1. Introduction
The preventive effect of the routine use of preoperative surgical antibiotic prophylaxis (SAP) on the occurrence of surgical site infections (SSI) prior to non-clean and implant surgery has long been recognized. However, the benefit of continued SAP after completion of the procedure is unclear. Increasing evidence shows that a single preoperative dose of SAP (and possible additional intraoperative doses according to the duration of the operation) may be non-inferior to additional postoperative multiple doses for the prevention of SSI. Despite this, surgeons still have a tendency to routinely continue SAP up to several days after surgery1,2.
The use and duration of postoperative prophylaxis has been specified in clinical practice guidelines issued by professional societies or national authorities. Several of these guidelines, such as those published by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA)3, and the American Society of Health Care Pharmacists (ASHP)4 recommend discontinuing SAP within 24 hours after surgery. The United States (US) Institute of Healthcare Improvement recommends discontinuing SAP within 24 hours in general and within 48 hours in cardiac surgery5. Other guidelines published by the United Kingdom (UK) National Institute for Health and Care Excellence (NICE)6, the Scottish Intercollegiate Guidelines Network (SIGN)7, the Royal College of Physicians of Ireland8 and the UK Department of Health9, recommend a single dose of preoperative SAP and no postoperative continuation with or without exceptions for specific surgical procedures.
2. PICO question
Does continued postoperative SAP reduce the risk of SSI compared with preoperative and (if necessary) intraoperative prophylaxis only?
Population: patients of any age undergoing surgical procedures who need to receive SAP
Intervention: continued postoperative antibiotic prophylaxis
Comparator: single-dose antibiotic prophylaxis only (and possible additional intraoperative doses according to duration of the operation)
Outcome: SSI, SSI-attributable mortality
3. Methods
The following databases were searched: Medline (PubMed); Cumulative Index to Nursing and Allied Health Literature (CINAHL); Cochrane Central Register of Controlled Trials (CENTRAL); and WHO regional medical databases. The time limit for the review was between 1 January 1990 and 1 October 2015. Language was restricted to English, German and Spanish. A comprehensive list of search terms was used, including Medical Subject Headings (MeSH) (Appendix 1).
Two independent reviewers screened titles and abstracts of retrieved references for potentially relevant studies. The full text of all potentially eligible articles was obtained. Two authors independently reviewed the full text articles for eligibility based on inclusion criteria. Duplicate studies were excluded. Only studies comparing the same agent in the same dosage (per administration) were included. The first dose was always administered preoperatively.
Two authors extracted data in a predefined evidence table (Appendix 2) and critically appraised the retrieved studies using the Cochrane collaboration tool10 for assessing risk of bias (Appendix 3). Any disagreements were resolved through discussion after consultation of the senior author, when necessary.
Meta-analyses of available comparisons of SAP were performed using Review Manager version 5.3 as appropriate11 (Appendix 4). Odds ratios (OR) and the mean difference with 95% confidence intervals (CI) were extracted and pooled for each comparison with a random effects model. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology12 (GRADE Pro software, http://gradepro.org/13) was used to assess the quality of the retrieved evidence (Appendix 5).
4. Study selection
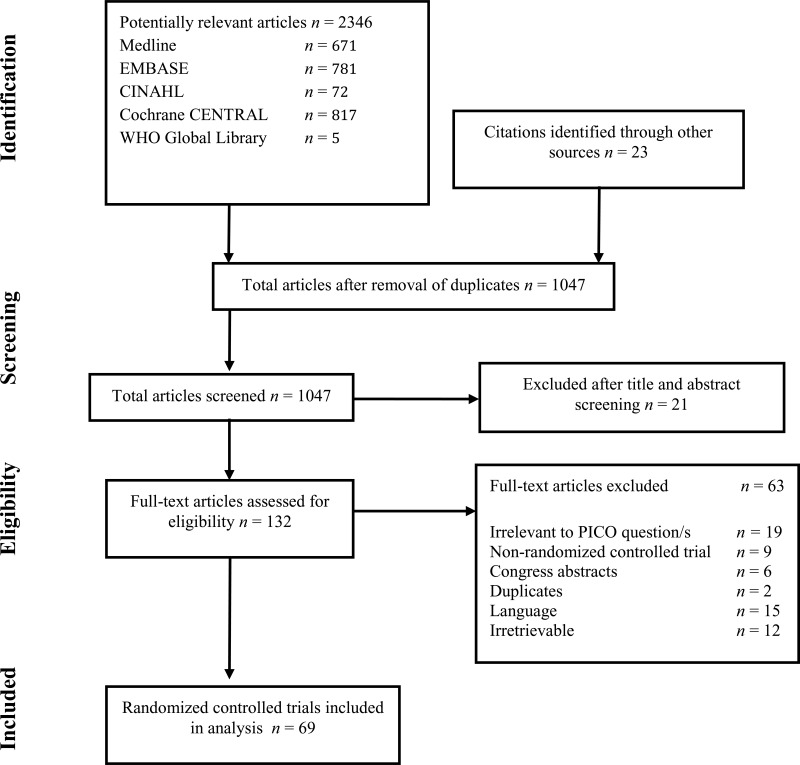
Flow chart of the study selection process
5. Summary of the findings
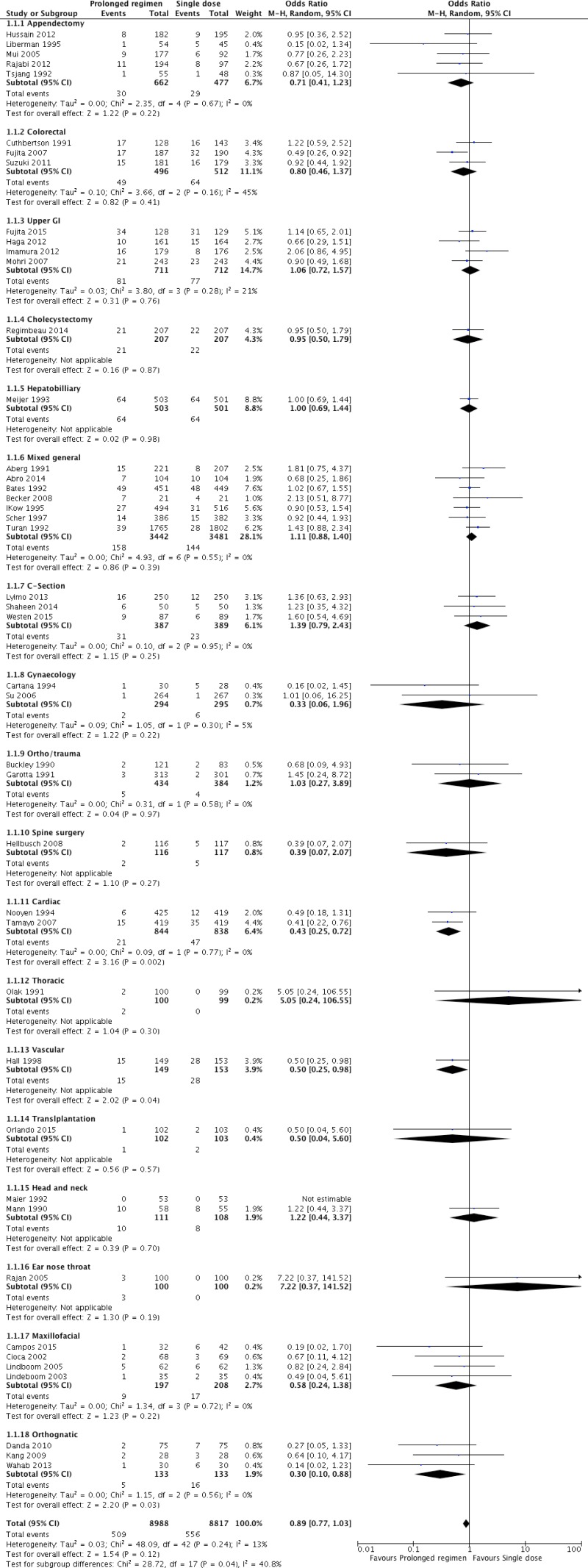
A total of 69 randomized controlled trials (RCTs)14–82 investigating the optimal duration of antibiotic prophylaxis in a variety of surgical procedures with an SSI outcome were identified. A total of 21 243 patients were included, mostly adults. Only 2 studies18,58 addressed specifically the paediatric population. Fifteen studies16,17,28,30,32,33,51,54,56,69,73–75,83,84 reported that some paediatric patients were included, but most were adult patients. Fourteen17,29,34–36,50,51,54,56,69,71,73,75,80 of the included studies were conducted in low- and middle-income countries.
Both the intervention and control group received the same preoperative regimen in all the included studies and only differed in the postoperative continuation of antibiotic prophylaxis. Only studies comparing the same antibiotic agent in the same dosage (per administration) were considered in order to prevent confounding by the type of antibiotic. The first dose of antibiotic prophylaxis was always administered preoperatively. In addition to the single dose, possible additional dose/s according to the duration of the operation were given, depending on the protocol used in the trial.
To investigate the optimal duration of antibiotic prophylaxis, the Guideline Development Group (GDG) agreed to not only assess trials comparing a continued postoperative antibiotic prophylaxis with a single dose antibiotic prophylaxis only (or repeated according to duration of the operation), but also to assess trials that compared different regimens of prolonged postoperative antibiotic prophylaxis.
Accordingly, the following comparisons were made:
Any prolonged regimen vs. no postoperative dose (44 RCTs)
A prolonged regimen less than 24 hours postoperative vs. a single postoperative dose (one RCT)
A prolonged regimen more than 24 hours postoperative vs. a prolonged regimen less than 24 hours postoperative (23 RCTs)
A prolonged regimen more than 48 hours postoperative vs. a prolonged regimen less than 48 hours postoperative (3 RCTs)
Type of procedure with a prolonged antibiotic regimen
Cardiac surgery
Vascular surgery
Orthognathic surgery
The results of the meta-analyses based on these comparisons are shown in Appendix 4.
Forty-four RCTs
14–56,82 including 17 805 patients and comparing any prolonged regimen of antibiotic prophylaxis with no postoperative antibiotic prophylaxis were identified. These studies included a variety of surgical procedures: appendectomy
14–18; colorectal surgery
19–21; upper gastrointestinal tract surgery
22–25; cholecystectomy
26; hepatobiliary surgery
85; mixed general surgery
28–33,82; caesarean section
34–36; gynaecological surgery
37,38; orthopaedic and trauma surgery
39,40; spine surgery
41; cardiac surgery
42,43; thoracic surgery
44; vascular surgery
45; transplantation surgery
46; head and neck surgery
47,86; ear, nose and throat surgery
49; maxillofacial surgery
50–53; and orthognathic surgery
54–56.
Only 3 trials
20,43,45 showed a decreased risk of SSI when antibiotic prophylaxis was prolonged postoperatively. The remaining 40 trials showed no difference in risk. The analysis was further stratified according to the type of procedure. No significant difference in the risk of SSI was found with the exception of cardiac, vascular and orthognathic surgery for which prolonged antibiotic prophylaxis resulted in a decreased risk of SSI (
Appendix 4, –).
Meta-analysis of the 44 RCTs
14–56,82 (
Appendix 4, ) demonstrated that prolonged postoperative antibiotic prophylaxis had no benefit when compared to a single dose of antibiotic prophylaxis in reducing SSI after surgery (OR: 0.89; 95% CI: 0.77–1.03).
The quality of the evidence for this comparison was moderate due to the risk of bias (
Appendix 5).
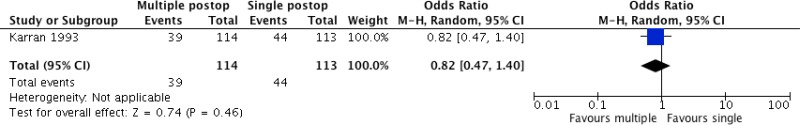
One study
57 including 227 patients undergoing colorectal surgery compared the continuation of antibiotic prophylaxis up to 16 hours postoperatively with a single postoperative dose. The trial
57 (
Appendix 4, ) demonstrated that the continuation of prolonged antibiotic prophylaxis up to a last dose at 16 hours postoperatively had no benefit in reducing SSI when compared to a single postoperative dose (OR: 0.82; 95% CI: 0.47–1.40).
The quality of evidence was very low due to risk of bias and imprecision (
Appendix 5).
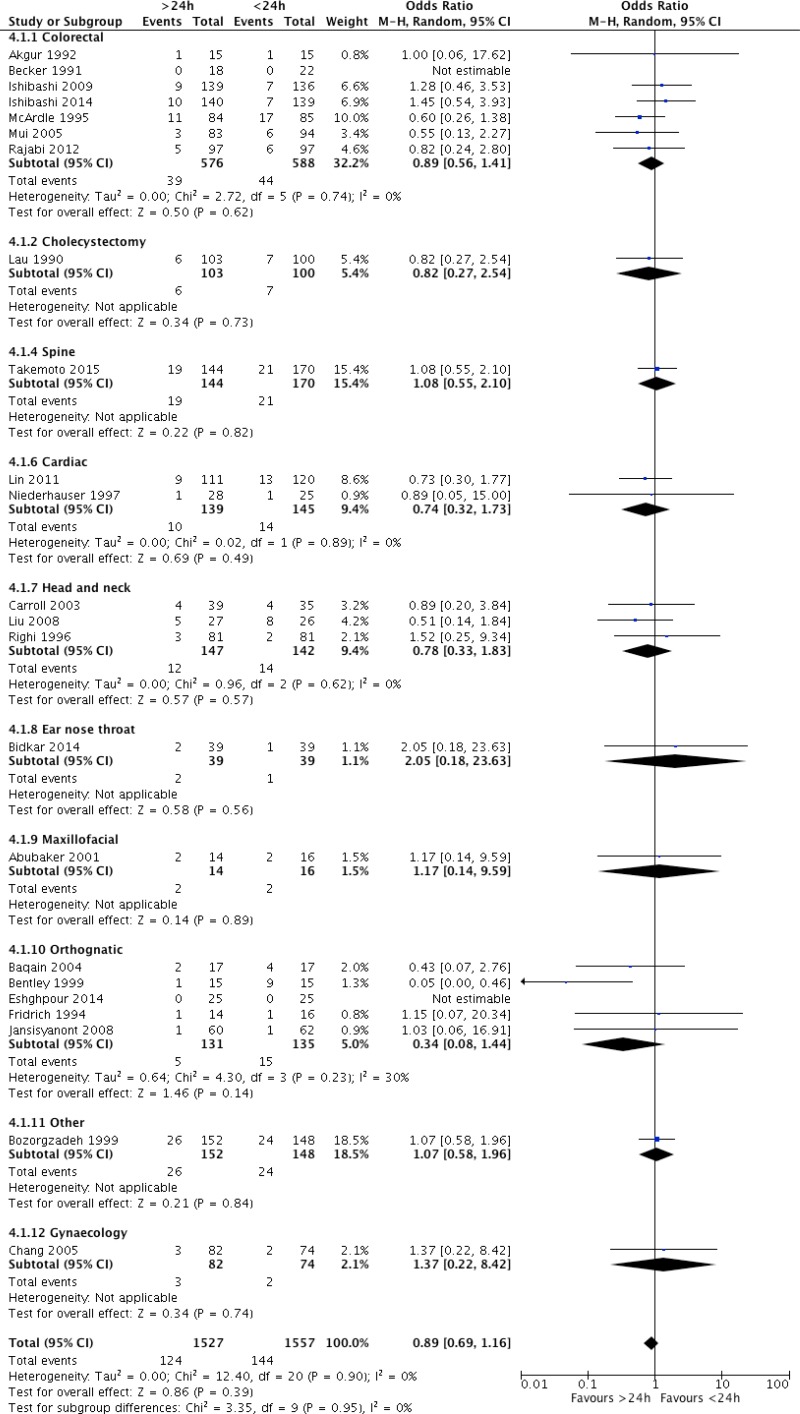
Twenty-three trials
16,17,58–78 including 3084 patients compared any prolonged regimen of more than 24 hours postoperatively with a postoperative regimen of less than 24 hours. These studies included a wide variety of surgical procedures: colorectal surgery
58–61,78; cholecystectomy
62; gynaecological surgery
77; spine surgery
63; cardiac surgery
64,65; head and neck surgery
66–68; ear, nose and throat surgery
69; maxillofacial surgery
70; orthognathic surgery
71–75; and others
76.
Only one trial
72 in orthognathic surgery showed a decreased risk of SSI when antibiotic prophylaxis was prolonged for more than 24 hours postoperatively. Twenty trials showed no difference in risk and 2 trials had no SSI events
73,78. The analysis was further stratified by the type of surgical procedure, but no significant difference in the risk of SSI was observed according to the procedure. Meta-analysis of these 23 RCTs
16,17,58–78 (
Appendix 4, comparison demonstrated that a prolonged antibiotic prophylaxis regimen of more than 24 hours postoperatively had no benefit in reducing SSI when compared to a prolonged regimen of less than 24 hours (OR: 0.89; 95% CI: 0.69–1.16).
The quality of evidence was moderate due to the risk of bias (
Appendix 5).
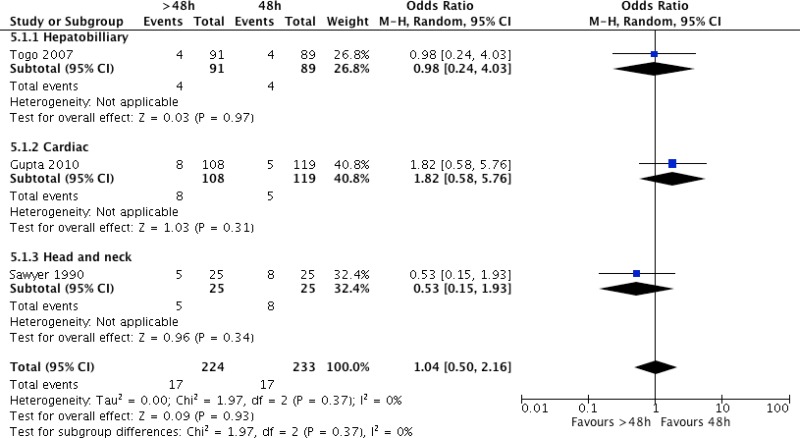
Three studies
79–81 including 457 patients undergoing hepatobiliary
79, cardiac
80 and head and neck surgery
81 compared any prolonged postoperative regimen of more than 48 hours with a prolonged regimen of less than 48 hours. The individual trials showed no difference in the risk of SSI. The analysis was further stratified by the type of surgical procedure, but no significant difference in the risk of SSI was observed according to the procedure. Meta-analysis of the trials
79–81 (
Appendix 4, ) demonstrated that a prolonged antibiotic prophylaxis regimen of more than 48 hours had no benefit when compared to a prolonged regimen for up to 48 hours in reducing SSI (OR: 1.04; 95% CI: 0.50–2.16).
The quality of evidence was very low due to the risk of bias and imprecision (
Appendix 5).
Types of procedure associated with a decreased risk of SSI with a prolonged antibiotic regimen.
- a)
Cardiac surgery
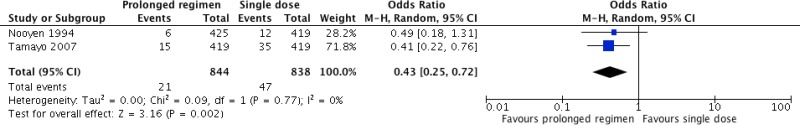
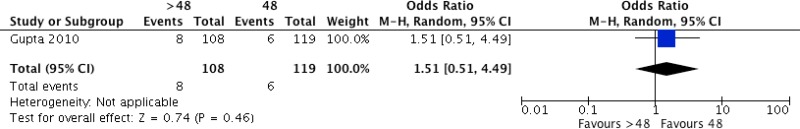
Five studies42,43,64,65,80 compared different postoperative antibiotic regimens in cardiac surgery. Among these, 2 studies42,43 compared any prolonged regimen with no postoperative antibiotic prophylaxis. Two other studies64,65 compared continuation of antibiotic prophylaxis for more than 24 hours postoperatively with continuation for less than 24 hours. One study80 compared continuation of postoperative antibiotic prophylaxis for longer than 48 hours with regimens continuing for less than 48 hours. Separate meta-analyses were performed for the three comparisons, when appropriate (Appendix 4, ).
Meta-analysis of the 2 RCTs
42,43 comparing any prolonged regimen with no postoperative antibiotic prophylaxis demonstrated that the former had a benefit in terms of reducing SSI (OR: 0.43; 95% CI: 0.25–0.76).
The quality of evidence was low due to the risk of bias and imprecision (
Appendix 5).
Meta-analysis of the 2 RCTs
64,65 comparing postoperative prophylaxis for more than 24 hours with continuation for less than 24 hours demonstrated that the former had no benefit in terms of reducing the risk of SSI (OR: 0.74; 95% CI: 0.32–1.73).
The quality of evidence was very low due to the risk of bias and imprecision (
Appendix 5).
One RCT
80 comparing the continuation of postoperative antibiotic prophylaxis for longer than 48 hours with continuation for less than 48 hours demonstrated that the former had no benefit in terms of reducing the risk of SSI (OR: 0.53; 95% CI: 0.15–1.93).
The quality of evidence was very low due to the risk of bias and imprecision (
Appendix 5).
- b)
Vascular surgery
One RCT45 in patients undergoing vascular surgery compared the continuation of antibiotic prophylaxis until all lines were removed with a single dose of antibiotic prophylaxis and demonstrated that the former had a significant benefit in terms of reducing the risk of SSI (Appendix 4, ; OR: 0.50; 95% CI: 0.25–0.98).
The quality of evidence was low due to the risk of bias and imprecision (Appendix 5).
- c)
Orthognathic surgery
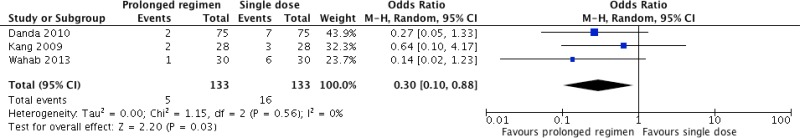
Eight studies54–56,71–75 compared different postoperative antibiotic regimens in orthognathic surgery. Among these, 3 studies54–56 compared any prolonged regimen with no postoperative prolongation of antibiotic prophylaxis. Five other studies71–75 compared continuation of antibiotic prophylaxis for more than 24 hours postoperatively with continuation for less than 24 hours. Meta-analyses were performed for each of these comparisons (Appendix 4, ).
Meta-analysis of the 3 RCTs
54–56 comparing any prolonged regimen with no postoperative antibiotic prophylaxis demonstrated that the former had a benefit in terms of reducing SSI (OR: 0.30; 95% CI: 0.10–0.88).
The quality of evidence was low due to the risk of bias and imprecision (
Appendix 5).
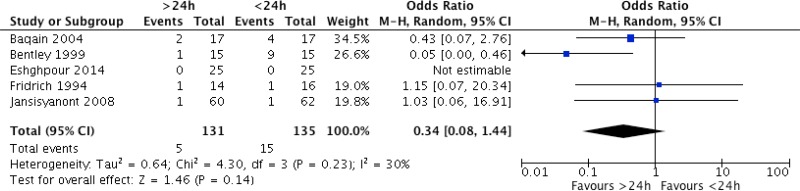
Meta-analysis of the 5 RCTs
71–75 comparing postoperative antibiotic prophylaxis for more than 24 hours with continuation for less than 24 hours demonstrated that the former had no benefit in terms of reducing the risk of SSI (OR: 0.34; 95% CI: 0.08–1.44).
The quality of evidence was very low due to the risk of bias and imprecision (
Appendix 5).
In conclusion, the retrieved evidence can be summarized as follows:
- -
Postoperative continuation of antibiotic prophylaxis vs. a single dose of antibiotic prophylaxis ():
Overall, a moderate quality of evidence shows that the postoperative continuation of antibiotic prophylaxis has neither benefit nor harm in reducing SSI rates when compared to a single dose of antibiotic prophylaxis.
In cardiac surgery (), a low quality of evidence shows that the continuation of antibiotic prophylaxis for up to 24 hours postoperatively has a benefit in reducing the SSI rate when compared to a single dose of antibiotic prophylaxis. A very low quality of evidence showed that continuation beyond 24 hours postoperatively has no benefit.
In vascular surgery (), a low quality of evidence shows that the continuation of antibiotic prophylaxis until all lines are removed has a benefit in reducing the SSI rate when compared to a single dose of prophylaxis.
In orthognathic surgery (), a low quality of evidence shows that the continuation of antibiotic prophylaxis for up to 24 hours postoperatively had a benefit in reducing the SSI rate when compared to a single dose of antibiotic prophylaxis. A very low quality of evidence showed that continuation beyond 24 hours postoperatively had no benefit in reducing SSI.
The included studies have some limitations. The quality of the included RCTs was moderate. Most studies had an unclear or high risk of bias in at least one or more domains. Differences and inconsistencies were noted in the SSI definitions, patient population and antibiotic regimen.
6. Other factors considered in the review
The systematic review team identified the following other factors to be considered.
Potential harms
Twenty-three studies16,17,21,22,24–26,31,33,37,47,49,52,54,55,57,66–69,73,80,81 described the presence or absence of possible harms and adverse events related to SAP prolongation. Five studies reported more adverse effects in the intervention group. Among these, one study16 reported a significantly higher number of cases of clostridial enterocolitis. Other studies reported a higher frequency of rash, erythema, phlebitis and hypotension57, unspecified local side-effects33 gastrointestinal disturbance69, or nausea, diarrhoea, skin rash or pruritus49. The remaining 18 studies17,21,22,24–26,31,37,47,52,54,55,66–68,73,81 reported that there were no adverse events attributable to the intervention in both groups. Although it is an important concern, the risk of antimicrobial resistance possibly due to the prolonged administration of antibiotics has not been assessed by any of the included studies.
Resource use
Studies addressing cost-effectiveness reported a cost reduction associated with shorter antibiotic prophylaxis regimens that varied from US$ 36,90 to US$ 166415,38,46,49,77,87 depending also on the treatment of side-effects and duration of hospitalization. There is a need to raise awareness and provide education on the rational use of antibiotics and antibiotic stewardship among both health care workers (surgeons in particular, with reference to this recommendation) and patients.
7. Key uncertainties and future research priorities
The systematic review team identified the following key uncertainties and future research priorities.
There is a need for further well-designed RCTs in cardiac and vascular surgery as well as in low- and middle-income countries and in the paediatric population. More research is needed to demonstrate the linkage between the prolongation of SAP and the emergence of antibiotic resistance. Furthermore, future trials should investigate the effect of prolonged antibiotic prophylaxis on the microbiome.
Appendices
Appendix 1. Search terms
Medline (through PubMed)
surgical wound infection“[Mesh] OR surgical site infection*[tiab] OR SSI[tiab] OR SSIs[tiab] OR surgical wound infection*[tiab] OR surgical infection*[tiab] OR post-operative wound infection*[tiab] OR postoperative wound infection*[tiab]
antibiotic prophylaxis“[Mesh] OR antimicrobial[tiab] OR antibiotic*[tiab]
(prolong*[tiab] OR duration[tiab] OR short[tiab] OR long[tiab] OR single dose*[tiab] OR single dosage*[tiab] OR single dosis[tiab] OR singular dose*[tiab] OR singular dosage*[tiab] OR singular dosis[tiab] OR multi dose*[tiab] OR multi dosage*[tiab] OR multi dosis[tiab] OR multiple dose*[tiab] OR multiple dosage*[tiab] OR multiple dosis[tiab])
trial[ti]) OR randomly[tiab]) OR clinical trial as topic[mesh:noexp]) OR placebo[tiab]) OR randomized[tiab]) OR controlled clinical trial[pt]) OR randomized controlled trial[pt]
1 AND 2 AND 3 AND 4
EMBASE
surgical infection/ or (SSI or SSIs).ti,ab,kw. or ((surg* or postoperat* or post-operat*) adj3 infect*).ti,ab,kw.
antibiotic prophylaxis/ or (antimicrobial or antibiotic*).ti,ab,kw.
exp drug dose/ or treatment duration/ or (prolong* or duration*).ti,ab,kw. or ((single or singular or multi*) adj3 (dose* or dosage* or dosis)).ti,ab,kw. or ((short* or long*) adj3 (duration* or course*)).ti,ab,kw.
controlled clinical trial/ or randomized controlled trial/ or exp “clinical trial (topic)”/ or (randomly or randomized or placebo).ti,ab,kw. or trial.ti.
1 and 2 and 3 and 4
Cochrane Central Register (CENTRAL)
MeSH descriptor: [surgical wound infection] explode all trees
SSI or SSIs:ti,ab,kw (word variations have been searched)
(surg* or postoperat* or post-operat*) near/3 infect*:ti,ab,kw (word variations have been searched)
#1 or #2 or #3
MeSH descriptor: [antibiotic prophylaxis] explode all trees
antimicrobial or antibiotic*:ti,ab,kw (word variations have been searched)
#5 or #6
prolong* or duration*:ti,ab,kw (word variations have been searched)
(single or singular or multi*) near/3 (dose* or dosage* or dosis):ti,ab,kw (word variations have been searched)
(short* or long*) near/3 (duration* or course*):ti,ab,kw (word variations have been searched)
#8 or #9 or #10
#4 and #7 and #11 in Trials
CINAHL
(MH “surgical wound infection”) OR (TI (surgical site infection* OR SSI OR SSIs OR surgical wound infection* OR surgical infection* OR post-operative wound infection* OR postoperative wound infection*) OR AB (surgical site infection* OR SSI OR SSIs OR surgical wound infection* OR surgical infection* OR post-operative wound infection* OR postoperative wound infection*))
(MH “antibiotic prophylaxis”) OR TI (antimicrobial OR antibiotic*) OR AB (antimicrobial OR antibiotic*)
(MH “treatment duration”) OR TI (prolong* OR duration OR short OR long OR single dose* OR single dosage* OR single doses OR singular dose* OR singular dosage* OR singular doses OR multi dose* OR multi dosage* OR multi doses OR multiple dose* OR multiple dosage* OR multiple doses) OR AB (prolong* OR duration OR short OR long OR single dose* OR single dosage* OR single doses OR singular dose* OR singular dosage* OR singular doses OR multi dose* OR multi dosage* OR multi doses OR multiple dose* OR multiple dosage* OR multiple doses)
(MH “randomized controlled trials”) OR (MH “clinical trials+”) OR TI trial OR (TI controll* AND trial*) OR AB (TI controll* AND trial*) OR (TI (randomly OR placebo OR randomi?ed) OR AB (randomly OR placebo OR randomi?ed))
S1 AND S2 AND S3 AND S4
WHO Global Health Library
(surgical site infection)
(wound infections)
(wound infection)
filter Subject [Mesh] antibiotic prophylaxis
- ti:
title;
- ab:
abstract;
- kw:
key word
Appendix 3. Risk of bias assessment of the included studies (Cochrane Collaboration tool)
View in own window
| RCT, author, year, reference | Sequence generation | Allocation concealment | Participants and caregivers blinded | Outcome assessors blinded | Incomplete outcome data | Selective outcome reporting |
|---|
| Appendectomy |
|---|
| Rajabi-Masshadi 201217 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
|---|
| Hussain 201214 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Mui 200516 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Liberman 199515 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Tsang 199218 | Low | High | High | Unclear | Low | Low |
|---|
| Colorectal |
|---|
| Ishibashi 201460 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Suzuki 201121 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Ishibashi 200959 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Fujita 200720 | Low | High | Unclear | Unclear | High | High |
|---|
| McArdle 199561 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Karran 199357 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Akgur 199258 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Cuthbertson 199119 | Low | Low | Unclear | Low | Low | High |
|---|
| Becker 199178 | Unclear | Unclear | Unclear | Low | Low | Low |
|---|
| Upper gastrointestinal tract |
|---|
| Fujita 201522 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Imamura 201224 | Low | High | High | High | Low | Low |
|---|
| Haga 201223 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Mohri 200725 | Low | Low | High | Low | Low | Low |
|---|
| Cholecystectomy |
|---|
| Regimbeau 201426 | Low | High | High | Unclear | Low | Low |
|---|
| Lau 199062 | Unclear | Unclear | Unclear | Low | Low | Low |
|---|
| Hepatobiliary |
|---|
| Meijer 199327 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Togo 200779 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
|---|
| Mixed general |
|---|
| Abro 201429 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Becker 200831 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Scher 199782 | Low | Low | Low | Low | Low | Low |
|---|
| Kow 199532 | Low | Low | Unclear | Unclear | Low | Low |
|---|
| Turano 199233 | Unclear | Unclear | High | High | Low | Low |
|---|
| Bates 199230 | Low | High | Unclear | Low | Low | Low |
|---|
| Aberg 199128 | Unclear | Unclear | High | High | Low | Low |
|---|
| Caesarean section |
|---|
| Westen 201536 | Low | Low | Unclear | Unclear | Low | Low |
|---|
| Shaheen 201435 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Lyimo 201334 | Low | Unclear | High | High | Low | Low |
|---|
| Gynaecological |
|---|
| Su 200538 | Low | High | Unclear | Unclear | Low | Low |
|---|
| Cartaña 199437 | Low | High | Unclear | Unclear | Low | Low |
|---|
| Chang 200577 | Low | Unclear | High | Unclear | Low | Low |
|---|
| Orthopaedic/trauma |
|---|
| Buckley 199039 | Unclear | Unclear | Unclear | Unclear | Low | High |
|---|
| Garotta 199140 | Low | High | Unclear | Unclear | Low | Low |
|---|
| Takemoto 201563 | Low | Unclear | Unclear | Unclear | Low | High |
|---|
| Hellbusch 200841 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Cardiac |
|---|
| Gupta 201080 | Low | Low | Low | Low | Low | High |
|---|
| Lin 201164 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Niederhauser 199765 | Low | High | High | High | Low | Low |
|---|
| Nooyen 199442 | Low | Low | Unclear | Low | Low | Low |
|---|
| Tamayo 200743 | Low | Unclear | Unclear | Unclear | Low | High |
|---|
| Vascular |
|---|
| Hall 199845 | Low | Low | Unclear | Unclear | Low | Low |
|---|
| Thoracic |
|---|
| Olak 199144 | Low | Unclear | Low | Unclear | Low | Unclear |
|---|
| Kidney transplant |
|---|
| Orlando 201546 | Low | Low | Unclear | Unclear | Low | Low |
|---|
| Head and neck |
|---|
| Liu 200867 | Low | High | Unclear | Unclear | Low | Low |
|---|
| Carroll 200366 | Unclear | Unclear | Unclear | Low | Low | High |
|---|
| Righi 199668 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Sawyer 199081 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
|---|
| Maier 199247 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
|---|
| Mann 199048 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
|---|
| Ear, nose and throat |
|---|
| Bidkar 201469 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Rajan 200549 | Low | Low | Unclear | Unclear | Low | Low |
|---|
| Maxillofacial |
|---|
| Campos 201550 | Unclear | Unclear | Unclear | Unclear | High | High |
|---|
| Lindeboom 200552 | Low | Unclear | Unclear | Low | Low | Low |
|---|
| Lindeboom 200353 | Low | High | Unclear | Low | Low | Low |
|---|
| Cioaca 200251 | Unclear | Unclear | Unclear | Low | Low | Low |
|---|
| Abubaker 200170 | Unclear | Low | Low | Low | Low | Low |
|---|
| Orthognathic |
|---|
| Eshghpour 201473 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Wahab 201356 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Danda 201054 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Kang 200955 | Low | Unclear | Unclear | Unclear | Low | Low |
|---|
| Jansisyanont 200875 | Unclear | Unclear | Low | Unclear | High | Low |
|---|
| Baqain 200471 | Low | Low | Low | Low | Low | Unclear |
|---|
| Bentley 199972 | Unclear | Unclear | Low | Low | Low | High |
|---|
| Fridrich 199474 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
| Other |
|---|
| Bozorgzadeh 199976 | Unclear | Unclear | Unclear | Unclear | Low | Low |
|---|
RCT: randomized controlled trial
References
- 1.
Kobayashi
M, Takesue
Y, Kitagawa
Y, Kusunoki
M, Sumiyama
Y. Antimicrobial prophylaxis and colon preparation for colorectal surgery: Results of a questionnaire survey of 721 certified institutions in Japan. Surg Today. 2011;41:1363–9. [
PubMed: 21922358]
- 2.
Bratzler
DW, Houck
PM, Richards
C, Steele
l, Dellinger
EP, Fry
DE, et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;140:174–82. [
PubMed: 15724000]
- 3.
Anderson
DJ, Podgorny
K, Berrios-Torres
SI, Bratzler
DW, Dellinger
EP, Greene
L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:605–27. [
PMC free article: PMC4267723] [
PubMed: 24799638]
- 4.
Bratzler
DW, Dellinger
EP, Olsen
KM, Perl
TM, Auwaerter
PG, Bolon
MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. [
PubMed: 23327981]
- 5.
Friese
S, Willems
FT, Loriaux
SM, Meewis
JM. Prophylaxis in gynaecological surgery: a prospective randomized comparison between single dose prophylaxis with amoxycillin/clavulanate and the combination of cefuroxime and metronidazole. J Antimicrob Chemother. 1989:24 (Suppl. B):213–6. [
PubMed: 2691483]
- 6.
Leaper
D, Burman-Roy
S, Palanca
A, Cullen
K, Worster
D, Gautam-Aitken
E, et al. Prevention and treatment of surgical site infection: summary of NICE guidance. BMJ. 2008;337:a1924. [
PubMed: 18957455]
- 7.
Scottish Intercollegiate Guidelines Network. Antibiotic prophylaxis in surgery. July
2008, updated April 2014. Edinburgh: Healthcare Improvement Scotland (
http://www.sign.ac.uk/pdf/sign104.pdf., accessed 10 May 2016).
- 8.
- 9.
- 10.
Higgins
JP, Altman
DG, Gotzsche
PC, Jüni
P, Moher
D, Oxman
AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [
PMC free article: PMC3196245] [
PubMed: 22008217]
- 11.
The Nordic Cochrane Centre TCC. Review Manager (RevMan). Version 5.3. Copenhagen: The Cochrane Collaboration; 2014.
- 12.
Guyatt
G, Oxman
AD, Akl
EA, Kunz
R, Vist
G, Brozek
J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. [
PubMed: 21195583]
- 13.
GRADEpro Guideline Development Tool. Summary of findings tables, health technology assessment and guidelines. GRADE Working Group, Ontario: McMaster University and Evidence Prime Inc.; 2015 (
http://www.gradepro.org, accessed 5 May 2016).
- 14.
Hussain
MI, Alam
MK, Al-Qahatani
HH, Al-Akeely
MH. Role of postoperative antibiotics after appendectomy in non-perforated appendicitis. J Coll Physicians Surg Pak. 2012;22:756–9. [
PubMed: 23217479]
- 15.
Liberman
MA, Greason
KL, Frame
S, Ragland
JJ. Single-dose cefotetan or cefoxitin versus multiple-dose cefoxitin as prophylaxis in patients undergoing appendectomy for acute nonperforated appendicitis. J Am Coll Surg. 1995;180:77–80. [
PubMed: 8000659]
- 16.
Mui
LM, Ng
CSH, Wong
SKH, et al. Optimum duration of prophylactic antibiotics in acute non-perforated appendicitis. ANZ J Surg. 2005;75:425–8. [
PubMed: 15943731]
- 17.
Rajabi-Mashhadi
MT, Mousavi
SH, Mh
KM, Ghayour-Mobarhan
M, Sahebkar
A. Optimum duration of perioperative antibiotic therapy in patients with acute non-perforated appendicitis: a prospective randomized trial. Asian Biomed. 2012;6:891–4.
- 18.
Tsang
TM, Tam
PK, Saing
H. Antibiotic prophylaxis in acute non-perforated appendicitis in children: single dose of metronidazole and gentamicin. J Royal Coll Surg Edinb. 1992;37:110–2. [
PubMed: 1377245]
- 19.
Cuthbertson
AM, McLeish
AR, Penfold
JCB, Ross
H. A comparison between single and double dose intravenous timentin for the prophylaxis of wound infection in elective colorectal surgery. Dis Colon Rectum. 1991;34:151–5. [
PubMed: 1993412]
- 20.
Fujita
S, Saito
N, Yamada
T, et al. Randomized, multicenter trial of antibiotic prophylaxis in elective colorectal surgery: single dose vs 3 doses of a second-generation cephalosporin without metronidazole and oral antibiotics. Arch Surg. 2007;142:657–61. [
PubMed: 17638804]
- 21.
Suzuki
T, Sadahiro
S, Maeda
Y, Tanaka
A, Okada
K, Kamijo
A. Optimal duration of prophylactic antibiotic administration for elective colon cancer surgery: A randomized, clinical trial. Surgery. 2011;149:171–8. [
PubMed: 20655559]
- 22.
Fujita
T, Daiko
H. Optimal duration of prophylactic antimicrobial administration and risk of postoperative infectious events in thoracic esophagectomy with three-field lymph node dissection: Short-course versus prolonged antimicrobial administration. Esophagus. 2015;12:38–43.
- 23.
Haga
N, Ishida
H, Ishiguro
T, Kumamoto
K, Ishibashi
K, Tsuji
Y, et al. A prospective randomized study to assess the optimal duration of intravenous antimicrobial prophylaxis in elective gastric cancer surgery. Int Surg. 2012;97:169–76. [
PMC free article: PMC3723203] [
PubMed: 23102084]
- 24.
Imamura
H, Kurokawa
Y, Tsujinaka
T, Inoue
K, Kimura
Y, Iijima
S, et al. Intraoperative versus extended antimicrobial prophylaxis after gastric cancer surgery: a phase 3, open-label, randomised controlled, non-inferiority trial. Lancet Infect Dis. 2012;12:381–7. [
PubMed: 22297080]
- 25.
Mohri
Y, Tonouchi
H, Kobayashi
M, Nakai
K, Kusunoki
M. Randomized clinical trial of single- versus multiple-dose antimicrobial prophylaxis in gastric cancer surgery. Br J Surg. 2007;94:683–8. [
PubMed: 17514671]
- 26.
Regimbeau
JM, Fuks
D, Pautrat
K, Mauvais
F, Haccart
V, Msika
V, et al. Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis: a randomized clinical trial. JAMA. 2014;312:145–54. [
PubMed: 25005651]
- 27.
Meijer
WS, Schmitz
PI. Prophylactic use of cefuroxime in biliary tract surgery: randomized controlled trial of single versus multiple dose in high-risk patients. Galant Trial Study Group. Br J Surg. 1993:917–21. [
PubMed: 8369939]
- 28.
Aberg
C, Thore
M. Single versus triple dose antimicrobial prophylaxis in elective abdominal surgery and the impact on bacterial ecology. J Hosp Infect. 1991;18:149–54. [
PubMed: 1678761]
- 29.
Abro
AH, Pathan
AH, Siddiqui
FG, Syed
F, Laghari
AA. Single dose versus 24 - Hours antibiotic prophylaxis against surgical site infections. J Liaquat Univ Med Health Sci. 2014;13:27–31.
- 30.
Bates
T, Roberts
JV, Smith
K, German
KA. A randomized trial of one versus three doses of augmentin as wound prophylaxis in at-risk abdominal surgery. Postgrad Med J. 1992;68:811–6. [
PMC free article: PMC2399526] [
PubMed: 1461853]
- 31.
Becker
A, Koltun
L, Sayfan
J. Impact of antimicrobial prophylaxis duration on wound infection in mesh repair of incisional hernia - preliminary results of a prospective randomized trial. Eur Surg. 2008;40:37–40.
- 32.
Kow
L, Toouli
J, Brookman
J, McDonald
PJ, Ronald
M, Nichols
RL. Comparison of cefotaxime plus metronidazole versus cefoxitin for prevention of wound infection after abdominal surgery. World J Surg. 1995;19:680–6. [
PubMed: 7571663]
- 33.
Turano
A. New clinical data on the prophylaxis of infections in abdominal, gynecologic, and urologic surgery. Multicenter study group. Am J Surg. 1992;164:16S-20S. [
PubMed: 1443354]
- 34.
Lyimo
FM, Massinde
AN, Kidenya
BR, Konje
ET, Mshana
SE. Single dose of gentamicin in combination with metronidazole versus multiple doses for prevention of post-caesarean infection at Bugando Medical Centre in Mwanza, Tanzania: a randomized, equivalence, controlled trial. BMC Pregnancy Childbirth
2013;13:123. [
PMC free article: PMC3681664] [
PubMed: 23721411]
- 35.
Shaheen
S, Akhtar
S. Comparison of single dose versus multiple doses of anitibiotic prophylaxis in elective caesarian section. J Postgrad Med Inst. 2014;28:83–6.
- 36.
Westen
EH, Kolk
PR, Van Velzen
CL, Unkels
R, Mmuni
NS, Hamisi
A, et al. Single-dose compared with multiple day antibiotic prophylaxis for cesarean section in low-resource settings, a randomized controlled, noninferiority trial. Acta Obstet Gynecol Scand. 2015;94:43–9. [
PubMed: 25263498]
- 37.
Cartana
J, Cortes
J, Yarnoz
MC, Rossello
JJ. Antibiotic prophylaxis in Wertheim-Meigs surgery. A single dose vs three doses. Europ J Gynaecol Oncol. 1994;15:14–8. [
PubMed: 8206064]
- 38.
Su
HY, Ding
DC, Chen
DC, Lu
MF, Liu
JY, Chang
FY. Prospective randomized comparison of single-dose versus 1-day cefazolin for prophylaxis in gynecologic surgery. Acta Obstet Gynecol Scand. 2005;84:384–9. [
PubMed: 15762971]
- 39.
Buckley
R, Hughes
GNF, Snodgrass
T, Huchcroft
SA. Perioperative cefazolin prophylaxis in hip fracture surgery. Can J Surg. 1990;33:122–5. [
PubMed: 2268811]
- 40.
Garotta
F, Pamparana
F. Antimicrobial prophylaxis with ceftizoxime versus cefuroxime in orthopedic surgery. Ceftizoxime Orthopedic Surgery Italian Study Group. J Chemother. 1991;3 (Suppl 2):34–5. [
PubMed: 2040899]
- 41.
Hellbusch
LC, Helzer-Julin
M, Doran
SE, Leibrock
LG, Long
DJ, PUccioni
MJ, et al. Single-dose vs multiple-dose antibiotic prophylaxis in instrumented lumbar fusion-a prospective study. Surg Neurol. 2008;70:622–7. [
PubMed: 18207532]
- 42.
Nooyen
SM, Overbeek
BP, Brutel de la Rivière
A, Storm
AJ, Langemeyer
JJ. Prospective randomised comparison of single-dose versus multiple-dose cefuroxime for prophylaxis in coronary artery bypass grafting. Europ J Clin Microbiol Infect Dis. 1994;13:1033–7. [
PubMed: 7889965]
- 43.
Tamayo
E, Gualis
J, Florez
S, Castrodeza
J, Eiros Bouza
JM, Alvarez
FJ. Comparative study of single-dose and 24-hour multiple-dose antibiotic prophylaxis for cardiac surgery. J Thorac Cardiovasc Surg. 2008;136:1522–7. [
PubMed: 19114201]
- 44.
Olak
J, Jeyasingham
K, Forrester-Wood
C, Hutter
J, Al-Zeerah
M, Brown
E. Randomized trial of one-dose versus six-dose cefazolin prophylaxis in elective general thoracic surgery. Ann Thorac Surg. 1991;51:956–8. [
PubMed: 2039326]
- 45.
Hall
JC, Christiansen
KJ, Goodman
M, et al. Duration of antimicrobial prophylaxis in vascular surgery. Am J Surg. 1998;175:87–90. [
PubMed: 9515521]
- 46.
Orlando
G, Manzia
TM, Sorge
R, et al. One-shot versus multidose perioperative antibiotic prophylaxis after kidney transplantation: A randomized, controlled clinical trial. Surgery. 2015;157:104–10. [
PubMed: 25304836]
- 47.
Maier
W, Strutz
J. [Perioperative single-dose prophylaxis with cephalosporins in ENT surgery. A prospective randomized study.] [Article in German]. Laryngorhinootologie. 1992;71:365–9. [
PubMed: 1497770]
- 48.
Mann
W, Maurer
J. [Perioperative short-term preventive antibiotics in head-neck surgery]. [Article in German]
Laryngorhinootologie. 1990;69:158–60. [
PubMed: 2187445]
- 49.
Rajan
GP, Fergie
N, Fischer
U, Romer
M, Radivojevic
V, Hee
GK. Antibiotic prophylaxis in septorhinoplasty? A prospective, randomized study. Plast Reconstr Surg. 2005;116:1995–8. [
PubMed: 16327614]
- 50.
Campos
GBP, Lucena
EES, da Silva
JSP, Gomes
PP, Germano
AR. Efficacy assessment of two antibiotic prophylaxis regimens in oral and maxillofacial trauma surgery: preliminary results. Int J Clin Exper Med. 2015;8:2846–52. [
PMC free article: PMC4402891] [
PubMed: 25932244]
- 51.
Cioaca
RE, Bucur
A, Coca-Nicolae
C, Coca
CA. [Comparative study of clinical effectiveness of antibiotic prophylaxis in aseptic mouth-jaw- and facial surgery.] [Article in German]
Mund Kiefer Gesichtschir. 2002;6:356–9. [
PubMed: 12448241]
- 52.
Lindeboom
JAH, Tuk
JGC, Kroon
FHM, van den Akker
HP. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone grafting procedures: single-dose clindamycin versus 24-hour clindamycin prophylaxis. Mund Kiefer Gesichtschir. 2005;9:384–8. [
PubMed: 16270222]
- 53.
Lindeboom
JA, Baas
EM, Kroon
FH. Prophylactic single-dose administration of 600 mg clindamycin versus 4-time administration of 600 mg clindamycin in orthognathic surgery: a prospective randomized study in bilateral mandibular sagittal ramus osteotomies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:145–9. [
PubMed: 12582352]
- 54.
Danda
AK, Wahab
A, Narayanan
V, Siddareddi
A. Single-dose versus single-day antibiotic prophylaxis for orthognathic surgery: a prospective, randomized, double-blind clinical study. J Oral Maxillofac Surg. 2010;68:344–6. [
PubMed: 20116706]
- 55.
Kang
SH, Yoo
JH, Yi
CK. The efficacy of postoperative prophylactic antibiotics in orthognathic surgery: a prospective study in Le Fort I osteotomy and bilateral intraoral vertical ramus osteotomy. Yonsei Med J. 2009;50:55–9. [
PMC free article: PMC2649850] [
PubMed: 19259349]
- 56.
Wahab
PUA, Narayanan
V, Nathan
S, Madhulaxmi. Antibiotic prophylaxis for bilateral sagittal split osteotomies: a randomized, double-blind clinical study. Int J Oral Maxillofac Surg. 2013;42:352–5. [
PubMed: 23265757]
- 57.
Karran
SJ, Sutton
G, Gartell
P, Karran
SE, Finnis
D, Blenkinsop
J. Imipenem prophylaxis in elective colorectal surgery. Br J Surg. 1993;80:1196–8. [
PubMed: 8402132]
- 58.
Akgur
FM, Tanyel
FC, Buyukpamukcu
N, Hicsonmez
A. Prophylactic antibiotics for colostomy closure in children: Short versus long course. Pediatr Surg Int. 1992;7:279–81.
- 59.
Ishibashi
K, Kuwabara
K, Ishiguro
T, Ohsawa
T, Okada
N, Miyazaki
T, et al. Short-term intravenous antimicrobial prophylaxis in combination with preoperative oral antibiotics on surgical site infection and methicillin-resistant
Staphylococcus aureus infection in elective colon cancer surgery: results of a prospective randomized trial. Surg Today. 2009;39:1032–9. [
PubMed: 19997797]
- 60.
Ishibashi
K, Ishida
H, Kuwabara
K, Ohsawa
T, Okada
N, Yokoyama
M, et al. Short-term intravenous antimicrobial prophylaxis for elective rectal cancer surgery: results of a prospective randomized non-inferiority trial. Surg Today. 2014;44:716–22. [
PMC free article: PMC3950565] [
PubMed: 23989910]
- 61.
McArdle
CS, Morran
CG, Pettit
L, Gemmell
CG, Sleigh
JD, Tillotson
GS. Value of oral antibiotic prophylaxis in colorectal surgery. Br J Surg. 1995;82:1046–8. [
PubMed: 7648148]
- 62.
Lau
WY, Yuen
WK, Chu
KW, Chong
KK, Li
AK. Systemic antibiotic regimens for acute cholecystitis treated by early cholecystectomy. Aust N Z J Surg. 1990;60:539–43. [
PubMed: 2113376]
- 63.
Takemoto
RC, Lonner
B, Andres
T, et al. Appropriateness of twenty-four-hour antibiotic prophylaxis after spinal surgery in which a drain is utilized: a prospective randomized study. J Bone Joint Surg (Am). 2015;97:979–86. [
PubMed: 26085531]
- 64.
Lin
MH, Pan
SC, Wang
JL, Hsu
SRB, Lin Wu
FL, Chen
YC, et al. Prospective randomized study of efficacy of 1-day versus 3-day antibiotic prophylaxis for preventing surgical site infection after coronary artery bypass graft. J Formos Med Assoc. 2011;110:619–26. [
PubMed: 21982465]
- 65.
Niederhauser
U, Vogt
M, Vogt
P, Genoni
M, Kunzli
A, Turina
MI. Cardiac surgery in a high-risk group of patients: is prolonged postoperative antibiotic prophylaxis effective?
J Thorac Cardiovasc Surg. 1997;114:162–8. [
PubMed: 9270631]
- 66.
Carroll
WR, Rosenstiel
D, Fix
JR, de la Torre
J, Solomon
JS, Brodish
B, et al. Three-dose vs extended-course clindamycin prophylaxis for free-flap reconstruction of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129:771–4. [
PubMed: 12874080]
- 67.
Liu
SA, Tung
KC, Shiao
JY, Chiu
YT. Preliminary report of associated factors in wound infection after major head and neck neoplasm operations--does the duration of prophylactic antibiotic matter?
J Laryngol Otol. 2008;122:403–8. [
PubMed: 17445309]
- 68.
Righi
M, Manfredi
R, Farneti
G, Pasquini
E, Cenacchi
V. Short-term versus long-term antimicrobial prophylaxis in oncologic head and neck surgery. Head Neck. 1996;18:399–404. [
PubMed: 8864730]
- 69.
Bidkar
VG, Jalisatigi
RR, Naik
AS, Shanbag
RD, Siddappa
R, Sharma
PV, et al. Perioperative only versus extended antimicrobial usage in tympanomastoid surgery: a randomized trial. Laryngoscope
2014;124:1459–63. [
PubMed: 24307502]
- 70.
Abubaker
AO, Rollert
MK. Postoperative antibiotic prophylaxis in mandibular fractures: a preliminary randomized, double-blind, and placebo-controlled clinical study. J Oral Maxillofac Surg. 2001;59:1415–9. [
PubMed: 11732026]
- 71.
Baqain
ZH, Hyde
N, Patrikidou
A, Harris
M. Antibiotic prophylaxis for orthognathic surgery: a prospective, randomised clinical trial. Br J Oral Maxillofac Surg. 2004;42:506–10. [
PubMed: 15544879]
- 72.
Bentley
KC, Head
TW, Aiello
GA. Antibiotic prophylaxis in orthognathic surgery: a 1-day versus 5-day regimen. J Oral Maxillofac Surg. 1999;57:226–30; discussion 30–2. [
PubMed: 10077192]
- 73.
Eshghpour
M, Khajavi
A, Bagheri
M, Banihashemi
E. Value of prophylactic postoperative antibiotic therapy after bimaxillary orthognathic surgery: a clinical trial. Iran J Otorhinolaryngol. 2014;26:207–10. [
PMC free article: PMC4196443] [
PubMed: 25320697]
- 74.
Fridrich
KL, Partnoy
BE, Zeitler
DL. Prospective analysis of antibiotic prophylaxis for orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1994;9:129–31. [
PubMed: 7989814]
- 75.
Jansisyanont
P, Sessirisombat
S, Sastravaha
P, Bamroong
P. Antibiotic prophylaxis for orthognathic surgery: a prospective, comparative, randomized study between amoxicillin-clavulanic acid and penicillin. J Med Assoc Thai. 2008;91:1726–31. [
PubMed: 19127796]
- 76.
Bozorgzadeh
A, Pizzi
WF, Barie
PS, et al. The duration of antibiotic administration in penetrating abdominal trauma. American Journal of Surgery
1999;177:125–31. [
PubMed: 10204554]
- 77.
Chang
WC, Hung
YC, Li
TC, Yang
TC, Chen
HY, Lin
CC. Short course of prophylactic antibiotics in laparoscopically assisted vaginal hysterectomy. J Reprod Med. 2005;50:524–8. [
PubMed: 16130850]
- 78.
Becker
JM, Alexander
DP. Colectomy, mucosal proctectomy, and ileal pouch-anal anastomosis. A prospective trial of optimal antibiotic management. Ann Surg. 1991;213:242–7. [
PMC free article: PMC1358335] [
PubMed: 1847796]
- 79.
Togo
S, Tanaka
K, Matsuo
K, Nagano
Y, Ueda
M, Morioka
D, et al. Duration of antimicrobial prophylaxis in patients undergoing hepatectomy: a prospective randomized controlled trial using flomoxef. J Antimicrob Chemother. 2007;59:964–70. [
PubMed: 17329271]
- 80.
Gupta
A, Hote
MP, Choudhury
M, Kapil
A, Bisoi
AK. Comparison of 48 h and 72 h of prophylactic antibiotic therapy in adult cardiac surgery: a randomized double blind controlled trial. J Antimicrob Chemother. 2010;65:1036–41. [
PubMed: 20332194]
- 81.
Sawyer
R, Cozzi
L, Rosenthal
DI, Maniglia
AJ. Metronidazole in head and neck surgery--the effect of lengthened prophylaxis. Otolaryngol Head Neck Surg. 1990;103:1009–11. [
PubMed: 2126114]
- 82.
Scher
KS. Studies on the duration of antibiotic administration for surgical prophylaxis. Am Surg. 1997;63:59–62. [
PubMed: 8985073]
- 83.
Liberman
MA, Greason
KL, Frame
S, Ragland
JJ. Single-dose cefotetan or cefoxitin versus multiple-dose cefoxitin as prophylaxis in patients undergoing appendectomy for acute nonperforated appendicitis. J Am Coll Surg. 1995;180:77–80. [
PubMed: 8000659]
- 84.
Bozorgzadeh
A, Pizzi
WF, Barie
PS, Khaneja
SC, LaMaute
HR, Mandava
N, et al. The duration of antibiotic administration in penetrating abdominal trauma. Am J Surg. 1999;177:125–31. [
PubMed: 10204554]
- 85.
Meijer
WS, Schmitz
PIM. Prophylactic use of cefuroxime in biliary tract surgery: Randomized controlled trial of single versus multiple dose in high-risk patients. Br J Surg. 1993;80:917–21. [
PubMed: 8369939]
- 86.
Mann
W, Maurer
J, Wolfensberger
M, Riechelmann
H, Daschner
F. [Perioperative preventive use of antibiotics in head and neck surgery.] [Article in German]
HNO. 1990;38:197–201. [
PubMed: 2199415]
- 87.
Wu
CC, Yeh
DC, Lin
MC, Liu
TJ, P’Eng
F K. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. Br J Surg. 1998;85:489–93. [
PubMed: 9607529]