1. Introduction
The invasive nature of surgery introduces a high risk for the transfer of pathogens that may cause bloodborne infections in patients and/or the surgical team, including postoperative surgical site infection (SSI). This risk may be reduced by implementing protective barriers, such as wearing surgical gloves.
The latest WHO guidelines for safe surgery published in 2009 (1) recommend that the operating team should cover their hair and wear sterile gowns and sterile gloves during the operation, but without any indication on single- or double-gloving. The gudielines of the Society for Healthcare Epidemiology of America (SHEA)/Infectious Diseases Society of America (IDSA) (2) recommend that all members of the operative team should double-glove and change gloves when perforation is noted. The modalities and frequency of the changing of gloves have not been included in any guidelines or recommendations (1–3).
A Cochrane Review (4) published in 2009 investigated whether additional glove protection reduces the number of SSI or bloodborne infections in patients or the surgical team and the number of perforations to the innermost pair of surgical gloves. There was no direct evidence that additional glove protection worn by the surgical team reduces SSI in patients. However, the review had insufficient power for this outcome as only 2 trials were found with the primary outcome of SSI, both of which reported no infections. No trials were found with transmitted bloodborne infections as the outcome in surgical patients or the surgical team in relation to the gloving method. Thirty-one randomized controlled trials (RCTs) were identified with the outcome of glove perforation, leading to the result that the use of a second pair of surgical gloves, triple-gloving, knitted outer gloves and glove liners significantly reduces perforations to the innermost gloves.
The objective of this review was to assess the evidence on the effectiveness of double-gloving, the criteria for changing gloves during the operation and the optimal type of gloves to be used to prevent SSI.
2. PICO questions
When is double-gloving recommended?
What are the criteria for changing gloves during an operation?
What type of gloves should be used?
Population: inpatients and outpatients of any age undergoing surgical operations (any type of procedure)
Intervention: (1) use of double gloves
(2) change of gloves
(3) other types of gloves: glove liners, coloured perforation indicator systems, cloth outer gloves, steel outer gloves, triple gloves
Comparator: (1) use of a single pair of gloves
(2) retaining gloves
(3) latex gloves
Outcomes: SSI, SSI-attributable mortality
3. Methods
The following databases were searched: Medline (PubMed); Excerpta Medica Database (EMBASE); Cumulative Index to Nursing and Allied Health Literature (CINAHL); Cochrane Central Register of Controlled Trials (CENTRAL); and WHO regional medical databases. The time limit for the review was between 1 January 1990 and 24 April 2014. Language was restricted to English, French and Spanish. A comprehensive list of search terms was used, including Medical Subject Headings (MeSH) (Appendix 1).
Two independent reviewers screened the titles and abstracts of retrieved references for potentially relevant studies. The full text of all potentially eligible articles was obtained and two authors then independently reviewed these for eligibility based on inclusion criteria. Duplicate studies were excluded.
The two authors extracted data in a predefined evidence table (Appendix 2) and critically appraised the retrieved studies. Quality was assessed using the Cochrane Collaboration tool to assess the risk of bias of randomized controlled studies (5) (Appendix 3a) and the Newcastle-Ottawa Quality Assessment Scale for cohort studies (6) (Appendix 3b). Any disagreements were resolved through discussion or after consultation with the senior author, when necessary.
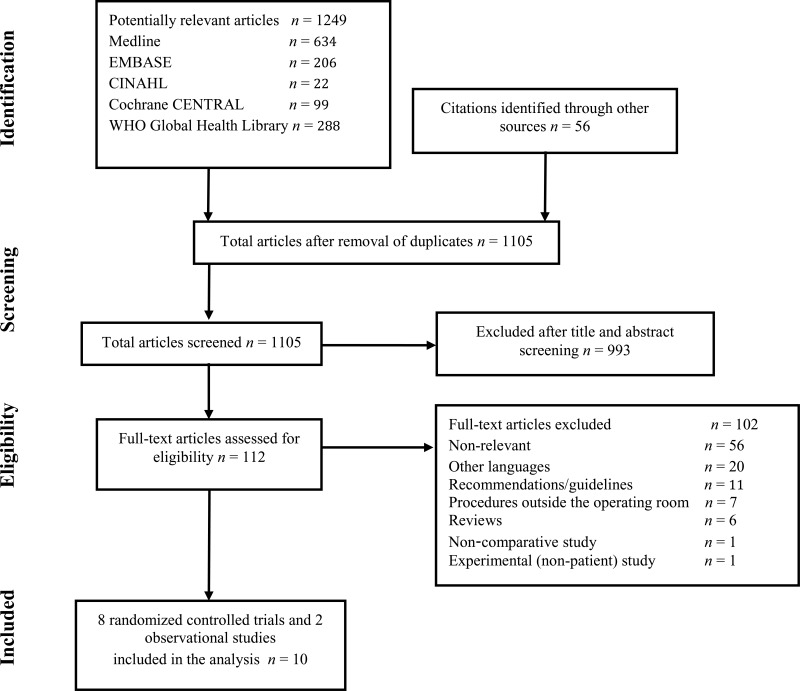
4. Study selection
Flow chart of the study selection proces
5. Summary of the findings and quality of the evidence
A total of 10 studies (8 randomized controlled trials [RCTs] (7–14) and 2 observational studies (15, 16)) were identified. Only 6 studies were identified with a SSI outcome (8–12, 15), one with cerebrospinal fluid (CSF) shunt infection (16). Thus, it was decided to include also studies with bacterial contamination as a surrogate outcome. Bacterial contamination was evaluated by making an impression of gloves on sterile culture media immediately before removal of each set of gloves. The culture plates were sent to a microbiology laboratory for incubation and the degree of contamination was evaluated by counting the number of bacterial colonies.
Due to heterogeneity among the selected studies regarding comparison, design and outcome, quantitative meta-analyses were not performed.
Findings related to PICO question 1: double-gloving vs. use of a single pair of gloves
Two observational studies (15, 16) comparing double-gloving vs. the use of a single pair of gloves were identified with an infectious outcome. Included patients were adults undergoing neurosurgery and hernia repair. One retrospective “before/after” study (16) investigated the effect of double-gloving on CSF shunt infection rates and showed that the overall infection rate was significantly higher in the single-gloved group compared to the double-gloved group (odds ratio [OR]: 2.48; 95% confidence interval [CI]: 1.50–4.22). Another non-randomized “before/after” study (15) found no difference in the risk of SSI between double- vs. single-gloving in patients undergoing hernia repair.
Findings related to PICO question 2: change of gloves vs. no change of gloves in the course of the operation
Three RCTs (8, 9, 12) comparing change of gloves vs. retaining gloves were identified with an SSI outcome. Included patients were adults undergoing caesarean section. Changing the entire surgical team’s gloves intraoperatively after delivery of the placenta or removing the external second glove by a circulating nurse after delivery of the fetus showed no difference in the rate of post-caesarean SSI and/or endometritis. All 3 studies addressed a specific question in a particular setting. Two studies had an additional intervention comparing different placental delivery techniques. None of the studies had superficial SSI as the primary outcome, but rather investigated endometritis.
One RCT (14) investigating a change of gloves vs. no change of gloves before the first contact with the vascular prosthesis in synthetic vascular graft surgery was identified with an SSI outcome. The authors reported 2 superficial SSIs in the glove change group and 5 superficial SSIs in the no change group (P<0.02). There were no acute graft infections in either group.
Two RCTs (13, 14) comparing a change of gloves vs. no change of gloves were identified with bacterial contamination as the outcome. One RCT (13) showed that in clean orthopaedic procedures, surgeons retaining the outer gloves one hour after the start of surgery had a subsequent positive glove contamination rate of 23% compared with 13% among surgeons who had exchanged their outer gloves (OR: 1.97; 95% CI: 1.02–3.80). The second RCT (14) investigated a change of gloves vs. no change of gloves before the first contact with the vascular prosthesis in synthetic vascular graft surgery and found the number of contaminated grafts to be similar in both groups. In a third RCT (7), a change of outer gloves after draping and prior to cementation during hip arthroplasty was implemented as standard of care in both groups. The authors investigated whether a systematic change of outer gloves at 20-minute intervals during surgery had an additional effect and found a significantly lower incidence of glove contamination in this group.
Findings related to PICO question 3: specific types of gloves vs. latex gloves
Two RCTs (10, 11) comparing 3 different types of gloves (double-gloving) in orthopaedic surgery were identified with an SSI outcome.
- -
An inner pair of standard latex gloves with cotton cloth outer gloves vs. 2 pairs of latex gloves (10).
- -
An inner pair of standard latex gloves with outer “orthopaedic” gloves vs. 2 pairs of latex gloves (11).
- -
Repel cloth gloves between 2 pairs of regular latex gloves vs. 2 pairs of latex gloves (11).
Neither of the trials reported any SSI in any of the groups.
The body of retrieved evidence focused on adult patients and no study was available in a paediatric population. The literature search did not identify any studies that reported on SSI-attributable mortality.
After discussion, 9 studies (17–25) were excluded. These concerned a comparison of sterile vs. non-sterile gloves in procedures performed outside the operating room, that is, dental extractions, dermatological procedures and emergency repair of uncomplicated traumatic lacerations, studies related to the impact of gloves on contamination by bloodborne infections (for example, human immunodeficiency virus, hepatitis, etc.), and studies investigating the impact of double-gloving on glove perforation. One non-comparative observational study (26) found a reduced risk of contamination and perforation of the outer gloves associated with systematic changes of the outer gloves at key situations during total hip arthroplasty operations. However, this study was excluded from further assessment due to a lack of comparison.
In conclusion, there is no relevant evidence to determine the effectiveness of wearing an additional pair of gloves, the criteria for changing gloves during an operation or of a specific type of gloving on the reduction of the SSI rate.
Of note, the identified studies have several limitations. The methodological quality of most studies was poor as the majority of the trials did not provide sufficient details of their process of randomization, allocation, sample size calculation and blinding. SSI definitions varied across the studies and there were few studies with SSI as the primary outcome. The selected studies with bacterial contamination as a surrogate outcome showed a great heterogeneity in the setting, design and outcome measures. There is no direct evidence demonstrating the link between bacterial contamination and SSI rates.
6. Key uncertainties and future research priorities
Well-designed RCTs investigating the effectiveness of double-gloving compared to the use of a single pair of gloves would be welcome, especially in low- and middle-income countries. RCTs evaluating whether a change of gloves during the operation is more effective in reducing the risk of SSI than no change of gloves are needed, including an assessment of the criteria for a change of gloves during an operation. To address the question of the optimal type of gloves to be used, it would be interesting to compare different types of gloving. All studies should focus on SSI as primary outcome, defined according to the United States Centers of Disease Control and Prevention criteria and subspecified as superficial, deep and organ/space occupying. Authors should use the CONSORT (Consolidating Standards of Reporting Trials) statement as a guideline for reporting parallel group randomized trials (27).
Appendices
Appendix 1. Search terms
MEDLINE (via PubMed)
“surgical wound infection”[Mesh] OR (surgical site infection* [TIAB] OR “SSI” OR “SSIs” OR surgical wound infection* [TIAB] OR surgical infection*[TIAB] OR post-operative wound infection* [TIAB] OR postoperative wound infection* [TIAB] OR wound infection*[TIAB])
glove [TIAB] OR gloves [TIAB] OR gloving [TIAB]
Step 1 AND Step 2
“cross infection”[MeSH] OR “nosocomial infection” OR “nosocomial infections” OR “hospital acquired infection” OR “hospital acquired infections” OR “hospital-acquired infection” OR “hospital-acquired infections” OR “health care associated infection” OR “health care associated infections” OR “health care-associated infection” OR “health-care-associated infections” OR “infection control”[MeSH] OR infection control [TIAB] OR “infection reduction” OR “reduction infection” OR colonization [TIAB] OR transmission [TIAB]
“gloves, surgical”[Mesh]
Step 4 AND Step 5
Step 3 OR Step 6
EMBASE
‘surgical infection’/exp OR ‘surgical site infection’:ti,ab OR ‘surgical site infections’:ti,ab OR ssis OR ‘surgical infection wound’:ti,ab OR ‘surgical infection wounds’:ti,ab OR ‘surgical infection’:ti,ab OR ‘postoperative wound infection’:ti,ab OR ‘postoperative wound infections’:ti,ab OR ‘post-operative wound infection’:ti,ab OR ‘post-operative wound infections’:ti,ab OR ‘wound infection’:ti,ab OR ‘wound infections’:ti,ab
‘surgical glove’/exp OR glove:ab,ti OR gloves:ab,ti OR gloving:ab,ti
‘cross infection’/exp OR ‘infection control’/exp OR ‘nosocomial infection’ OR ‘nosocomial infections’ OR ‘hospital acquired infection’ OR ‘hospital acquired infections’ OR ‘hospital-acquired infection’ OR ‘hospital-acquired infections’ OR ‘health care associated infection’ OR ‘health care associated infections’ OR ‘health care-associated infection’ OR ‘health-care-associated infections’ OR ‘infection control’:ti,ab OR ‘infection reduction’ OR “reduction infection” OR colonization:ti,ab OR colonization:ti,ab OR transmission:ti,ab
‘surgical glove’/exp
STEP 3 AND STEP4
STEP 1 AND STEP 2
STEP 5 OR STEP 6
CINAHL
(MH surgical wound infection) OR (AB surgical site infection* OR AB SSI OR AB SSIs OR AB surgical wound infection* OR AB surgical infection* OR AB post-operative wound infection* OR AB postoperative wound infection* OR AB wound infection*)
AB glove OR AB gloves OR AB gloving
Step 1 AND Step 2
((MH “cross infection+”) OR (MH “infection control+”) OR (MH “infection preventionists”) OR (MH “infection control (Saba CCC)+”) OR (MH “infection control (Iowa NIC)”) OR AB nosocomial infection OR AB nosocomial infections OR AB hospital acquired infection OR AB hospital acquired infections OR AB hospital-acquired infection OR AB hospital-acquired infections OR AB health care associated infection OR AB health care associated infections OR AB health care-associated infection OR AB health-care-associated infections OR AB infection control OR AB infection reduction OR AB reduction infection OR AB colonization OR AB transmission)
(MH gloves)
Step 4 AND Step 5
Step 3 OR Step 6
Cochrane CENTRAL
MeSH descriptor: [surgical wound infection] explode all trees
surgical site infections or SSI or SSIs or surgical wound infection* or surgical infection* or post-operative wound infection* or postoperative wound infection* or wound infection*:ti,ab,kw (word variations have been searched)
#1 or #2
glove or gloves or gloving:ti,ab,kw (word variations have been searched)
#3 and #4
MeSH descriptor: [infection control] explode all trees
MeSH descriptor: [cross infection] explode all trees
“nosocomial infection” or “nosocomial infections” or “hospital acquired infection” or “hospital acquired infections” or “hospital-acquired infection” or “hospital-acquired infections” or “health care associated infection” or “health care associated infections” or “health care-associated infection” or “health-care-associated infections” or infection control or “infection reduction” or “reduction infection” or colonization or transmission:ti,ab,kw (word variations have been searched)
#6 or #7 or #8
MeSH descriptor: [gloves, surgical] explode all trees
#9 and #10
#5 or #11
WHO Global Health Library
((ssi) OR (surgical site infection) OR (surgical site infections) OR (wound infection) OR (wound infections) OR (postoperative wound infection)) AND ((glove) OR (gloves)
- ti:
title;
- ab:
abstract.
Appendix 2. Evidence table
Appendix 2a. Studies related to double- vs. single-gloving: SSI outcome
Download PDF (194K)
Appendix 2b. Studies related to changing of gloves vs. retaining gloves: SSI outcome
Download PDF (132K)
Appendix 2c. Studies related to changing of gloves vs. retaining gloves - bacterial contamination as primary outcome*
Download PDF (288K)
Appendix 2d. Studies related to different types of gloving - SSI outcome
Download PDF (220K)
Appendix 3. Risk of bias assessment of the included studies
Appendix 3a. Risk of bias in the included randomized controlled studies (Cochrane Collaboration tool)
View in own window
| Author, year, reference | Sequence generation | Allocation concealment | Participants blinded | Care-providers blinded | Outcome assessors blinded | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|
| Ventolini 2004 (12) | Low risk | Low risk | Unclear | High risk | Unclear | Low risk | Low risk | Follow-up period unknown.
No validated SSI definition. |
| Cernadas 1998 (9) | Low risk | Low risk | Unclear | High risk | Low risk | Low risk | Low risk | Follow-up period unknown.
No validated SSI definition. |
| Atkinson 1996 (8) | Low risk | Low risk | Unclear | High risk | Unclear | Unclear | High risk | No clear inclusion and exclusion criteria.
Follow-up period unknown.
No validated SSI definition. |
| Ward 2014 (13) | Unclear | Unclear | Unclear | High risk | Unclear | Unclear | Low risk | Study period unknown.
No clear inclusion and exclusion criteria.
No a priori sample size calculation. |
| Al-Maiyah, 2005 (7) | Low risk | Low risk | Unclear | High risk | Unclear | Unclear | High risk | Study period unknown.
No a priori sample size calculation. |
| Zdanowski 2000 (14) | Unclear | Unclear | Unclear | High risk | Unclear | Unclear | High risk | Study period unknown.
No clear inclusion and exclusion criteria.
No a priori sample size calculation. |
| Sanders 1990 (10) | Low risk | Low risk | Unclear | High risk | Unclear | Unclear | High risk | No clear inclusion and exclusion criteria.
Follow-up period unknown.
No validated SSI definition.
No a priori sample size calculation. |
| Sebold 1993 (11) | Low risk | Low risk | Unclear | High risk | Unclear | Unclear | High risk | No clear inclusion and exclusion criteria.
Follow-up period unknown.
No validated SSI definition.
No a priori sample size calculation.
Number of patients lost to follow-up unknown. |
SSI: surgical site infection.
Appendix 3b. Risk of bias assessment of the included observational studies (Newcastle-Ottawa quality assessment scale)
View in own window
| Author, year, reference | Representativeness of cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start | Comparability of cohorts | Assessment of outcome | Follow-up long enough | Adequacy of follow-up of cohorts |
|---|
| Tulipan 2006 (16) | C. Selected group of interventions (only one surgeon, the same for non-exposed and exposed patients). | A. (*) Drawn from the same community as the exposed cohort. | A. (*) Secure records (computerized database). | B. No | A. (*) | B. (*). Record linkage. | B. No (6-month follow-up, not 1 year) | D. No statement.
Number of patients lost to follow-up unknown. |
| Dodds 1990 (15) | D. No description of the derivation cohort. | A. (*) Drawn from the same community as the exposed cohort. | D. No description. | B. No | A. (*) | D. No description: “the wounds were inspected for signs of infection at 7–10 days”. | B. No | A. (*)
Complete follow-up – all subjects accounted for. |
References
- 1.
- 2.
Anderson
DJ, Podgorny
K, Berrios-Torres
SI, Bratzler
DW, Dellinger
EP, Greene
L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–27. [
PMC free article: PMC4267723] [
PubMed: 24799638]
- 3.
Alexander
JW, Solomkin
JS, Edwards
MJ. Updated recommendations for control of surgical site infections. Ann Surg. 2011;253(6):1082–93. [
PubMed: 21587113]
- 4.
- 5.
Higgins
JP, Altman
DG, Gotzsche
PC, Jüni
P, Moher
D, Oxman
AD, et al. The Cochrane Collaboration‘s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [
PMC free article: PMC3196245] [
PubMed: 22008217]
- 6.
Wells
GA, Shea
B, O’Connell
D, Peterson
J, Welch
V, Losos
M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Toronto: The Ottawa Hospital Research Institute; 2014 (
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, accessed 13 May 2016).
- 7.
Al-Maiyah
M, Bajwa
A, Mackenney
P, Port
A, Gregg
PJ, Hill
D, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556–9. [
PubMed: 15795210]
- 8.
Atkinson
MW, Owen
J, Wren
A, Hauth
JC. The effect of manual removal of the placenta on post-cesarean endometritis. Obstet Gynecol. 1996;87(1):99–102. [
PubMed: 8532276]
- 9.
Cernadas
M, Smulian
JC, Giannina
G, Ananth
CV. Effects of placental delivery method and intraoperative glove changing on postcesarean febrile morbidity. J Matern Fetal Med. 1998;7(2):100–4. [
PubMed: 9584823]
- 10.
Sanders
sR, Fortin
P, Ross
E, Helfet
D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914–7. [
PubMed: 2195035]
- 11.
Sebold
EJ, Jordan
LR. Intraoperative glove perforation. A comparative analysis. Clin Orthop Rel Res. 1993(297):242–4. [
PubMed: 8242939]
- 12.
Ventolini
G, Neiger
R, McKenna
D. Decreasing infectious morbidity in cesarean delivery by changing gloves. J Reprod Med. 2004;49(1):13–6. [
PubMed: 14976789]
- 13.
Ward
WG, Cooper
JM, Lippert
D, Kablawi
RO, Neiberg
RH, Sherertz
RJ. Glove and gown effects on intraoperative bacterial contamination. Ann Surg. 2014;259(3):591–7. [
PubMed: 24045444]
- 14.
Zdanowski
Z, Danielsson
G, Jonung
T, Norgren
L, Ribbe
E, Thorne
J, et al. Intraoperative contamination of synthetic vascular grafts. Effect of glove change before graft implantation. A prospective randomised study. Europ J Vasc Endovasc Surg. 2000;19(3):283–7. [
PubMed: 10753692]
- 15.
Dodds
RD, Barker
SG, Morgan
NH, Donaldson
DR, Thomas
MH. Self protection in surgery: the use of double gloves. Br J Surg. 1990;77(2):219–20. [
PubMed: 2317684]
- 16.
Tulipan
N, Cleves
MA. Effect of an intraoperative double-gloving strategy on the incidence of cerebrospinal fluid shunt infection. J Neurosurg. 2006;104(1 Suppl.):5–8. [
PubMed: 16509473]
- 17.
Beldame
J, Lagrave
B, Lievain
L, Lefebvre
B, Frebourg
N, Dujardin
F. Surgical glove bacterial contamination and perforation during total hip arthroplasty implantation: when gloves should be changed. Orthop Traumatol Surg Res. 2012;98(4):432–40. [
PubMed: 22578871]
- 18.
Adeyemo
WL, Ogunlewe
MO, Ladeinde
AL, Bamgbose
BO. Are sterile gloves necessary in nonsurgical dental extractions?
J Oral Maxillofac Surg. 2005;63(7):936–40. [
PubMed: 16003618]
- 19.
Cheung
LK, Chow
LK, Tsang
MH, Tung
LK. An evaluation of complications following dental extractions using either sterile or clean gloves. Int J Oral Maxillofac Surg. 2001;30(6):550–4. [
PubMed: 11829239]
- 20.
Chiu
WK, Cheung
LK, Chan
HC, Chow
LK. A comparison of post-operative complications following wisdom tooth surgery performed with sterile or clean gloves. Int J Oral Maxillofac Surg. 2006; 35(2):174–9. [
PubMed: 16154315]
- 21.
Giglio
JA, Rowland
RW, Laskin
DM, Grenevicki
L, Roland
RW. The use of sterile versus nonsterile gloves during out-patient exodontia. Quintessence Int. 1993;24(8):543–5. [
PubMed: 8272491]
- 22.
Mehta
D, Chambers
N, Adams
B, Gloster
H. Comparison of the prevalence of surgical site infection with use of sterile versus non-sterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234–9. [
PubMed: 24446695]
- 23.
Rhinehart
BM, Murphy
ME, Farley
MF, Albertini
JG. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surgery. 2006;32(2):170–6. [
PubMed: 16442035]
- 24.
Xia
Y, Cho
S, Greenway
HT, Zelac
DE, Kelley
B. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37(5):651–6. [
PubMed: 21457390]
- 25.
Rogues
AM, Lasheras
A, Amici
JM, Guillot
P, Beylot
C, Taieb
A, et al. Infection control practices and infectious complications in dermatological surgery. J Hosp Infect. 2007;65(3):258–63. [
PubMed: 17244515]
- 26.
Perelman
VS, Francis
GJ, Rutledge
T, Foote
J, Martino
F, Dranitsaris
G. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004; (3):362–70. [
PubMed: 14985664]
- 27.