1. Introduction
Although surgeons have progressively paid more attention to the control of operative wound contamination during surgical procedures, incisional surgical site infection (SSI) is still a frequent postoperative adverse event jeopardizing patient safety and increasing health care costs. A scrupulous aseptic surgical technique and the administration of adequate antibiotic prophylaxis prevent operative wound infection by decreasing contamination and eliminating the microorganisms that invade the surgical site, despite the efforts of the surgical team.
Conventional surgical drapes are commonly used by surgeons to limit the aseptic surgical area and to cover the freshly-made wound edges. Nevertheless, this non-fixed mechanical barrier may become dislodged or potentially contaminated. To better reinforce the aspects related to wound edge isolation, surgical wound protectors (WP) have been fabricated and marketed, unlike newly developed drugs that need different controlled studies before approval by regulatory bodies. These new surgical devices are comprised of a non-adhesive plastic sheath attached to a single or double rubber ring that firmly secures the plastic sheath to the wound edges, which can facilitate the retraction of the incision during surgery without the need for additional mechanical retractors and cloths. Theoretically, commercially-available WPs are intended to reduce wound edge contamination to a minimum during abdominal surgical procedures, including contamination from outside (clean surgery) and inside the peritoneal cavity (clean-contaminated, contaminated and dirty surgery). Although these surgical devices are already on the market, their real usefulness and cost-effectiveness warrants additional evidence-based analysis.
Few organizations have issued recommendations regarding the use of WP devices. The United Kingdom-based National Institute for Health and Care Excellence states that wound-edge protection devices may reduce SSI rates after open abdominal surgery, but no recommendation is given due to the lack of further high quality evidence (1). The guidelines of the Society for Healthcare Epidemiology of America (SHEA)/Infectious Diseases Society of America (IDSA) recommend the use of impervious plastic WPs for gastrointestinal and biliary tract surgery (2).
2. PICO question
Does the use of WP devices reduce the rate of SSI in open abdominal surgery?
Population: inpatients and outpatients of any age undergoing either elective or urgent abdominal surgery through conventional open access
Intervention: use of single or double plastic ring WP devices
Comparator: conventional wound protection, mainly through placing wet towels between the wound edge and steel type retractors
Outcomes: SSI, SSI-attributable mortality
3. Methods
The following databases were searched: Medline (PubMed); EMBASE; Cumulative Index to Nursing and Allied Health Literature (CINAHL); Cochrane Central Register of Controlled Trials (CENTRAL); and WHO regional medical databases. The time limit for the review was between 1 January 1990 and 28 November 2014. Language was restricted to English, French and Spanish. A comprehensive list of search terms was used, including Medical Subject Headings (MeSH) (Appendix 1).
Two independent reviewers screened the titles and abstracts of retrieved references for potentially relevant studies. The full text of all potentially eligible articles was obtained and two authors then independently reviewed these for eligibility based on inclusion criteria. Duplicate studies were excluded.
The two authors extracted data in a predefined evidence table (Appendix 2) and critically appraised the retrieved studies. Quality was assessed using the Cochrane Collaboration tool to assess the risk of bias of randomized controlled studies (RCTs) (3) (Appendix 3). Any disagreements were resolved through discussion or after consultation with the senior author, when necessary.
Meta-analyses of available comparisons were performed using Review Manager version 5.3 as appropriate (4) (Appendix 4). Adjusted odds ratios (OR) with 95% confidence intervals (CI) were extracted and pooled for each comparison with a random effects model. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (5) (GRADE Pro software) (6) was used to assess the quality of the body of retrieved evidence (Appendix 5).
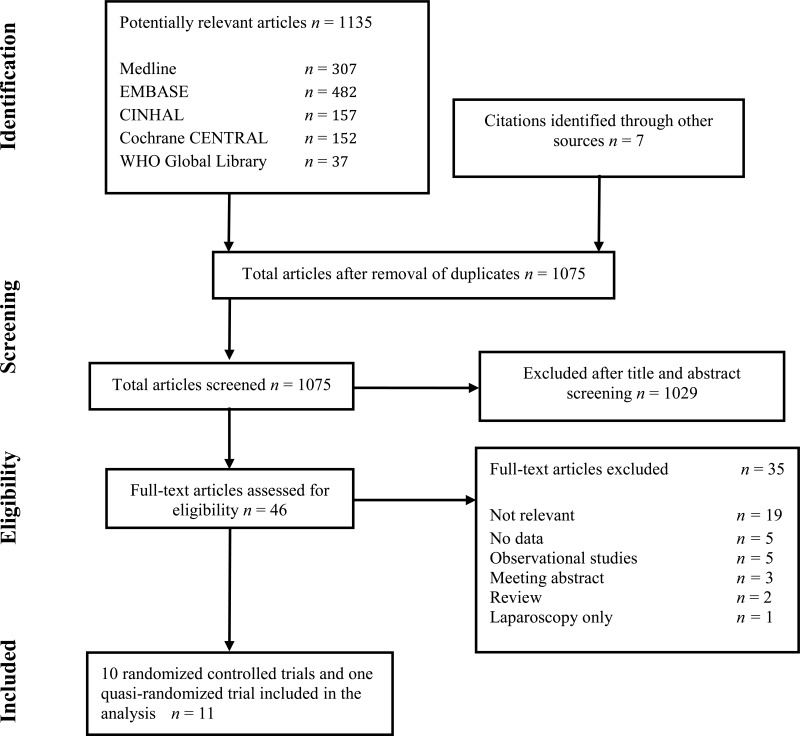
4. Study selection
Flow chart of the study selection process
5. Summary of the findings and quality of the evidence
Eleven studies (7–17) including 10 RCTs (7–16) and one prospective controlled trial (17) comparing the use of a WP device vs. conventional wound protection were identified with an SSI outcome. Patients were adults undergoing abdominal surgical procedures with laparotomy.
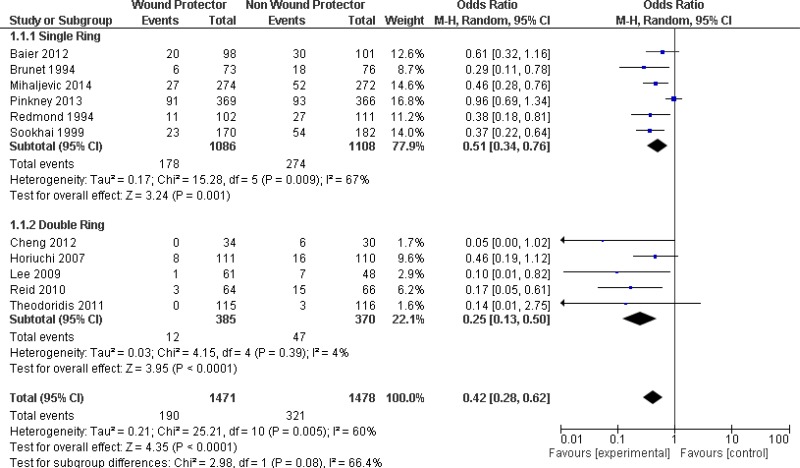
Six studies (7, 11–13, 15, 17) used a single-ring WP device as the intervention. Among these, 4 trials (11, 13, 15, 17) demonstrated that the use of a single-ring WP led to a significant reduction of the SSI rate when compared with standard wound protection. However, 2 trials showed no difference of risk (7, 12). A meta-analysis of this subgroup (Appendix 4, comparison 1) showed the benefit of a single-ring WP in reducing the SSI rate when compared with standard wound protection (OR: 0.51; 95% CI; 0.34–0.76).
Five (8–10, 14, 16) of the 11 studies used a double-ring WP device as the intervention. In 2 trials (10, 14), there was a significant reduction of the SSI rate when using a double-ring WP. Three trials (8, 9, 16) showed no difference in risk. A meta-analysis of this subgroup (Appendix 4, comparison 1) showed the benefit of a double-ring WP in reducing the rate of SSI (OR: 0.25; 95% CI: 0.13–0.50).
The overall meta-analysis (Appendix 4, comparison 1) including all 11 studies showed the benefit of using a WP device when compared with standard wound protection in reducing the rate of SSI (OR: 0.42; 95% CI: 0.28–0.62). In meta-regression analysis, there was no strong evidence for a difference in the effect between a single- and double-ring WP (P=0.107). A sensitivity analysis comparing the RCTs and the prospective controlled trial (17) indicated that there was no difference in the results, irrespective of whether the quasi-randomized trial was included or not.
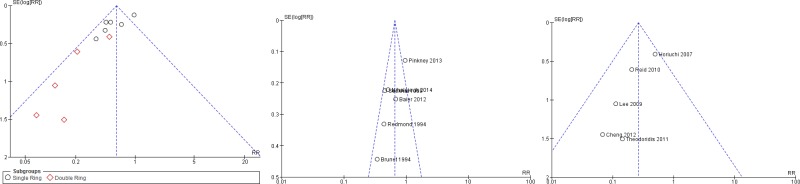
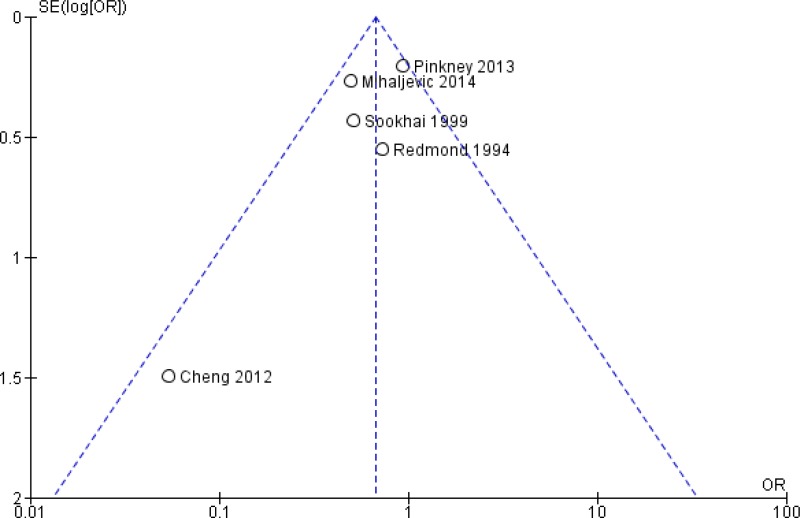
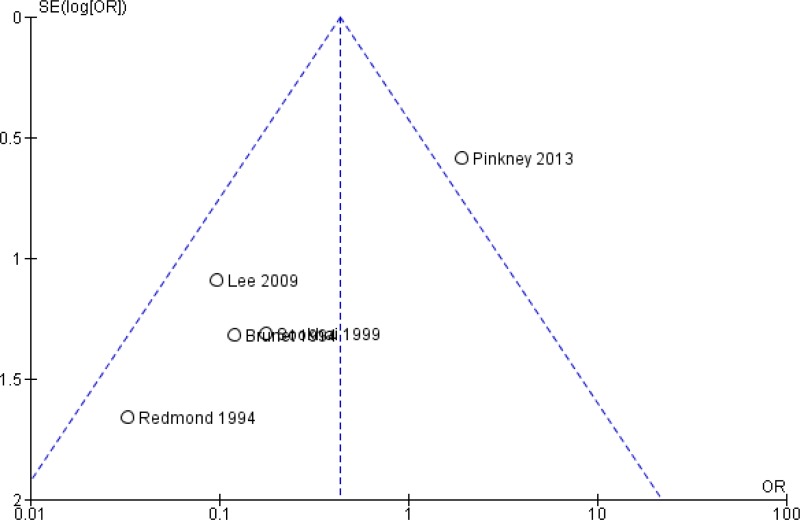
The quality of the evidence for this comparison was very low due to risk of bias, inconsistency and publication bias (Appendix 5). Most studies had an unclear to high risk in random sequence generation and unclear allocation concealment. There was considerable asymmetry observed in the funnel plot, compatible with the preferential publication of small studies demonstrating a benefit (Appendix 4, ).
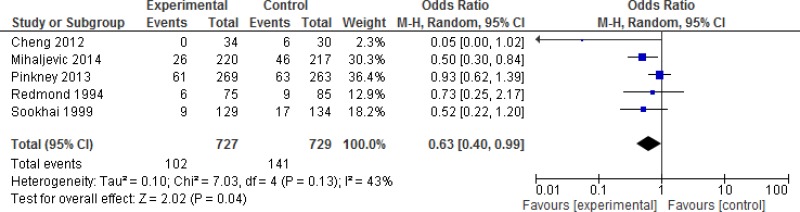
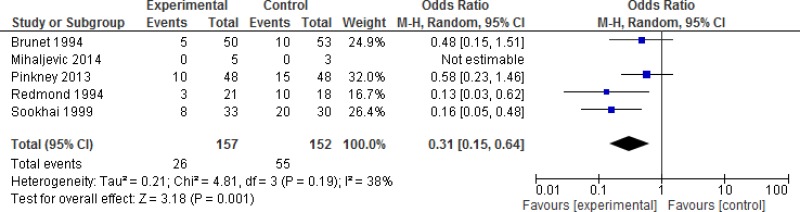
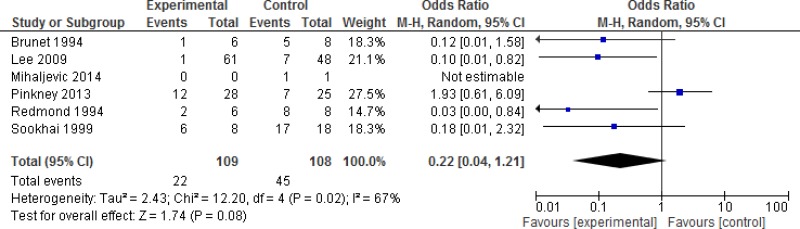
A subgroup analysis discriminating between different degrees of wound contamination in abdominal surgery (clean-contaminated, contaminated and dirty) showed that the use of a WP device is beneficial in reducing the SSI rate when compared to standard wound protection in clean-contaminated (OR: 0.63; 95% CI: 0.4–0.99) and contaminated (OR: 0.31; 95% CI: 0.15–0.64) procedures, but not in dirty (OR: 0.22; 95% CI: 0.04–1.21) surgery (Appendix 4, –). However, in meta-regression analysis, there was no evidence that the effect differed between clean-contaminated (P=0.244) or contaminated (P=0.305) or dirty (P=0.675) surgery and other surgery.
The body of retrieved evidence focused on adult patients and no study was available in the paediatric population. The literature search did not identify any studies that reported SSI-attributable mortality.
In conclusion, the available evidence can be summarized as follows.
- –
WP device vs. standard wound protection (comparison 1)
Overall, a very low quality of evidence shows that a single- or double-ring WP device has a benefit in reducing the rate of SSI compared with regular wound protection and retraction.
Of note, the included studies have some limitations. Blinding of care providers was not feasible due to the nature of the intervention and therefore it is likely to be a source of bias. The authors reported an appropriate random sequence generation process in only 4 of the 11 studies (10–12, 14). In addition, only 4 studies described allocation concealment (8, 11, 12, 14). The combined baseline SSI rate of the control group in the studies investigating the single-ring WP device was twice as high as in those investigating the double-ring device. This was believed to be due to differences in participating centres, surgeons, etc. There was considerable asymmetry observed in the funnel plot, compatible with the preferential publication of small studies demonstrating a benefit. SSI definitions and follow-up varied across studies. Although the outcomes of interest are incisional (superficial plus deep) SSIs with the use of a WP device, the type of SSI was reported differently across studies.
6. Other factors considered in the review of studies
The systematic review team identified the following other factors to be considered.
Potential harms
In patients with abdominal adhesions, the insertion of a WP device might be difficult and lead to the need to enlarge the incision, to injuries to the small bowel and to the prolongation of the procedure. There may be also limited space to access the surgical field after insertion of the device. Therefore, the operating surgeon needs to be familiar with handling a WP device during placement, in the operative phase and upon removal.
Values and preferences
No study was found on patient values and preferences with regards to this intervention. Patients will certainly prefer to be treated by surgeons who are familiar with the use of WP devices in order to reduce the risk of complications.
Resource use
Few studies addressed the cost-effectiveness of the intervention. Two small studies found the use of WPs to be cost-effective (10, 15), while one larger trial did not (18). Sookhai and colleagues (15) calculated that the use of wound edge protectors would have led to a potential saving of US$ 319 913 at a total cost of US$ 1620 per patient per procedure. Lee and colleagues (10) calculated a potential saving of US$ 430 per patient per procedure. Cheng and colleagues (8) concluded that UK£ 350 are required to prevent one probable superficial incisional SSI that costs UK£ 117 to treat. However, the authors found the additional costs too difficult to quantify, for example, regular outpatient consultations with medication, repeated travel to the hospital for dressings and absenteeism from work. A cost-effective analysis of a RCT comparing a single-ring WP with standard wound protection showed that the ratio of incremental cost per quality-adjusted life year (QALY) gained was not worthwhile (18). The use of a WP device was more costly and equally effective compared to standard care, but there was significant uncertainty around incremental costs and QALYs.
7. Key uncertainties and future research priorities
The systematic review team identified the following key uncertainties and future research priorities.
The prevalence of mostly low quality small studies highlights the need for properly designed multicentre RCTs. The SSI outcome should be defined according to the United States Centers for Disease Prevention and Control criteria and subspecified as superficial, deep and organ/space occupying. Specific and relevant surgical procedures should be included regarding the level of wound contamination and the rate of incisional SSI, for example, colorectal surgery and laparotomy for peritonitis. Investigators should consider comparing single- with double-ring WP devices. Trials should report adverse events related to the intervention. Cost-effectiveness studies are also needed.
Appendices
Appendix 1. Search terms
Medline (through PubMed)
“surgical wound infection”[Mesh] OR surgical site infection* [TIAB] OR “SSI” OR “SSIs” OR surgical wound infection* [TIAB] OR surgical infection*[TIAB] OR post-operative wound infection* [TIAB] OR postoperative wound infection* [TIAB] OR wound infection*[TIAB]
(wound protect*) OR wound retractor) OR Alexis retractor) OR Alexis wound protector) OR wound protector) OR wound protector device) OR “protective devices”[MeSH Terms]
Step 1 AND Step 2
“colony count, microbial”[Mesh] or colonization [TIAB] OR transmission [TIAB] OR contamination [TIAB]
* ((((wound retractor) OR Alexis retractor) OR Alexis wound protector) OR wound protector) OR wound protector device
Step 4 AND Step 5
Step 3 OR Step 6
AND (“1990/01/01”[PDat]: “2014/12/31”[PDat])))
*Excluded protective devices (mesh) due to the large amount of extra not relevant hits (1600)
EMBASE
surgical infection/ or (surgical site infection* or SSI or SSIs or surgical wound infection* or surgical infection* or post-operative wound infection* or postoperative wound infection*).ti,ab, kw.
wound retractor.mp. or exp retractor/ OR wound protector.mp. OR protective device.mp. or exp protective equipment/ OR Alexis retractor.mp.
Step 1 AND Step 2
surgery.ti,ab,kw.
colony count, microbial.ti,ab,kw. OR colonization.ti,ab,kw. OR contamination.ti,ab,kw. OR transmission.ti,ab, kw.
Step 2 AND Step 4 AND Step 5
Step 3 OR Step 6
limit 7 to yr=“1990 - current”
CINAHL
- S1.
MH “wound infection+”) OR “wound infection” OR (MH “surgical wound infection”)
- S2.
(MH “surgical instruments”) OR (MH “surgical mesh”) OR “wound protector” OR “wound retractor”
- S3.
S2 AND S1
Cochrane CENTRAL
wound infection:ti,ab,kw
surgical wound infection:ti,ab,kw
wound protector OR wound retractor OR (protective device AND surgery) OR wound protection
1 or 2
4 AND 3
WHO Global Regional Medical Databases
((SSI) OR (surgical site infection) OR (surgical site infections) OR (wound infection) OR (wound infections)) AND ((wound protector) OR (wound retractor) OR (protective device) OR (wound protection)
- ti:
title;
- ab:
abstract;
- kw:
keyword
Appendix 2. Evidence tables
Appendix 2a. Studies related to single-ring wound protectors
Download PDF (259K)
Appendix 2b. Studies related to double-ring wound protectors
Download PDF (300K)
Appendix 3. Risk of bias assessment of the included studies
View in own window
| Type of study | Author, year, reference | Random sequence generation | Allocation concealment | Blinding of participants | Blinding for care providers | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|
| Single-ring WP | Baier, 2012 (7) | HIGH | UNCLEAR | HIGH | HIGH | UNCLEAR | HIGH | LOW | - |
| Brunet, 1994 (17) | HIGH1 | UNCLEAR | LOW | HIGH | HIGH | HIGH | LOW | HIGH2 |
| Mihaljevic, 2014 (11) | LOW | LOW | LOW | HIGH | LOW | LOW | LOW | - |
| Pinkney, 2013 (12) | LOW | LOW | LOW | HIGH | LOW | LOW | LOW | - |
| Redmond, 1994 (13) | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW | UNCLEAR | LOW | |
| Sookhai, 1999 (15) | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW | UNCLEAR | UNCLEAR | - |
| Double-ring WP | Cheng, 2012 (8) | UNCLEAR | LOW | LOW | HIGH | UNCLEAR | LOW | HIGH | HIGH3 |
| Horiuchi, 2007 (9) | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW | UNCLEAR | UNCLEAR | - |
| Lee, 2009 (10) | LOW | UNCLEAR | LOW | HIGH | LOW | LOW | LOW | - |
| Reid, 2010 (14) | LOW | LOW | LOW | HIGH | LOW | LOW | LOW | HIGH4 |
| Theodoridis, 2011 (16) | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | UNCLEAR | UNCLEAR | LOW | - |
- 1
Quasi-randomized controlled trial.
- 2
Antibiotic prophylaxis was given in elective cases only for colorectal operations.
- 3
3 cases per month in a university hospital department: restricted/low rate of recruitment. The number of procedures per surgeon is not provided, thus a potential bias might be derived from such a possible imbalance if poor performers are in the standard group.
- 4
The proportion of operations was unequally distributed among all surgeons who participated in the study. The surgeon may contribute to different SSI rates.
Appendix 4. Comparisons
Comparison 1. Wound protector device (single- and double-ring) vs. conventional wound protection in abdominal surgery - SSI outcome
Comparison 2. Wound protector vs. no wound protector. Subgroup analysis of degree of wound contamination (clean-contaminated, contaminated and dirty) - SSI outcome
References
- 1.
A summary of selected new evidence relevant to NICE clinical guideline 74 ‘Prevention and treatment of surgical site infection’ (2008). Evidence update 43. June 2013. Manchester: National Institute for Health and Care Excellence; 2013 (
http://www.nice.org.uk/guidance/cg74/evidence, accessed 12 May 2016).
- 2.
Anderson
DJ, Podgorny
K, Berrios-Torres
SI, Bratzler
DW, Dellinger
EP, Greene
L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–27. [
PMC free article: PMC4267723] [
PubMed: 24799638]
- 3.
Higgins
JP, Altman
DG, Gotzsche
PC, Juni
P, Moher
D, Oxman
AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [
PMC free article: PMC3196245] [
PubMed: 22008217]
- 4.
The Nordic Cochrane Centre TCC. Review Manager (RevMan). Version 5.3. Copenhagen: The Cochrane Collaboration
2014.
- 5.
Guyatt
G, Oxman
AD, Akl
EA, Kunz
R, Vist
G, Brozek
J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [
PubMed: 21195583]
- 6.
GRADEpro Guideline Development Tool. Summary of findings tables, health technology assessment and guidelines. GRADE Working Group, Ontario: McMaster University and Evidence Prime Inc.; 2015 (
http://www.gradepro.org, accessed 5 May 2016).
- 7.
Baier
P, Kiesel
M, Kayser
C, Fischer
A, Hopt
UT, Utzolino
S. Ring drape do not protect against surgical site infections in colorectal surgery: a randomised controlled study. Int J Colorectal Dis. 2012;27(9):1223–8. [
PubMed: 22584293]
- 8.
Cheng
KP, Roslani
AC, Sehha
N, Kueh
JH, Law
CW, Chong
HY, et al. ALEXIS O-Ring wound retractor vs conventional wound protection for the prevention of surgical site infections in colorectal resections(1). Colorectal Dis. 2012;14(6):e346–51. [
PubMed: 22568647]
- 9.
Horiuchi
T, Tanishima
H, Tamagawa
K, Matsuura
I, Nakai
H, Shouno
Y, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62(1):212–5. [
PubMed: 17215757]
- 10.
Lee
P, Waxman
K, Taylor
B, Yim
S. Use of wound-protection system and postoperative wound-infection rates in open appendectomy: A randomized prospective trial. Arch Surg. 2009;144(9):872–5.
- 11.
Mihaljevic
AL, Schirren
R, Ozer
M, Ottl
S, Grun
S, Michalski
CW, et al. Multicenter double-blinded randomized controlled trial of standard abdominal wound edge protection with surgical dressings versus coverage with a sterile circular polyethylene drape for prevention of surgical site infections: a CHIR-Net trial (BaFO; NCT01181206). Ann Surg. 2014;260(5):730–7; discussion 7–9. [
PubMed: 25379844]
- 12.
Pinkney
TD, Calvert
M, Bartlett
DC, Gheorghe
A, Redman
V, Dowswell
G, et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). BMJ. 2013;347:f4305. [
PMC free article: PMC3805488] [
PubMed: 23903454]
- 13.
Redmond
HP, Meagher
PJ, Kelly
CJ, Deasy
JM. Use of an impervious wound-edge protector to reduce the postoperative wound infection rate. Br J Surg. 1994;81(1811).
- 14.
Reid
K, Pockney
P, Draganic
B, Smith
SR. Barrier wound protection decreases surgical site infection in open elective colorectal surgery: a randomized clinical trial. Dis. Colon Rectum. 2010;53(10):1374–80. [
PubMed: 20847618]
- 15.
Sookhai
S, Redmond
HP, Deasy
JM. Impervious wound-edge protector to reduce postoperative wound infection: a randomised, controlled trial. Lancet. 1999;353(9164):1585. [
PubMed: 10334259]
- 16.
Theodoridis
TD, Chatzigeorgiou
KN, Zepiridis
L, Papanicolaou
A, Vavilis
D, Tzevelekis
F, et al. A prospective randomized study for evaluation of wound retractors in the prevention of incision site infections after cesarean section. Clin Exp Obstet Gynecol. 2011;38(1):57–9. [
PubMed: 21485728]
- 17.
Brunet
P, Bounoua
F, Bugnon
PY, Gautier-Benoit
C. Intérêt des champs à anneau en chirurgie abdominale [Article in French]. Lyon Chir. 1994;90(6):438–41.
- 18.
Gheorghe
A, Roberts
TE, Pinkney
TD, Bartlett
DC, Morton
D, Calvert
M. The cost-effectiveness of wound-edge protection devices compared to standard care in reducing surgical site infection after laparotomy: an economic evaluation alongside the ROSSINI trial. PloS One. 2014;9(4):e95595. [
PMC free article: PMC3991705] [
PubMed: 24748154]