1016/j .scitotenv.2022.160255 . [PubMed: 36402341] - Zeevi David, Korem Tal, Zmora Niv, Israeli David, Rothschild Daphna, Weinberger Adina, Ben-Yacov Orly, Lador Dar, Avnit-Sagi Tali, Lotan-Pompan Maya, Suez Jotham, Ali Mahdi Jemal, Matot Elad, Malka Gal, Kosower Noa, Rein Michal, Zilberman-Schapira Gili, Dohnalová Lenka, Pevsner-Fischer Meirav, Bikovsky Rony, Halpern Zamir, Elinav Eran, Segal Eran. “Personalized Nutrition by Prediction of Glycemic Responses” Cell. 2015;163(5):1079–1094. https://doi
.org/10.1016/j .cell.2015.11.001 . [PubMed: 26590418] - Zhang Hai-Yang, Bissett Andrew, Aguilar-Trigueros CarlosA, Liu Hong-Wei, Powell JeffR. “Fungal Genome Size and Composition Reflect Ecological Strategies Along Soil Fertility Gradients” Ecology Letters. 2023;26(7):1108–1118. https://doi
.org/10.1111/ele.14224 . [PubMed: 37078433] - Zheng Danping, Liwinski Timur, Elinav Eran. “Interaction Between Microbiota and Immunity in Health and Disease” Cell Research. 2020;30(6):492–506. https://doi
.org/10.1038 /s41422-020-0332-7 . [PMC free article: PMC7264227] [PubMed: 32433595] - Zheng Yanfen, Han Xiaobin, Zhao Donglin, Wei Keke, Yuan Yuan, Li Yiqiang, Liu Minghong, Zhang Cheng-Sheng. “Exploring Biocontrol Agents from Microbial Keystone Taxa Associated to Suppressive Soil: A New Attempt for a Biocontrol Strategy” Frontiers in Plant Science. 2021;12 https://doi
.org/10.3389/fpls.2021.655673 . [PMC free article: PMC8095248] [PubMed: 33959142] - Zhou Dongrui, Zhang Honglin, Bai Zhimao, Zhang Aidi, Bai Futian, Luo Xing, Hou Yue, Ding Xiao, Sun Beili, Sun Xiao, Ma Ning, Wang Cuifen, Dai Xiaoniu, Lu Zuhong. “Exposure to Soil, House Dust and Decaying Plants Increases Gut Microbial Diversity and Decreases Serum Immunoglobulin E Levels in BALB/c Mice” Environmental Microbiology. 2016;18(5):1326–1337. https://doi
.org/10.1111/1462-2920.12895 . [PubMed: 25958920] - Zhou Dongrui, Bai Zhimao, Zhang Honglin, Li Na, Bai Zhiyu, Cheng Fudong, Jiang Haitao, Mao Chuanbin, Sun Xiao, Lu Zuhong. “Soil Is a Key Factor Influencing Gut Microbiota and Its Effect Is Comparable to That Exerted by Diet for Mice” F1000Research. 2018;7:1588. https://doi
.org/10.12688/f1000research .15297.1 .
- Microbiomes and the Soil–Human Health Continuum - Exploring Linkages Between Soi...Microbiomes and the Soil–Human Health Continuum - Exploring Linkages Between Soil Health and Human Health
Your browsing activity is empty.
Activity recording is turned off.
See more...