Analysis of Individual-patient Data of Patients on treatment for isoniazid-resistant tuberculosis
- Under Review for Publication -
Authors of summary report: Federica Fregonese and Dick Menzies, on behalf of the IPD in INHR Group.
Members of the IPD in INHR Group: Ahuja S, Akkerman OW, Baghaei P, Bang D, BanuRekha VV, Bastos M, Benedetti A, Bonnet M, Cattamanchi A, Cegielski P, Chien J-Y, Cox H, Dedicoat M, Elliott A, Erkens C, Escalante P, Falzon D, Fregonese F, Garcia-Prats A, Gegia MCT, Glynn JR, Goldberg S, Hesseling A, Huyen MNC, Jacobson KR, Johnston J, Jones-Lopez E, Khan A, Kim YHS, Koh W, Kritski A, Lan Z, Lee H, Lee JH, Levin J, Li PZ, Maciel EL, Menzies D, Merle CSC, Munang M, Nahid P, Narendran G, Ohkado AA, Park JS, Phillips PPJ, Ponnuraja C, Quy H, Romanowski K, Schaaf S, Seaworth B, Seung KJ, Skrahina A, Sundari M, Swaminathan S, Tabarsi P, Trajman A, Trieu L, Viet Nhung N, Vikklepp P, Wang J-Y, Yoshiyama T.
Background and rationale: One of the major challenges impeding global tuberculosis (TB) control is drug-resistant TB (DR-TB). The World Health Organization (WHO) has estimated that approximately 10% of all TB cases have isoniazid-resistant TB without resistance to rifampicin (Hr-TB) (1). The impact of Hr-TB on treatment outcomes is not as serious as MDR-TB but combined failure and relapse in randomized trials of first-line therapy are around 12-13%. In a recent systematic review (2), failure and relapses were significantly higher in patients with Hr-TB than with drug-susceptible TB. Building upon this systematic review, and the experience that the McGill group acquired with the individual-patient data (IPD) analysis for MDR-TB treatment (3), we realised an IPD meta-analysis for treatment outcome of patients with Hr-TB in whom resistance to rifampicin had been excluded.
Methods: The Hr-TB treatment IPD was used to inform the WHO treatment policy for the treatment of this form of TB.1 Ahead of the WHO Guideline Development Group discussions on Hr-TB treatment, the expert panel involved developed a series of PICO2 questions to guide the evidence review (Annex 3). The outcomes of interest were: 1) Cured or completed (“success”) by end of treatment; 2) Failure and/or relapse; 3) Death from any cause during treatment; 4) Adverse reactions from anti-TB medicines (severity, type, organ class); and 5) Acquisition (amplification) of drug resistance for rifampicin. Relapse was defined as any recurrence of disease within two years after successful treatment completion. Studies of Hr-TB treatment were primarily identified from the 2016 systematic review (2). The search was done using PubMed, with no language restrictions, looking for publications since 1994 which reported treatment outcomes in patients with Hr-TB, either cohorts (with at least 20 Hr-TB subjects) or RCT. The review was updated until February 2016. Authors were invited to submit a set of standardized variables for patient-level data.
Outcomes were compared in patients on regimens grouped by the major elements of their composition (isoniazid [H], rifampicin [R], ethambutol [E], pyrazinamide [Z], fluoroquinolone [FQ], streptomycin [S]) and duration (6 months or >6 months). Relapse was combined with treatment failure for all analyses and “success” was compared with this composite outcome. Death was reported in all studies, but could not be analysed as a function of regimen length because in many studies treatment was individualized and thus the impact of death on its duration could not be reliably calculated from the information available. Mortality and success analysis were done in different populations, as deaths and loss to follow-up were removed from the population utilised in success analysis. The “actual” duration of treatment was used whenever this was reported by the authors; otherwise the “planned ” duration was used. Pooled analysis of adverse events was not done owing to the different ways in which this was recorded in the datasets.
We used propensity score matching (caliper method with difference of 0.02 allowed, with replacement) to estimate the adjusted odds ratios of outcome and their 95% confidence intervals. Patients were considered clustered within studies; hence intercepts and slopes of the main exposure variables were allowed to vary across studies. This is to account for otherwise unmeasured inter-study differences in patient populations, as well as centre-specific differences in data ascertainment, measurement and other factors. This analysis method provides very good matching of covariates. Risk differences were calculated with fixed effects generalized linear mixed effects models. Results were summarised in GRADE tables (see Online Annex 5).
Estimates of effect of each treatment parameter for each dataset were adjusted for the following covariates: age, gender, HIV co-infection, AFB smear positivity, cavitation on chest identified in radiography, past history of TB treatment and resistance to other first-line medicines beside isoniazid (if drug is used).
All analysis was performed using SAS, version 9.4 (SAS Institute, Cary, N.C.).
This project was approved by the Research Ethics Board of the Montréal Chest Institute, McGill University Health Centre, and also (if deemed necessary) by local ethics boards of originally approved studies.
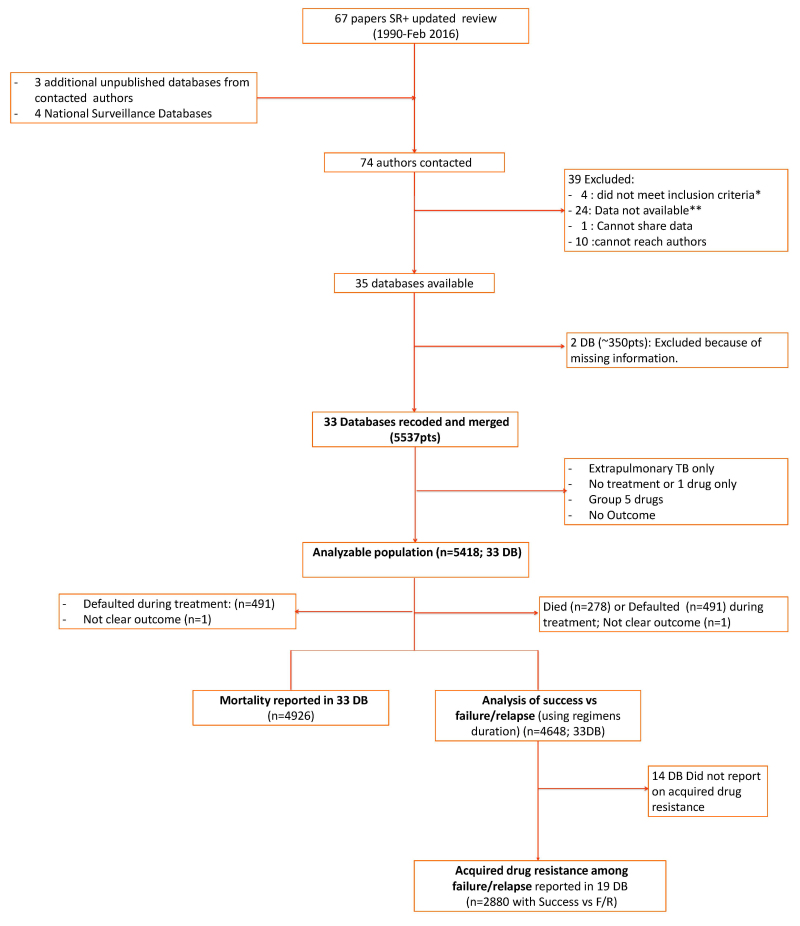
Results: Data on 5537 patients from 33 databases were combined and 5418 patients with adequate information were included in the analysis (Figure a; Table a).
Only 2% were children (0-14 years); a specific analysis on children was therefore not possible. Study characteristics are summarised in Table 3.
PICO 1. 6(H)REZ vs >6(H)REZ
6REZ was associated with a marginally statistically significant higher likelihood of treatment success when compared with 7-9 months of REZ (the effect was no longer statistically significant when a single large study was excluded). No added benefit from adding H to the regimen could be shown. Duration was not associated with a statistically significant difference in the acquisition of rifampicin resistance in Hr-TB patients.
PICO 2. 6 months or more (H)RE plus <4 months Z plus FQ vs 6 months or more (H)REZ
Use of FQ was associated with higher odds of treatment success when Z was given for less than 4 months, although not significant: a small number of patients received this regimen (n=118; 105 of whom received levofloxacin, moxifloxacin or gatifloxacin) and the comparator group also had a high level of success (crude success rates were 99% in FQ group and 93% in comparator group) (Data not shown in GRADE tables).
PICO 3. 6 months or more (H)REZ plus FQ vs 6 months or more (H)REZ
The addition of FQ to 6 months or more of (H)REZ is moderately associated with an improvement in success rates (97.6% vs 92.8%; aOR=2.8 95%CI 1.1-7.3), an effect which remains statistically significant even in the absence of H. Given the high success rate of the comparator regimen, it was unclear if FQ added benefit when Z was given for the full duration (See also PICO 2 above). Use of FQ was associated with lower odds of dying (in patients without H) and of acquired resistance to rifampicin.
PICO 4. 6 months or more (H)RE plus <4 months Z plus 3 months S vs 6 months or more (H)REZ
Treatment with 3 months or less of Z was consistently associated with worse outcomes, even when S was added. The WHO retreatment regimen (“Category 2”; 2SHREZ/1HREZ/5HRE) had significantly and substantially worse treatment success compared to 6REZ (83.4% vs 92.8%; aOR=0.4 95%CI 0.2-0.7). The odds of dying were lower for patients on S-containing regimens, although this effect was not statistically significant when the analysis was restricted to the studies included for the analysis of treatment success. Stratified analysis did not show differences in the odds of dying by S resistance, regardless of the inclusion of S in the regimens.
Conclusions: In summary, it appears that 6 REZ can result in high levels of treatment success in Hr-TB patients. The addition of FQ to these regimens appears to lower the risk of death and acquisition of rifampicin resistance; it may increase the likelihood of success even when Z is used for <4 months, which could thus reduce the risk of hepatotoxicity associated with this medicine. In contrast, the use of S was not associated with improved outcomes. The data could not identify which patient subgroups (e.g. extensive disease, polydrug resistance) could benefit most from prolonging the treatment beyond 6 months or by adding H or FQ or S to REZ.
Figure aStudy selection flowchart
Notes.–* No treatment information; or sample size <20 patients. ** Data unavailable as studies done in the 1990s, authors have changed institutions; data inaccessible (damaged in storage etc).
Table a
Analysable data in included databases.
Footnotes
- 1
[Public notice - Guideline Development Group (GDG) meeting - 27 April 2017] WHO treatment guidelines for isoniazid-resistant tuberculosis. Available at: http://www
.who.int/tb /areas-of-work/drug-resistant-tb /treatment /gdg-meeting-izoniazid-resistant-tb /en/ - 2
PICO is an acronym for Population, Intervention, Comparator, Outcome.
References
- 1)
- Global tuberculosis report 2014 (WHO/HTM/TB/2014.08). Geneva, World Health Organization. 2014. Available from: http://www
.who.int/tb /publications/global_report /gtbr14_main_text.pdf - 2)
- Gegia M, Winters N, Benedetti A et al. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017 Feb;17(2):223-234. doi: 10.1016/S1473-3099(16)30407-8. [PubMed: 27865891] [CrossRef]
- 3)
- Ahuja SD, et al. for the Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9(8):e1001300 [PMC free article: PMC3429397] [PubMed: 22952439]
Publication Details
Copyright
Sales, rights and licensing. To purchase WHO publications, see http://apps.who.int/bookorders. To submit requests for commercial use and queries on rights and licensing, see http://www.who.int/about/licensing.
Third-party materials. If you wish to reuse material from this work that is attributed to a third-party, such as tables, figures or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests solely with the user.
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “The translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”.
Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization.
Publisher
World Health Organization, Geneva
NLM Citation
WHO treatment guidelines for isoniazid-resistant tuberculosis: Supplement to the WHO treatment guidelines for drug-resistant tuberculosis [Internet]. Geneva: World Health Organization; 2018. Annex 4, Summary of unpublished data used for the recommendations.