NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and Other Influenza Viruses. Geneva: World Health Organization; 2010 Feb.

WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and Other Influenza Viruses.
Show details1. Treatment of Seasonal or Pandemic Influenza
1.1. Use of oseltamivir – treatment
Oseltamivir, a neuraminidase inhibitor, is available for oral administration as hard capsules (75mg, 45mg, and 30mg) or as a powder for reconstitution (12mg/ml suspension). Extemporaneous preparation for nasogastric administration has been described and enteric absorption appears to be comparable between critically ill and ambulatory influenza patients (Ariano et al., In press; Taylor et al., 2008). Treatment is now indicated for infants <1 year when treating pandemic influenza; dosage and administration are described elsewhere (see Annexes 7 and 8).
There are no systematic reviews or randomized controlled trials assessing the efficacy and safety of antivirals for pandemic influenza A (H1N1) 2009 infection. There are, however, a number of recent observational studies addressing a range of outcomes for antiviral use, with oseltamivir the most commonly used antiviral (see ‘Observational data – pandemic influenza’ below for a summary of these studies). Given the lack of clinical trial evidence specifically addressing pandemic influenza, a description of evidence for seasonal influenza is provided below.
Systematic review/clinical trial evidence – seasonal influenza
A recent systematic review of neuraminidase inhibitors (Jefferson et al., 2009) provides an updated assessment of the efficacy and safety of oseltamivir for the treatment of influenza in adults and a second systematic review (Shun-Shin et al., 2009) provides an assessment of the use of neuraminidase inhibitors in children (see Section 2.1 for prophylactic evidence and Sections 1.2 and 2.2 for zanamivir evidence).
The Jefferson et al. (2009) review included five trials of oseltamivir used for treatment of influenza in otherwise healthy adults. The results of these trials indicated a statistically significant advantage for oseltamivir compared to placebo in the alleviation of symptoms (HR=1.20; 95% CI: 1.06, 1.35; see Table A5.1, Annex 5). However the reduction in duration of illness is less than a day, which suggests a modest treatment benefit (Jefferson et al., 2009). The evidence presented by Jefferson (2009), although limited to healthy adults instead of the additional at-risk, children, and elderly populations assessed by Burch et al. (2008), concurs with the results reported by Burch (2008), which formed the basis of the evidence used in the formulation of the WHO Pharmacological Guidelines (August 2009).
The Jefferson (2009) review excludes some of the evidence used in the previous review by Kaiser et al. (2003). Eight of the 10 trials included in the Kaiser (2003) meta-analysis remain unpublished, resulting in inaccessibility of data for re-evaluation of outcomes presented in the Kaiser (2003) paper. The remaining available evidence addressing safety of oseltamivir indicates that oseltamivir induced nausea (OR=1.79; 95% CI: 1.10, 2.93; see Table A5.1, Annex 5) and did not significantly reduce influenza-related lower respiratory tract infections (RR=0.55; 95% CI: 0.22, 1.35; see Table A5.1, Annex 5). This evidence is based on a relatively small number of trials (three for lower respiratory tract complications and two for nausea). Jefferson (2009) states that it is possible there is publication bias, however a funnel plot was not undertaken given that there are only three trials.
There are no new reviews of the efficacy and safety of oseltamivir in at-risk patients and, as such, the evidence provided in the August 2009 Guidelines, which indicated a reduction of slightly less than a day in duration of illness (-22.75 hours), remains current (Burch et al., 2008).
The Shun-Shin (2009) review included two trials assessing the efficacy of oseltamivir for the treatment of seasonal influenza in children. The authors did not pool efficacy results from these trials due to inadequate reporting and heterogeneity of trial data. The results of the two oseltamivir trials indicated a median reduction of 0.4 to 1.5 days in time to illness resolution. The trials were pooled for some adverse event outcomes, which showed that oseltamivir significantly increased vomiting (RD=0.05; 95% CI: 0.02, 0.09; p=0.007; see Table A5.2, Annex 5), however there was no difference in occurrence of nausea and diarrhoea. There were also no data available on serious complications such as pneumonia or hospitalizations.
The Jefferson (2009) review and available randomized comparative trials do not provide any information regarding the outcomes of mortality, progression to severe disease, or hospitalization. There are, however, several observational studies of fatal outcomes and hospitalization as discussed below (see seasonal observational data and Annex 6).
Observational data – seasonal influenza
A summary of observational data for the use of antivirals in seasonal influenza is provided in Table A6.1 in Annex 6. The studies vary in terms of design, patient population, outcomes assessed, and analyses conducted. Most assessed the use of oseltamivir, with a few assessing zanamivir use and one study assessing the use of amantadine.
Some studies indicated advantages associated with the use of oseltamivir, however some conflicting results were observed. For example, Kawai et al. (2009), in a retrospective review of Japanese influenza patients receiving a neuraminidase inhibitor, reported that the mean duration of fever was longer for oseltamivir-treated patients than those treated with zanamivir (p<0.001). However, these results are based on a small population of 164 patients and were specifically for infection with 2008-09 H1N1 influenza, which is a predominantly oseltamivir-resistant (H275Y) strain. The impact of oseltamivir versus zanamivir on time to afebrile state may depend upon the influenza strain in question. A further report demonstrated no significant difference in fever duration for seasonal H1N1, but a shorter fever when treating H3N2 with oseltamivir and when treating influenza B with zanamivir (Kawai et al., 2009). Earlier data also demonstrated this lower clinical effectiveness of oseltamivir against influenza B compared to influenza A infection (Sugaya et al., 2007).
In an analysis of observational data for oseltamivir use, Freemantle and Calvert (2009) reviewed nine post-marketing studies of oseltamivir. The authors concluded that although the studies were of variable quality, they generally supported the conclusion that oseltamivir may reduce the incidence of pneumonia and other complications of influenza in healthy adults. Freemantle and Calvert (2009) highlight that these events are rare; therefore, treatment of influenza with oseltamivir is not likely to be clinically important for otherwise healthy adults. The authors also discuss the potential biases in the studies, in particular the studies' selection criteria, which excluded those who received oseltamivir later than the recommended time frame, so may not represent real world use. Differences in baseline comorbidity or geographical distribution were present in several studies and the direction of bias from confounding by indication was uncertain. These factors, or similar factors, may impact upon all observational studies; therefore, the results of the observational data provided should be critically assessed to consider potential sources of bias.
Several observational studies address the impact of oseltamivir on outcomes such as hospitalization and death in seasonal influenza. It was reported in August that oseltamivir may be associated with significant reductions in pneumonia, otitis media, and hospitalization compared to unmatched controls (Blumenthals et al., 2007; Gums et al., 2008). Two observational studies, McGeer et al (2007) and Lee et al. (2008), indicate a reduction in mortality in seasonal influenza, with odds ratios of 0.21 and 0.26, respectively, for impact of antiviral treatment on mortality. There is also a new observational study (Hanshaoworakul et al., 2009) which assessed the impact of oseltamivir treatment on fatal outcomes in hospitalized patients with severe influenza in Thailand. The study found that when cardiovascular disease and hypertension were controlled, oseltamivir was associated with increased survival (OR=0.13; 95% CI: 0.04, 0.38 for cardiovascular disease and OR=0.14; 95% CI: 0.04, 0.44 for hypertension, see Table A5.3, Annex 5). This study was a retrospective review of medical charts and, as such, may be open to bias and does not allow for the establishment of causal relationships.
Following are descriptions of recent observational studies of oseltamivir.
Piedra et al. (2009) assessed influenza-related complications in children with chronic medical conditions. This retrospective review of a medical database in the US covering six influenza seasons found that oseltamivir was associated with a statistically significant reduction in the risk of respiratory illnesses other than pneumonia (OR=0.74; 95%CI, 0.63–0.87), otitis media (OR=0.69; 95%CI, 0.48–0.99), and all-cause hospitalization (OR=0.33; 95%CI, 0.13–0.83) at 14 and 30 days following influenza diagnosis in children with chronic medical conditions (see Table A5.4, Annex 5). This study is based on the same database reported by Blumentals et al. (2007) previously reviewed by the Guidelines Panel, which noted that the observational data are derived from cohorts in the US; therefore, they may not be representative of the occurrence of these events in other populations or locations. In addition, the authors of the current study acknowledge a number of limitations of the study, including the fact that the database is limited primarily to patients covered by employer-sponsored health insurance; the use of diagnostic coding for influenza was assigned on basis of physicians' clinical diagnoses alone; it was impossible to confirm if patients began antiviral treatment within the recommended timeframe; and patients were not assigned randomly nor matched with respect to propensity to be given oseltamivir. Although there were few clinically significant differences between the two cohorts and multivariate analyses were used to adjust for differences, the results of this study should still be interpreted with caution.
Another observational study assessing safety (Casscells et al., 2009) was a retrospective review of administrative data for members of the US Department of Defense, which assessed occurrence of cardiovascular events in patients with a history of vascular disease (see Table A5.3, Annex 5). This study found that oseltamivir provided a statistically significant protective effect against recurrent cardiovascular events in patients with a history of vascular disease (OR=0.417; 95% CI: 0.349, 0.498). Given the study design, the authors acknowledge that the study is susceptible to a number of sources of confounding, including omission of potentially important variables such as severity and prior duration of patient's symptoms, presence of specific comorbidities, prior prophylactic treatment, subject compliance with critical medications, or death due to causes unrelated to influenza. As such, the results, which are only relevant to patients with vascular disease, should also be interpreted with caution.
There are no new data available regarding the use of oseltamivir in pregnant women in seasonal influenza. Evidence previously presented showed that the use of oseltamivir in pregnant women (Tanaka et al., 2009) has not indicated any additional dangers. The Tanaka study reported on a population of 90 pregnant Japanese women who received oseltamivir and found that the incidence of malformation (1.1%) was within the incidence of major malformations in the general population. Oseltamivir does not appear to have a negative impact on breastfeeding, although the only data available are based on the report of one lactating woman (Wentges-van Holthe et al., 2008).
There are no published randomized controlled trials assessing the efficacy and safety of oseltamivir in children aged <1 year. However a recent retrospective chart review (Kimberlin et al., 2009) assessed the comparative safety of oseltamivir, rimantadine, and amantadine in 180 infants treated with antivirals. This review found that children <1 year of age treated with oseltamivir were significantly less likely to develop abnormalities in the head/eyes/ears/nose/throat system, such as otitis media, compared to children treated with rimantadine or amantadine (1.7% versus 15.4%; p<0.01; see Table A5.5, Annex 5). However, there were no statistically significant differences in the occurrence of neurologic, pulmonary, gastrointestinal, cardiovascular, dermatologic, systemic response, genitourinary, musculoskeletal, hematologic/lymphatic, hepatobillary/pancreatic, and endocrine/metabolic abnormalities in children treated with oseltamivir or one of the adamantanes. A second retrospective chart review (Siedler et al., 2009) investigated the frequency of side-effects and duration of fever by time to oseltamivir treatment in infants <1 year (n=157). All except one infant completed the 5-day course. Seventy-eight infants experienced mild additional symptoms, of which vomiting (39%) and diarrhoea (22%) were the most common. These reviews are based on small numbers of subjects (n=180 and 157) and are open to bias given the lack of randomization, control group, or blinding of outcome assessment.
Observational data – pandemic influenza
Table 1.1 below provides a summary of the available observational data addressing the use of neuraminidase inhibitors for pandemic (H1N1) 2009 infection. All of these studies included ill or severely ill patients. Most of the studies did not specify which neuraminidase inhibitor was used; however, the only drug mentioned is oseltamivir and it is likely it was the most commonly used antiviral.
Table 1.1
Available observational data for pandemic influenza A (H1N1) 2009.
Some studies showed advantages associated with neuraminidase treatment (e.g. Dominguez-Cherit et al., 2009), such as indicating that neuraminidase treatment compared to no treatment was associated with improved survival (OR=7.4; 95% CI: 1.8, 31.0). However, all studies, except Echevarria-Zuno et al. (2009), had relatively small sample sizes and were likely to be open to a number of sources of bias.
In vitro and animal studies have demonstrated the efficacy of oseltamivir against pandemic (H1N1) 2009 virus (Itoh et al., 2009; MMWR, 1 May 2009).
Several observational studies have demonstrated the impact of time to treatment on disease progression and outcome for pandemic (H1N1) 2009 infection. Cao et al. (2009) identified treatment delays of greater than 48 hours as an independent risk factor for prolonged viral replication. Several retrospective studies reported fatal cases as rarely receiving treatment within 48 hours (Echevarria-Zuno et al., 2009; Jain et al., 2009; Jamieson et al, 2009; Libster et al., 2010), though no statistical comparison was made to other outcome groups. One case control study demonstrated that time to antiviral therapy was the strongest correlate of disease severity, with an odds ratio for ICU versus community cases of 12.0 (4.65–30.7) for an interval from symptom onset to antiviral treatment of more than 48 hours as compared to less than 48 hours (Zarachynski et al. 2010). In addition, a chart review has indicated that patients treated within 48 hours of symptom onset experience shorter median hospitalization. Much of the data presented is uncontrolled, retrospective clinical data; therefore, results should be interpreted with caution.
One observational study has been conducted with regard to the use of oseltamivir in pregnancy for pandemic influenza (Louie et al., 2009b). This study indicated that treatment initiation more than 48 hours after illness onset was associated with ICU admission or death. No data on adverse events from antiviral use were reported.
The WHO's Weekly Epidemiological Record (WER 2009) reported 39 cases of oseltamivir-resistant pandemic (H1N1) 2009 virus up to October 2009; a subsequent WER reported cumulative cases of 190 up to January 2010. WHO concluded that the relatively small number of oseltamivir-resistant pandemic viruses does not constitute a public health threat at this point and there is no evidence that such viruses are circulating at a community level, although transmission has occurred in local settings. Further discussion on antiviral sensitivity of circulating strains of influenza virus is in Part I, Section 5. Of relevance is the recent publication by Kawai et al. (2009) demonstrating that oseltamivir is clinically less effective in treatment of infection by oseltamivir-resistant viruses carrying the H275Y mutation. Lack of oseltamivir efficacy for oseltamivir-resistant seasonal H1N1 containing the same H275Y mutation was also noted in animal models (Itoh et al., 2009) and observational clinical studies (Gooskens et al., 2009; van der Vries et al., 2008).
With the exception of the two studies looking at adherence and adverse effects associated with prophylactic oseltamivir in UK school children (Kitching et al., 2009; Wallensten et al., 2009; see Section 4.1), as well as the observational data described here, there is a relative absence of data based directly on the use of oseltamivir in pandemic (H1N1) 2009. While the seasonal influenza data may be applicable to pandemic influenza infection, the similarities and differences between the two types of influenza should be considered when applying treatment recommendations.
Initial recommendations for dose and duration of oseltamivir treatment for pandemic (H1N1) 2009 influenza were based upon data from seasonal, uncomplicated influenza. However, the extent to which this is applicable to the pandemic strain is uncertain, given the high incidence of severe disease and longer viral replication experienced in pandemic influenza (Lee et al., 2009; Li et al., 2010; Witkop et al., 2009; de Serres et al., 2009; Lye et al., 2009).
1.2. Use of zanamivir - treatment
Zanamivir, also a neuraminidase inhibitor, is administered as an inhaled powder (10mg twice daily). It is licensed for adults and children aged 5 years and above.
As for oseltamivir, there are no systematic reviews or randomized controlled trials assessing the efficacy of zanamivir for pandemic (H1N1) 2009 infection. However, there are individual case reports of intravenous zanamivir use in the treatment of the severely ill, often immunocompromised patients with proven or suspected oseltamivir-resistant pandemic (H1N1) 2009 illness. As a result, seasonal clinical trial evidence and observational data are presented, alongside case studies of intravenous zanamivir.
Systematic review/clinical trial evidence – seasonal influenza
Jefferson et al.'s (2009) recent systematic review of neuraminidase inhibitors includes an assessment of zanamivir treatment in otherwise healthy adults with naturally occurring influenza (see Section 2.2 for prophylactic evidence and Sections 1.1 and 2.1 for oseltamivir evidence). A second systematic review (Shun-Shin et al., 2009) provides an assessment of the use of zanamivir in children.
The Jefferson et al. (2009) review includes a total of 8 treatment trials, 2 of which were linked to the others, leaving 6 separate trials. There was a statistically significant advantage of zanamivir compared to placebo for the alleviation of symptoms (HR=1.24; 95% CI: 1.13, 1.36; see Table A5.6, Annex 5). However, as with oseltamivir, the reduction of illness was less than a day. The Shun-Shin et al. (2009) review included two trials of zanamivir treatment in children. As for oseltamivir, the authors did not pool these trials for efficacy outcomes due to inadequate reporting and heterogeneity of data. The NA130009 trial (published as Hedrick et al., 2000) showed a median reduction of 1.25 days (95%CI: 0.5, 2.0; p<0.001) to resolution or alleviation of symptoms when comparing zanamivir to placebo for treatment of confirmed influenza. For the treatment of clinical influenza a significant reduction remained associated with zanamivir, but it decreased to 0.5 days (95% CI: 0.0, 1.5; p=0.011). The second zanamivir trial included in the Shun-Shin review is unpublished and it showed a similar reduction of 0.5 days in median time to resolution of symptoms, but did not report confidence intervals or a p-value. The Hedrick et al. (2000) trial also showed that children with confirmed or clinical influenza returned to school or normal activity one day sooner than those treated with placebo (p=0.019 and p=0.022, respectively). Overall, the data summarized by Jefferson et al. (2009) and Shun-Shin (2009) indicates the same as had been previously reported for zanamivir (Burch et al., 2008): a reduction of less than a day for alleviation of symptoms.
The trials included in the Jefferson (2009) review showed there was no occurrence of statistically significant adverse events associated with zanamivir. Similar results were reported for the zanamivir treatment trials in children in the Shun-Shin (2009) review, with no significant difference in the number of withdrawals due to adverse events between zanamivir and placebo. In addition, the Hedrick (2000) trial reported no significant difference in asthma exacerbations between zanamivir and placebo (difference=-0.01; 95% CI: -0.03, 0.01; p=0.30).
Observational data – seasonal influenza
There are no new data available addressing the outcomes of mortality, progression to severe disease or hospitalization. As reported in the August 2009 Guidelines, an observational study conducted in the US indicated that the occurrence of complications is similar between those treated with zanamivir and untreated controls (Cole et al., 2002). A retrospective analysis of published trials assessing the impact of zanamivir on the occurrence of respiratory events leading to the use of antibiotics found that zanamivir reduced the number of antibiotic prescriptions (Kaiser et al., 2000). However the number of patients with respiratory events was small and the post-hoc nature of the study indicates the results should be interpreted with caution.
There remains no publicly available data describing the use of zanamivir in children aged <1 year. There is no additional data regarding the use of zanamivir in pregnant women beyond the Tanaka (2009) report described in the August Guidelines, which illustrated the outcomes of four pregnant women who were exposed to zanamivir (one spontaneous miscarriage, one termination, and two healthy births). The Tanaka (2009) paper also concluded that the amount of zanamivir that would be ingested by a 5kg infant is much lower than the recommended dose for children.
Observational data – pandemic influenza
The body of clinical trials and reviews addressing the use of zanamivir are all for seasonal influenza. However, there are several published case reports summarizing the use of intravenous zanamivir in severely ill patients with confirmed pandemic (H1N1) 2009 infection (Kidd et al., 2009; Englund et al., 2009; Gaur et al., 2009). The patient reported by Kidd was neutropenic following chemotherapy for Hodgkin's disease and was not responding to oseltamivir or nebulized zanamivir. Intravenous zanamivir (600mg twice daily) was started in conjunction with methylprednisolone and the patient's condition improved within 48 hours. The authors concluded that although the data presented was a single case report and direct cause and effect cannot be confirmed, the improvement associated with intravenous zanamivir treatment warrants further investigation, both alone and in combination with methylprednisolone. The Englund case report detailed the treatment of a leukemia patient on immunosuppressive therapy. After identification of oseltamivir-resistant pandemic H1N1 2009, and poor tolerance to inhaled ribavirin and zanamivir, the patient received IV zanamivir and oral ribavirin. This case, however, was ongoing at time of print, so the impact of IV zanamivir was unknown. The Gaur correspondence reports a case of prolonged oseltamivir-resistant infection in a 10 year old with leukemia. The patient was given 600mg IV zanamivir every 12 hours for 15 days, during which viral load substantially decreased and, after 10 days, the patient was weaned off ventilation. No zanamivir-related adverse effects were observed.
As discussed for oseltamivir, dosing and duration recommendations for zanamivir are based on data from seasonal, uncomplicated influenza. However, due to the different experiences of clinical severity and duration of viral shedding in pandemic influenza, different treatment regimens may also be considered for zanamivir (Li et al., 2009; Lee et al., 2009).
1.3. Use of amantadine - treatment
Systematic review/clinical trial evidence – seasonal influenza
The reviews by Jefferson (2006) and Alves Galvao et al. (2008) are the most current source of information regarding the efficacy of amantadine. These reviews demonstrated that amantadine is superior to placebo in terms of a reduction in duration of fever for both adults and children, with a decrease in fever duration of a day for adults (MD=-0.99; 95% CI: -1.26, -0.71) and fewer cases of fever for children (see Table A5.7, Annex 5). There was no statistically significant difference demonstrated between amantadine and placebo in the occurrence of adverse events in the randomized trials.
Observational data – seasonal influenza
A retrospective chart review by Kimberlin et al. (2009) assessed the comparative safety of oseltamivir and the adamantanes rimantadine and amantadine in 180 infants treated with antivirals. As reported above for oseltamivir (see Section 1.1), the review found that children <1 year of age treated with oseltamivir were significantly less likely to develop abnormalities in the head/eyes/ears/nose/throat system, such as otitis media, compared to children treated with rimantadine or amantadine (1.7% versus 15.4%; p<0.01; see Table A5.5, Annex 5). However, there were no statistically significant differences in the occurrence of body system abnormalities in infants treated with oseltamivir or one of the adamantanes. This review is based on a small number of subjects (n=180) and is open to bias given the lack of randomization and lack of blinding of outcome assessment. A comparison of M2 inhibitors for prophylaxis in elderly patients concluded that amantadine was much less well-tolerated than rimantadine (Keyser et al., 2000). There remains no new published comparison of the safety of amantadine in adults.
There are also no published data assessing the outcomes of mortality, progression to severe disease or hospitalization, or the use of amantadine in pregnant women. Nor are there any published data assessing the use of amantadine in pandemic (H1N1) 2009 infection.
1.4. Use of rimantadine - treatment
Systematic review/clinical trial evidence – seasonal influenza
As for amantadine, the reviews by Jefferson (2006) and Alves Galvao et al. (2008) are the most current source of information regarding the efficacy of rimantadine. The reviews demonstrated that rimantadine is superior to placebo in terms of a reduction in duration of fever for adults of greater than a day (MD= -1.24; 95% CI: -1.71, -0.76) and fewer cases of fever for children (see Table A5.8, Annex 5). There was no statistically significant difference demonstrated between rimantadine and placebo in the occurrence of adverse events in the randomized trials.
Observational data – seasonal influenza
As noted above in Sections 1.1 and 1.3, the Kimberlin (2009) review found that children <1 year of age who were treated with oseltamivir were significantly less likely to develop abnormalities in the head/eyes/ears/nose/throat system, such as otitis media, compared to children treated with rimantadine or amantadine (1.7% versus 15.4%; p<0.01; see Table A5.5, Annex 5). However there were no statistically significant differences in the occurrence of body system abnormalities in children treated with oseltamivir or one of the adamantanes. In addition, the Keyser (2000) study indicates that rimantadine is better tolerated than amantadine.
There have been no further publications assessing the safety of rimantadine nor is there any information available regarding the outcomes of mortality, progression to severe disease, or hospitalization. Rimantadine is not recommended for use in pregnant women.
1.5. Use of peramivir - treatment
Peramivir, an investigational neuraminidase inhibitor, has received an Emergency Use Authorization (EUA) in the US and market authorization in Japan. The US authorization was based on a review by the Food and Drug Administration (FDA) of four trials assessing intravenous peramivir. These trials have not yet been published and there are no current publications assessing the use of intravenous peramivir in humans. A discussion of the EUA for peramivir (Birnkrant and Cox, 2009) provides some information regarding the peramivir data.
A total of 1891 patients have received peramivir in a variety of doses, formulations (intravenous or intramuscular), and/or durations. The usual adult dose is 600mg/day administered intravenously for 5 to 10 days. Birnkrant and Cox (2009) report one trial demonstrating that alleviation of symptoms was approximately one day sooner with peramivir than with placebo in otherwise healthy adults with uncomplicated seasonal influenza, similar to the effects observed with oseltamivir and zanamivir. Two trials were conducted using oseltamivir as the comparator, however the results did not indicate that peramivir was superior and, since a clinically meaningful non-inferiority margin has not been established, no conclusions can be drawn about the trial results. The fourth trial demonstrated no statistically significant distinctions between two different doses or single and multiple doses of peramivir.
The most commonly reported adverse events in the clinical trials were diarrhoea, nausea, vomiting, and neutropenia. The Birnkrant and Cox (2009) report does not provide any further details on adverse events.
No paediatric patients have received peramivir in clinical trials, although the Birnkrant and Cox (2009) report states that a limited number of paediatric patients have received peramivir under the earlier FDA Emergency Investigational New Drug procedures. The report does not provide any information regarding the use of peramivir in these paediatric patients.
There have been no trials of peramivir in patients with pandemic (H1N1) 2009 virus. The Birnkrant and Cox (2009) report indicates that peramivir was granted EUA as it is reasonable to believe that it may be effective in patients with pandemic influenza given the available evidence in seasonal influenza, the serious nature of the disease, and the lack of alternative treatment options.
1.6. Use of arbidol
Arbidol is a Russian-made antiviral that is widely used in Russia and China. A review by Boriskin et al. (2008) provides a summary of the studies of arbidol, although little detailed information is provided regarding the trials.
According to Boriskin (2008), arbidol taken at a dose of 200mg/day for 5 to 10 days was reported to reduce the duration of influenza by about 1.7 to 2.65 days. This is a greater increase than that observed for the neuraminidase inhibitors; however, no information is available regarding the size or design of the trials from which this result was derived. Boriskin (2008) also states that arbidol has been shown to prevent the development of post-influenza complications and lower the frequency of re-infection. The table below provides a summary of the trials reported by Boriskin (2008).
Table 1.6
Summary of arbidol data, as reported by Boriskin (2008).
While the results described by Boriskin (2008) report some efficacy and safety of arbidol, the lack of information regarding trial design, trial numbers, and comparative analyses indicates the results should be interpreted with caution.
The use of prophylactic arbidol to prevent acute viral respiratory infections and complications in over 4000 Russian servicemen (Shuster 2004) demonstrated a lower infection rate (14.1%) compared to placebo (30.8%). Arbidol also lowered the rate of viro-bacterial pneumonia. The authors conclude the results demonstrate that the use of arbidol allows for lowering the rate of infection of influenza and also lowering the rate of viro-bacterial pneumonia.
Kolobukhina et al. (2009) reports on a comparison of ingavirin and arbidol in adult patients with influenza. This trial included 105 patients with confirmed uncomplicated influenza. The results indicated that duration of fever with ingavirin (34.5 hours) was significantly lower compared to duration of fever with arbidol (48.4 hours). There were no side effects observed and no complications reported in patients treated with ingavirin.
1.7. Use of ribavirin
Ribavirin is a broad-spectrum antiviral agent, active in vitro against various RNA and DNA viruses. Ribavirin treatment of hepatitis C and respiratory syncytial virus infections has been approved in many countries, but no wide-scale authorizations have been made for its use against influenza.
The table below provides a summary of the available ribavirin data for influenza. The available randomized placebo-controlled trials provide inconsistent results. Symptomatic improvement was significant in studies by Knight (Knight et al., 1981; MEDA 2009), Stein (1987) and Rodriguez (1994), whereas Schiff (MEDA 2009) and Bernstein (1988) reported no statistical difference between ribavirin and placebo. Impact on viral load is uncertain, as case reports of intravenous (Hayden et al., 1996) and one trial of aerosolized ribavirin (Knight et al., 1981) suggest an antiviral-induced reduction, whereas two RCTs of oral ribavirin and one of aerosolized ribavirin report no impact on viral load (Smith et al., 1980; Stein et al., 1987; Berstein et al., 1988).
Table 1.7
Summary of Ribavirin studies and reviews for influenza.
All of the ribavirin efficacy trials had small sample sizes, with most trials having less than 35 patients and only the Rodriguez trial having more than 50 patients (n=62). Data for the clinical efficacy of ribavirin against influenza virus are limited, particularly due to small sample sizes, incomplete trial information and incompatible protocols for meta-analysis.
Pharmacokinetic trials in rats and monkeys have been conducted using oral, inhaled, and intravenous administration routes. Bioavailability of 45-65% has been reported upon oral administration (eMC 2009). High lung and plasma concentrations have been reported for inhaled and intravenous administration, respectively (MEDA 2009).
Adverse effects recorded in humans include mild to moderate haemolytic anaemia, reversible upon cessation of therapy. Animal data also indicate possible genotoxicity, carcinogenicity, and teratogenicity (MEDA 2009).
1.8. Other products
Intranasal interferons
In vitro data indicate no major cytokine dysregulation due to pandemic (H1N1) 2009 virus. Therefore, whether immunomodulators such as interferons are useful as an adjunctive therapy is uncertain, with the possible exception of individual severe cases (Woo et al., 2010). However, Osterlund et al. (2009) demonstrated the sensitivity of pandemic (H1N1) 2009 virus to the antiviral effects of interferons. Other influenza viruses vary in their in vitro interferon sensitivity. Thus, uncertainty remains regarding the potential value of interferons for treatment of influenza. Animal data show constraint of viral replication and prevention of transmission by intranasal interferons (Steel et al., 2009).
There are no published clinical randomized controlled trials or observational studies of current intranasal interferon preparations for the treatment of influenza. Other routes of administration, such as suppositories and sublingual tablets, were not considered in this review.
Immunoglobulins
Although monoclonal antibodies have been tested in pre-clinical models, there are no published, randomized controlled trials or observational data for the use of immunoglobulins in the treatment or prophylaxis for influenza.
1.9. Anti-inflammatory products
Aspirin
The association between Reye's syndrome and salicylates in children and adolescents (<18 years) is well established. A series of five key case control studies informed recognition of this association in 1980, which has been followed by extensive published epidemiological and observational data over the last thirty years (Starko et al., 1980; Halpin et al., 1982; Waldman et al., 1982; CDC MMWR, 1980). U.S. surveillance data demonstrate the likely impact on incidence of Reye's syndrome due to the reduction in aspirin use since the association was first identified. Reported cases rapidly descended from a peak of 555 cases in 1980, to less than 36 per annum since 1987 (Belay et al., 2009).
Corticosteroids
Corticosteroids, such as methylprednisolone and hydrocortisone, are occasionally used as an adjunctive therapy for the treatment of ARDS in severe influenza due to their immunomodulatory properties. The influenza virus mechanisms of cytokine dysregulation, and the action of corticosteroids to potentially correct this, are incompletely understood (Carter et al., 2008). A summary of key corticosteroid literature for influenza is provided in the table below (Table 1.9).
Table 1.9
Clinical data for corticosteroids in influenza.
Recently published retrospective observational studies suggest that corticosteroid treatment of influenza is associated with a higher likelihood of ICU admission and mortality as clinical outcomes (Jain et al., 2009; Liem et al., 2009). In addition, two observational studies demonstrate that corticosteroid use is associated with slower viral clearance, significantly increased odds of persistent viral replication 7 days after symptom onset (Lee et al., 2009), and a longer duration of viral shedding with increased corticosteroid dose (Nichols et al., 2004).
Dosage recommendations have changed as new data have emerged, but consensus on whether corticosteroids should be used for the treatment of influenza and, if so, at what dosage, has still not been attained. High dose methylprednisolone has been demonstrated as ineffective in ARDS (Bernard et al. 1987), though results from several studies and reviews suggest a positive impact on ARDS by long duration low-dose corticosteroids (Sessler et al., 2008; Quispe-Laime et al., 2009). However, there are no placebo-controlled clinical trials specifically assessing the impact of low-dose corticosteroids in patients with serious influenza. Therefore, the evidence base for the treatment of influenza with corticosteroids is largely extrapolated from trials conducted for ARDS resulting from different aetiologies (Annane et al., 2004; Tang et al., 2009). One such trial for late-stage ARDS demonstrated the impact of treatment timing on clinical outcome. Corticosteroids 7-13 days after ARDS onset reduced mortality, whereas after 13 days is associated with increased mortality (Steinberg et al., 2006), indicating possible harms from the use of corticosteroids.
In addition to the scarcity of influenza-specific trial data, many existing studies are limited by low participant numbers, lack of a control group, and confounding.
2. Chemoprophylaxis of Influenza
2.1. Use of oseltamivir - chemoprophylaxis
Systematic review/clinical trial evidence – seasonal influenza
There are no new trials available addressing the chemoprophylactic use of oseltamivir. The updated Jefferson (2009) review reported that the two trials of prophylactic use of oseltamivir in adults demonstrated that oseltamivir reduced the chance of symptomatic, laboratory-confirmed influenza (RR=0.39; 95% CI: 0.18, 0.85; see Table A5.1x, Annex 5). The trials did not support or refute the impact of oseltamivir on ILI (RR=1.28; 95% CI: 0.45, 3.66; see Table A5.1, Annex 5). Two trials assessing post-exposure prophylaxis demonstrated significant protection for households.
The Shun-Shin (2009) review of the use of neuraminidase inhibitors in children reported on one post-exposure prophylactic trial of oseltamivir. This trial demonstrated a reduction in the risk of developing confirmed symptomatic influenza after introduction of an index case into the household (RD=-0.12; 95% CI: -0.21, -0.03).
A systematic review by Khazeni et al. (2009) assessed the safety and efficacy of extended duration (>4 weeks) of chemoprophylaxis with neuraminidase inhibitors. Pooled results of the four oseltamivir trials demonstrated a decreased incidence of symptomatic influenza (RR=0.236; 95% CI: 0.144, 0.387; see Table A5.9, Annex 5). The Khazeni (2009) review also provides results for oseltamivir and zanamivir combined – these results follow the same pattern as those observed for the individual drugs (see Table A5.10, Annex 5). There was no statistically significant difference between the efficacy of oseltamivir and zanamivir (p=0.64). However, the review provides no information regarding the methodology used to indirectly compare the two drugs to obtain this result.
Based on the same four trials, there was no statistically significant advantage for oseltamivir compared to placebo for asymptomatic influenza (RR=0.781; 95% CI: 0.563, 1.082, see Table A5.9, Annex 5). There were no serious adverse events reported with oseltamivir in prophylactic treatment, although this is based on only one trial in the Khazeni (2009) review. Oseltamivir was associated with an increased risk for nausea and vomiting based on the results of four trials, compared with placebo (RR=1.48; 95% CI: 1.86, 2.33). There was no statistically significant difference between oseltamivir and zanamivir in the occurrence of adverse events (p=0.32).
The results presented by Khazeni (2009) should be interpreted with caution, given the risk of publication bias. The authors noted that although assessments for publication bias were limited by the small sample size, a funnel-plot analysis was asymmetric and the Begg method suggested bias (p=0.009). Figure 2.1 below provides the funnel plot assessing publication bias in the Khazeni (2009) review.
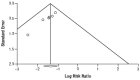
Figure 2.1
Funnel plot for symptomatic influenza.
The results described above for the Khazeni (2009) review are consistent with the evidence provided by the Tappenden et al. (2009) review summarized in the August 2009 Guidelines, which found that in adults there were statistically significantly fewer cases of laboratory confirmed infection in patients receiving oseltamivir compared to placebo (RR=0.27, 95% CI: 0.09, 0.83; see Table A5.11, Annex 5). In mixed households, including adults and children, post-exposure prophylaxis resulted in fewer cases of infection (RR=0.19; 95% CI: 0.08, 0.45). The Tappenden et al., (2009) review also reported that for elderly individuals there were statistically significantly fewer cases of infection (RR=0.08; 95% CI: 0.01, 0.63) with oseltamivir use.
Khazeni (2009) reported that antiviral therapy is contraindicated for only two weeks after live attenuated vaccination (LAIV) due to the possibility of limiting viral replication, therefore interfering with the response to vaccination. They also reported that, if the use of LAIV increases, it will be unclear whether individuals receiving LAIV could safely receive neuraminidase prophylaxis during a pandemic. The authors encouraged randomized controlled trials to study the efficacy and safety of neuraminidase inhibitors administered two weeks after LAIV.
Observational data – pandemic influenza
Two studies (Kitching et al., 2009; Wallensten et al., 2009) report on surveys of treatment adherence and adverse events associated with the prophylactic use of oseltamivir for H1N1 influenza in the UK. Wallensten et al. (2009) reported on 248 students (11-12 year olds) who received prophylaxis with oseltamivir. Over three-quarters of children (77.2%) reported that they took the full 10-day course of prophylaxis, while 91.9% reported they took the medication for at least 7 days. Half of the children (50.8%) reported they felt unwell while taking oseltamivir and 50.6% reported at least one symptom compatible with side effects of oseltamivir. Headaches were reported by 24.3% and stomach ache by 21.1%. The report states that although some children were ill with flu-like symptoms, none of the children tested had pandemic H1N1 infection. The proportion of subjects reporting adverse events was considerably higher than that reported in clinical trials (Tappenden et al., 2009), where less than 10% of patients reported adverse events with prophylactic use of oseltamivir.
The survey reported by Kitching et al. (2009) was sent to 256 schoolchildren and 103 (40%) responded. Of the responders, 95 were offered oseltamivir prophylaxis, of which 85 (89%) took any of the drug. Less than half of the primary school children (48%) took a full course, while 76% of secondary school children completed a full course. More than half of all children (53%) reported side effects, with gastrointestinal symptoms reported by 40% of children, nausea by 29%, and mild neuropsychiatric side effects reported by 18%.
Unlike Wallensten (2009), Kitching (2009) found low adherence with prophylaxis. This may be related to the fact that the Wallensten (2009) review was the first school affected by the pandemic (H1N1) 2009 outbreak in the UK and media attention was high at the time. The results of both surveys should be interpreted with caution given that the numbers are relatively small and responses may have been influenced by a number of sources. Both surveys indicated a relatively high proportion of adverse events; however, the severity of these events does not appear to be high.
2.2. Use of zanamivir – chemoprophylaxis
Systematic review/clinical trial evidence – seasonal influenza
There are no new trials available addressing the prophylactic use of zanamivir. The updated Jefferson (2009) review reported that the two trials of prophylactic use of zanamivir in adults demonstrated a reduction in the likelihood of symptomatic laboratory-confirmed influenza (RR=0.38; 95% CI: 0.17, 0.85; see Table A5.6, Annex 5). The trials did not support or refute the impact of zanamivir on ILI (RR=1.51; 95% CI: 0.77, 2.95; see Table A5.6, Annex 5). Two trials assessing post-exposure prophylaxis demonstrated significant protection for households.
The Shun-Shin (2009) review in children reported that two trials of post-exposure prophylactic zanamivir were associated with a reduction in the risk of developing confirmed symptomatic influenza following introduction of an index case in the household (RD=-0.07; 95% CI: -0.12, -0.02; RD=-0.08; 95% CI: -0.14, -0.03). When the zanamivir and oseltamivir trials were pooled, the absolute risk reduction was 8% (RD=-0.08; 95% CI: -0.12, -0.05).
The systematic review by Khazeni (2009) (described in Section 2.1 above) reported a decreased risk of the incidence of symptomatic influenza with zanamivir prophylaxis (RR=0.256; 95% CI: 0.179, 0.367; see Table A5.9, Annex 5), with no significant advantage for zanamivir for asymptomatic influenza (RR=1.402; 95% CI: 0.900, 1.983). There was no statistically significant difference between zanamivir and placebo in the occurrence of serious adverse events (RR=0.952; 95% CI: 0.525, 1.728).
The results of the recent reviews concur with those in the Tappenden review (2009) presented in the August 2009 Guidelines, which demonstrated a statistically significant benefit for zanamivir prophylaxis compared to placebo in all populations (except for the elderly), with protective efficacy ranging from 70% to just over 80% (see Tables A5.12-A5.13, Annex 5).
2.3. Use of amantadine - chemoprophylaxis
Systematic review/clinical trial evidence – seasonal influenza
There are no new trials or reviews addressing chemoprophylactic use of amantadine for influenza. The Tappenden review (2009) assessed the use of amantadine for chemoprophylaxis of influenza A, only reporting individual trial results, given the between-trial heterogeneity. Amantadine demonstrated advantages in post-exposure chemoprophylaxis; however, the authors state that the results should be interpreted with caution given the age and quality of the amantadine trials. The occurrence of adverse events was usually similar between amantadine and placebo, however two trials demonstrated a greater occurrence of adverse events in amantadine-treated patients, with severe adverse effects more frequent for those given amantadine chemoprophylaxis compared to placebo.
2.4. Use of rimantadine - chemoprophylaxis
Systematic review/clinical trial evidence – seasonal influenza
As for amantadine, there are no new trials or reviews addressing chemoprophylactic use of rimantadine. The data provided in the Jefferson (2006) review and the Alves Galvao (2008) review directionally favour rimantidine compared to placebo, with protective efficacy of 70% in adults and 50% in children. However, the results were not statistically significant. Assessment of the occurrence of adverse events in the Jefferson (2006) review revealed a statistically significant increase with rimantadine compared to placebo.
Footnotes
- 1
Updated January 2010.
- Summary of Evidence for Benefits and Harms - WHO Guidelines for Pharmacological ...Summary of Evidence for Benefits and Harms - WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and Other Influenza Viruses
Your browsing activity is empty.
Activity recording is turned off.
See more...