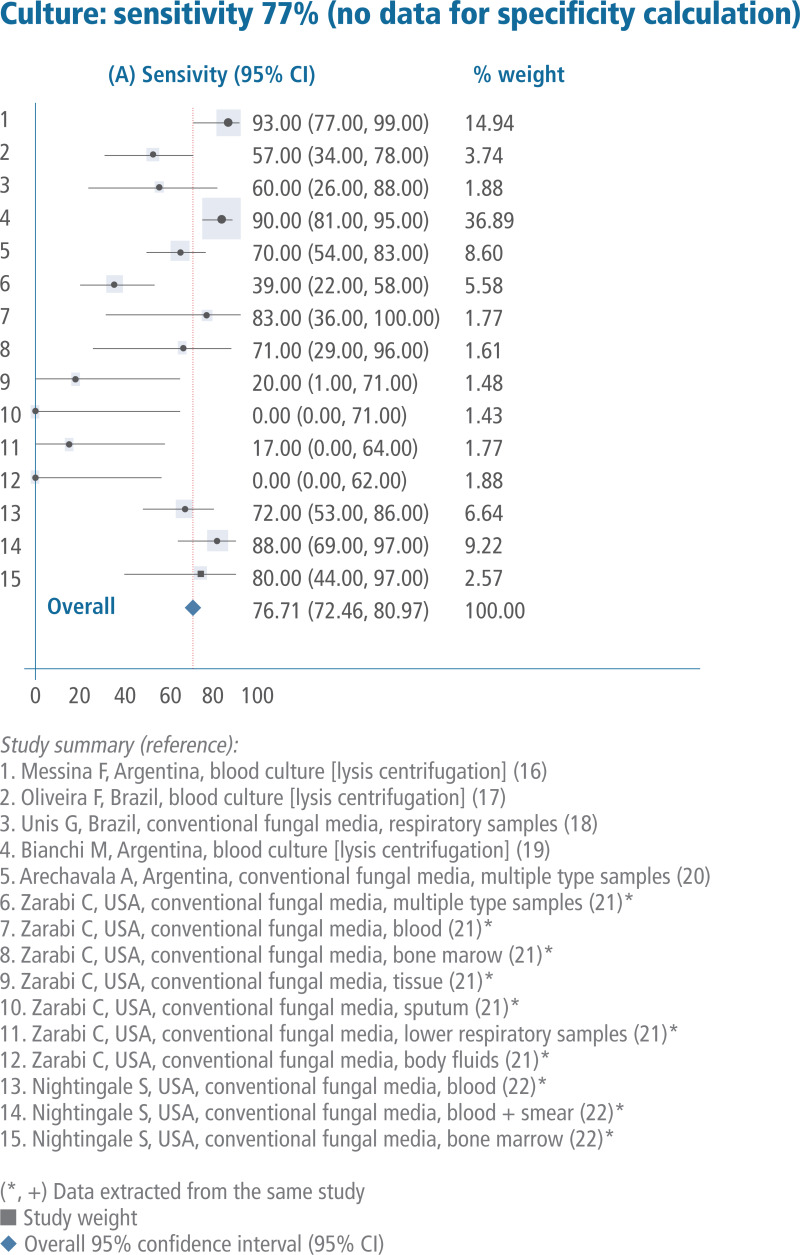
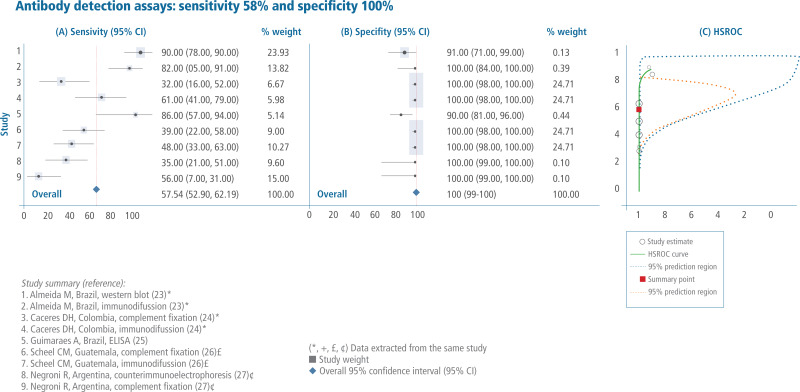
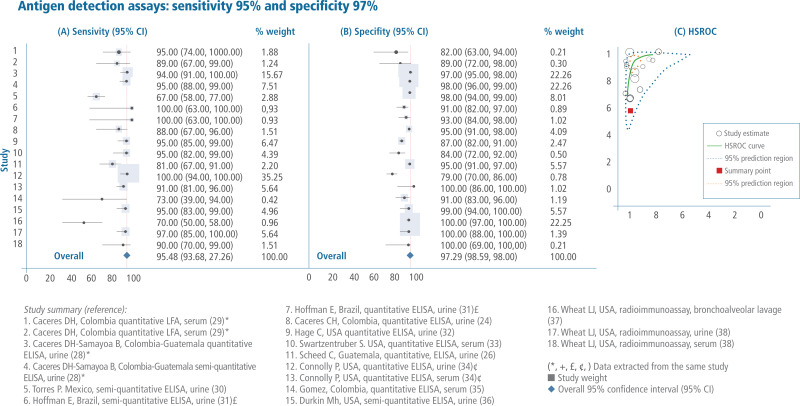
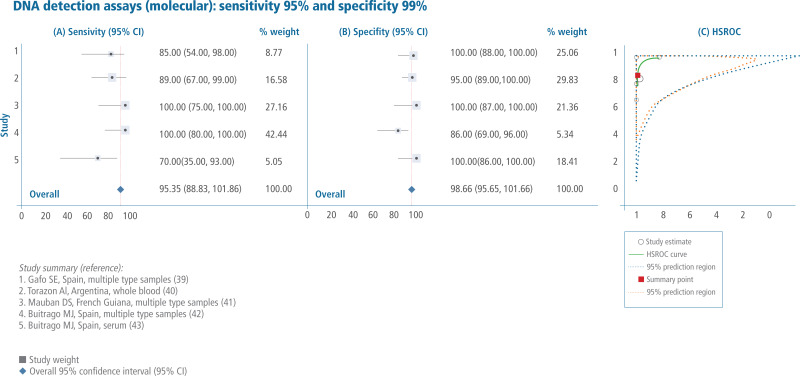
Sales, rights, and licensing. To purchase PAHO publications, visit http://publications.paho.org. To submit requests for commercial use and queries on rights and licensing, visit http://www.paho.org/permissions.
Third-party materials. If material that is attributed to a third party, such as tables, figures, or images, is reused from this work, it is the user’s responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned material or component from this work rests solely with the user.
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO license (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this license, this work may be copied, redistributed, and adapted for non-commercial purposes, provided the new work is issued using the same or equivalent Creative Commons license and it is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that the Pan American Health Organization (PAHO) and the World Health Organization (WHO) endorses any specific organization, product, or service. Use of the PAHO and WHO logo is not permitted.