2.1. ESTABLISHMENT OF THE GUIDELINE DEVELOPMENT GROUP
A Guideline Development Group (GDG) was established in June 2012 comprising members working on sexual health in low- and middle-income countries, from all WHO regions and with equal gender representation. The GDG included academics, psychologists, doctors, public health specialists, lawyers and social scientists, all with expertise in developing programmes or offering clinical services to promote sexual health and well-being. It also included representatives of key constituencies with overlapping sexual health and rights expertise. They were from organizations focused on the rights of women, men who have sex with men, and transgender persons. See Annex 2 for the names of those who participated in this guideline document development process.
2.1.1. Declaration of interest by Guideline Development Group members and peer reviewers
All GDG members completed a declaration of interests form. These forms were reviewed by the responsible officer at WHO, Igor Toskin, Medical Officer, Department of Reproductive Health and Research, before finalization of the group composition and invitation to attend the first GDG meeting. All non-WHO participants signed and submitted a Declaration of Conflict of Interest form. One potential conflict was declared. Dr Marlene Wasserman, DHS Clinical Sexologist, South Africa, declared being contracted by pharmaceutical companies Adcock Ingram, AstraZeneca, Bayer, Lilly, Novartis and Pfizer prior to the time of the GDG establishment, but this was assessed by the WHO Secretariat, presented to the meeting participants, and deemed not significant enough to preclude Dr Wasserman's participation in the consultations and in the process of formulating the recommendations. For the others, it was agreed that there was no conflict of interest. The peer reviewers also submitted a declaration of interest form, and these were similarly reviewed before their selection was finalized. Procedures for management of conflicts of interest were based on the WHO handbook for guideline development (150).
2.2. IDENTIFYING, APPRAISING AND SYNTHESIZING THE AVAILABLE EVIDENCE
No external funding for this guideline was obtained. WHO funded this guideline document's development entirely.
2.2.1. The GRADE framework
WHO follows the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach for the development and review of recommendations (150). This approach is increasingly being adopted by organizations worldwide to rate the quality of evidence and strength of various types of recommendations (61). GRADE emphasizes a structured, explicit and transparent approach to grading and consensus-building (64).
GRADE separates the rating of the quality of evidence from the grading of the recommendation. In the context of recommendations, quality reflects the confidence that the estimates of effect are adequate to support a particular recommendation (9). The GRADE system classifies the quality of evidence into one of four levels: high, moderate, low or very low (58, 59, 60, 62, 63).
The strength of a recommendation reflects the extent to which we can be confident that the desirable effects of an intervention outweigh the undesirable effects (64). The GRADE system classifies recommendations into two strengths: strong and conditional. A recommendation can also be either in favour of or against the intervention of interest. The strength and direction of a recommendation are affected by the quality of evidence, balance of benefits and harms, values and preferences, resource use and feasibility of the intervention.
One good practice recommendation is also included in the framework. This is a type of recommendation that does not require supporting evidence (see Chapter 3) and thus its development does not follow the above-described process (57).
2.2.2. Search strategy
An independent researcher conducted a systematic review based on the population, intervention, comparator and outcomes (PICO) questions. The following electronic databases were searched: PubMed, ProQuest, Cumulative Index to Nursing & Allied Health (CINAHL), Jstor, Scopus/Science Direct, Cochrane Library, EBSCO, PsychINFO and Web of Knowledge. The search was reformatted from a Medical Subject Headings (MeSH)-based approach to a keyword search in order to focus on other databases and increase the number of unique citations. Keyword searches on Summon (covering ProQuest, CINAHL, Jstor, Scopus/Science Direct, Cochrane Library, EBSCO, CINAHL, Ovid Medline/PubMed, PsycINFO and Web of Knowledge) were performed using the following terms: sexual health, primary care, counselling, sexual dysfunction, sexual distress, sexual concerns, sexual misconceptions, STIs, HIV, unintended pregnancy, abortion, sexual violence, harmful practices, knowledge increase, well-being, autonomy, pleasure and training. No language or date restrictions were applied. Reference sections of included articles were also searched. Grey literature was retrieved from New York Academy of Medicine Grey Literature Report. Both published and unpublished articles were searched.
2.2.3. Study selection
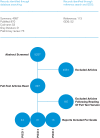
Studies were included that examined elements, outcomes and/or techniques of brief communication or counselling interventions within a public health or primary care setting and which sought to prevent or address sexual difficulties, concerns, distress and/or misconceptions; STI/HIV; unintended pregnancy and abortion; sexual violence; harmful practices; and sexual health knowledge. Articles were also considered that examined the elements, outcomes and/or techniques of brief interventions that encourage sexual well-being. Finally, other articles considered described the elements of training programmes for primary health-care providers to increase their knowledge and skill in sexuality counselling and communication. Abstracts were reviewed by two independent readers and one WHO reader (see ). For those articles that were considered relevant, full-text versions were retrieved. Studies were included that satisfied the requirements for the PICO criteria described in Annex 2. Criteria for the first two PICO questions required that the research was a controlled study and the intervention was between 15 and 60 minutes and could feasibly occur at the primary care level. For the third PICO question, the requirement was any evaluated training or sensitization intervention with the aim of improving primary health-care providers' ability to communicate about sexual health-related issues. Data from articles were extracted for setting, population, types of study, randomization, blinding, intervention, comparator, outcome and results. The evidence was assessed by GDG according to the outcomes.
SYSTEMATIC REVIEW SELECTION PROCESS.
The systematic review sought evidence of whether BSC was an effective approach – that is, whether there is a rationale for BSC. It did not assess the strengths and weaknesses of different techniques of BSC, but rather its overall effectiveness in improving sexual health services outcomes. The GDG saw this process as a necessary first step before specific clinical techniques of BSC could be assessed. The next step would be the development of a technical clinical guideline for BSC, based on a systematic review of the effectiveness of different BSC techniques.
2.3. THE PROCESS OF DEVELOPING THIS GUIDELINE DOCUMENT
The first face-to-face meeting of the GDG was conducted on 10–12 October 2012. The main outcomes of the meeting were decisions on:
- →
the scope of this guideline document;
- →
the use of PICO questions to govern the systematic search of the evidence (see Annex 2), and the evidence-retrieval strategy; and
- →
the modus operandi of the group and a common understanding of the process for the development of a guideline according to WHO requirements.
The GDG held several telephone conferences during this process as well as email conversations. These enabled the WHO Secretariat to finalize the outcomes in relation to each PICO question. Group members then scored the relative importance of each outcome on a scale from 1 to 9, where 7–9 indicates that the outcome is critical to a decision, 4–6 indicates that it is important, and 1–3 indicates that it is of low importance for decision-making. The average score for each outcome was used to determine the relative importance of each outcome. Outcomes and ratings are presented in Annex 3.
The GDG had its second meeting on 10–12 July 2013 to develop recommendations based on the available evidence as well as on the group's own technical expertise by following the GRADE process. The GDG set the evidence into context, considering: the relative benefits and harms of possible recommendations; the likely values and preferences of health-care providers and clients, as well as human rights standards such as the rights to information, respect and dignity; costs and resource use, as well as other relevant feasibility issues of providers, including in low- and middle-income settings and diverse social and cultural contexts.
The recommendations were shaped with a consideration of the diversity of target groups for this guideline document, including health-care policy-makers and decision-makers in health training institutions. Agreements were reached by unanimous consensus. If the group could not reach consensus, voting was conducted by hand raising (hence not anonymous), and a simple majority rule was applied.
Where there was a need for guidance but only low- to very low-quality research evidence was available, a recommendation was developed using the expertise of the GDG and the considerations given above (Recommendation 2).
The decisions of the GDG were then used to draft this guideline document. The first draft was reviewed by the GDG. All comments were collated by the Secretariat, with each comment reviewed and responses added to the comments in a table format. Relevant changes were then made to the document before the revised version was sent back to the members of the GDG for final review.
2.4. DOCUMENT PREPARATION AND PEER REVIEW
A second draft of the BSC guideline was reviewed by GDG members and peer reviewers of the various constituencies with a direct interest in this guideline document. They are listed in Appendix 2. Peer reviewers indicated that they found this guideline document relevant, appropriate and timely. Relevant revisions suggested by the GDG and peer reviewers and agreed upon by the Secretariat were made. The Guideline Review Committee subsequently reviewed this document and further revisions were made.