Abstract
Cardiovascular diseases are caused by pathological cardiac remodeling, which involves fibrosis, inflammation and cell dysfunction. This includes autophagy, apoptosis, oxidative stress, mitochondrial dysfunction, changes in energy metabolism, angiogenesis and dysregulation of signaling pathways. These changes in heart structure and/or function ultimately result in heart failure. In an effort to prevent this, multiple cardiovascular outcome trials have demonstrated the cardiac benefits of sodium-glucose cotransporter type 2 inhibitors (SGLT2is), hypoglycemic drugs initially designed to treat type 2 diabetes mellitus. SGLT2is include empagliflozin and dapagliflozin, which are listed as guideline drugs in the 2021 European Guidelines for Heart Failure and the 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America Guidelines for Heart Failure Management. In recent years, multiple studies using animal models have explored the mechanisms by which SGLT2is prevent cardiac remodeling. This article reviews the role of SGLT2is in cardiac remodeling induced by different etiologies to provide a guideline for further evaluation of the mechanisms underlying the inhibition of pathological cardiac remodeling by SGLT2is, as well as the development of novel drug targets.
Keywords: SGLT2 inhibitors, cardiac remodeling, myocardial hypertrophy, cardiac fibroblasts, molecular mechanisms
1. Introduction
In the event of external triggers, cardiac insufficiency responds to adaptive alterations in both the structure and function of the heart, commonly referred to as cardiac remodeling. These alterations include changes in genomic expression levels, cell morphology and abnormal interstitial secretion (1,2). Cardiac remodeling is divided into physiological and pathological types. Physiological cardiac remodeling is a reversible adaptive reaction that primarily occurs during growth, exercise and pregnancy (3). Where pathological cardiac remodeling is an irreversible adaptive response caused by numerous conditions, including myocardial infarction (MI), ischemia/reperfusion (I/R) injury, pressure loading, inflammation and oxidative stress (4,5). Direct manifestations of cardiac remodeling include myocardial hypertrophy and cardiac fibrosis and continued poor remodeling can lead to heart failure (6–8). Thus, determining the mechanisms that lead to cardiac remodeling and preventing undesirable remodeling is essential.
Sodium-glucose cotransporter type 2 inhibitors (SGLT2is) are hypoglycemic medications that inhibit SGLT2 in the renal tubules, decreasing glucose reabsorption, lowering the renal glucose threshold and initiating glucose excretion in the urine (9). Compared with other traditional hypoglycemic drugs, SGLT2is are primarily used for treating type 2 diabetes but have also been reported to exert cardiovascular benefits. According to cardiovascular outcome studies, SGLT2is reduce the incidence of hospitalization due to heart failure (10–14). The ‘new tetrad’ of cornerstone heart failure medications has replaced the original ‘golden triangle’ and now includes beta-blockers, aldosterone receptor antagonists, renin-angiotensin system inhibitors and SGLT2is (15,16).
The four SGLT2 inhibitors widely used in clinical treatment are Empagliflozin (EMPA), Dapagliflozin (DAPA), Canagliflozin (CANA) and Ertugliflozin; these have been approved by the US Food and Drug Administration and the European Medicines Agency (17). EMPA and DAPA are widely used for heart failure prevention and treatment (15,16). Existing studies have confirmed the lack of SGLT2 expression in cardiac tissue, thereby necessitating the investigation of the myocardial protective effect of SGLT2is (18). It has been suggested that SGLT2is exerts a diuretic effect via glomerular reabsorption, reducing blood volume and cardiac load and protecting the heart by reducing myocardial oxygen consumption. However, as this diuretic effect is dependent on blood glucose concentration, the cardiac benefit in non-diabetic patients has not been determined (19). Therefore, the protective effect of SGLT2is on the myocardium may be exerted directly on the heart. Several studies have shown that the anti-heart failure effect of SGLT2is may be mediated by inhibiting or reversing cardiac remodeling (20–22).
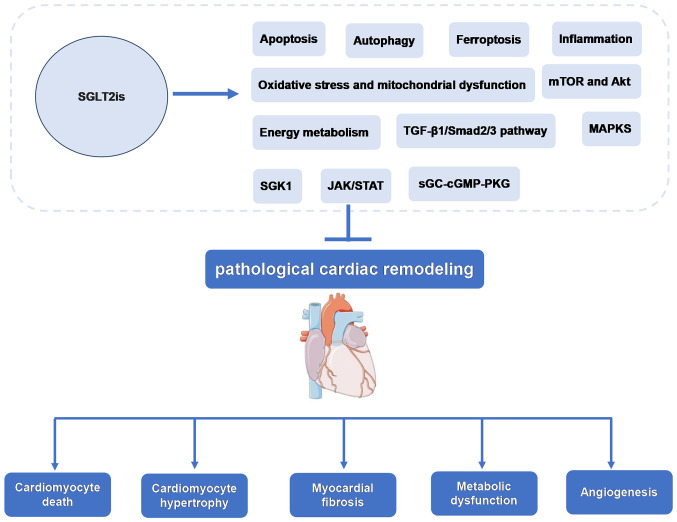
Currently, the molecular mechanisms and signaling pathways of SGLT2is in cardiac remodeling are being investigated. The present review provides a foundation and supports the investigation of novel mechanisms of cardiac remodeling and heart failure, as well as the development of novel drug targets based on the function and molecular mechanisms of SGLT2is-mediated inhibition of pathological cardiac remodeling (Fig. 1).
Figure 1.
Possible role and mechanism of SGLT2is in inhibiting pathological cardiac remodeling. SGLT2is, sodium-glucose cotransporter type 2 inhibitors; JAK, Janus kinase; STAT, signal transducer and activator of transcription; SGK1, Serum/glucocorticoid regulated kinase 1; sGC, soluble guanylate cyclase enzyme; cGMP, cyclic guanosine monophosphate; PKG, cGMP-dependent protein kinase.
2. Effects of SGLT2is on cardiac structure and function
Pathological cardiac remodeling is often manifested by changes in the morphology and size of the left ventricle. In addition, the left ventricular (LV) mass index (LVMI) and LV ejection fraction (LVEF) are used as evaluation indexes of cardiac structural function (23,24). Studies have reported that SGLT2isThe improved cardiac function is mainly manifested as increased LVEF and decreased LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), left atrial volume index (LAVI) and LVMI (22,25–35). However, LV diastolic dysfunction (LVDD) often manifests as altered LV diastolic filling and is assessed based on the peak mitral E wave velocity to early mitral or septal annular tissue Doppler velocity ratio (E/e'), peak mitral E wave velocity to A wave velocity ratio and LAVI (36). SGLT2is also improved ejection fraction, LVEDV, LVESV and diastolic dysfunction in animal models of heart failure (37–46).
Regarding cardiac structure, the LV mass (LVM), LV wall thickness and LV wall thickness-to-cavity radius can be used to determine the structural and morphological changes of the LV. Specifically, increased LVM has been considered a marker of clinical LV hypertrophy (47). Several studies have demonstrated that the cardiovascular benefits of SGLT2is may be achieved through reduced LVM, as it occurs without a decline in the volume, which reflects the decrease in ventricular wall thickness (26,29,30,48). However, the mechanisms of decreasing wall thickness are yet to be elucidated. In the present review, the effects of SGLT2is on cardiac structure and function in patients with cardiovascular disease and animal models were summarized (Tables I and II).
Table I.
Effects of SGLT2 inhibitors on cardiac structure and function in patients with cardiovascular disease.
| Author(s), year | Clinical trial number/type | Patient characteristics | SGLT2i | Dose and action time | Diagnostic method | Outcomes | (Refs.) |
|---|---|---|---|---|---|---|---|
| Soga et al, 2018 | UMIN000019789 | Patients with type 2 | DAPA | 5 mg, qd; 6 months | Echocardiography | LAVI↓; LVMI↓; | (29) |
| diabetes mellitus with | E/e'↓ | ||||||
| chronic heart failure | |||||||
| (n=58) | |||||||
| Verma et al, 2019 | NCT02998970 | Patients with type 2 | EMPA | 10 mg, qd; 6 months | CMRI | LVM↓; LVEF↑; | (26) |
| diabetes mellitus and | LVMI↓ | ||||||
| coronary artery disease | |||||||
| (n=49) | |||||||
| Brown et al, 2020 | NCT02956811 | Left ventricular | DAPA | 10 mg, qd; 12 months | CMRI | LVM↓ | (30) |
| hypertrophy in indivi- | |||||||
| duals with type two | |||||||
| diabetes (n=66) | |||||||
| Santos- | NCT03485222 | Non-diabetic patients | EMPA | 10 mg, qd; 6 months | CMRI | LVM↓; LVEF↑; | (25) |
| Gallego et al, | with heart failure and | LVEDV↓; | |||||
| 2021 | reduced ejection fraction | LVESV↓ | |||||
| (n=48) | |||||||
| Lee et al, 2021 | NCT03485092 | Left ventricular volumes | DAPA | 10 mg, qd; 36 weeks | CMRI | LVEF↑; LVMI↓; | (22) |
| in patients with type 2 | LVESVI↓; | ||||||
| diabetes, or prediabetes | LVEDVI↓; | ||||||
| and heart failure with | LAVI↓ | ||||||
| reduced ejection fraction | |||||||
| (n=105) | |||||||
| Omar et al, 2021 | NCT03198585 | Patients with heart failure | EMPA | 10 mg, qd; 12 weeks | Echocardiography | LVEF↑; LVESVI↓; | (27) |
| with reduced ejection | LVEDVI↓; | ||||||
| fraction (n=95) | LAVI↓ | ||||||
| Lan et al, 2021 | Prospective | Acute coronary syndrome | EMPA | 10 or 25 mg, qd; | Echocardiography | LVMI↓; LAVI↓; | (31) |
| observational | in patients with type 2 | after 3–6 months | E↓; E/e'↓ | ||||
| study | diabetes (n=44) | ||||||
| von | NCT03087773 | Patients with acute MI | EMPA | 10 mg, qd; 32 weeks | Echocardiography | LVEF↑; E/e'↓; | (32) |
| Lewinski et al, | (n=800) | LVEDV↓; | |||||
| 2022 | LVESV↓; | ||||||
| NT-proBNP↓ | |||||||
| Ersbøll et al, | The SIMPLE | Patients with type 2 | EMPA | 25 mg, qd; 13 weeks | Echocardiography | LVMI↓; E/e'↓ | (33) |
| 2022 | randomized | diabetes at high | |||||
| clinical trial | cardiovascular risk | ||||||
| (n=45) | |||||||
| Palmiero et al, | A pilot prospective study. | Patients with type 2 | EMPA | 10 mg, qd; 6 months | Echocardiography | LVEF↑; LV-GLS↑ | (34) |
| 2023 | GLISCAR study | diabetes mellitus and | |||||
| reduced ejection fraction | |||||||
| heart failure (n=31) | |||||||
| Russo et al, 2023 | Real World Study | Patients with type 2 | EMPA/ | 10 mg, qd; 6 months | Echocardiography | LVEF↑; LV-GLS↑ | (35) |
| diabetes mellitus (n=35) | DAPA |
CMRI, cardiac magnetic resonance imaging; qd, quaque die; LAVI, left atrial volume index; LVMI, left ventricular mass index; E/e', ratio peak early diastolic mitral velocity to mitral annulus early diastolic velocity; LVM, left ventricular mass; LVEF, left ventricular ejection fraction; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; E, early diastolic filling velocity; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LV-GLS, LV global longitudinal strain; SGLT2is, sodium-glucose cotransporter type 2 inhibitors; EMPA, Empagliflozin; DAPA, Dapagliflozin.
Table II.
Effects of SGLT2is on cardiac structure and function in animal models.
| Author(s), year | Animal model | SGLT2i | Dose and action time | Diagnostic method | Outcome | (Refs.) |
|---|---|---|---|---|---|---|
| Habibi et al, 2017 | Diabetic models in female rodents | EMPA | 10 mg/kg/day orally for 5 weeks | Echocardiography | E/e'↓; | (40) |
| Zhang et al, 2019 | Pig model of heart failure with preserved ejection fraction | DAPA | Oral administration of 10 mg/day was started at the 9th week from the beginning of modeling for 9 weeks | Echocardiography | LVMI↓ | (41) |
| Lee et al, 2019 | Hypertensive heart failure rats | EMPA | 20 weeks after modeling began, 12 mg/kg/day was administered for 12 weeks | Echocardiography | Ves↓; Ved↓; +dP/dt↑; -dP/dt↓ | (42) |
| Santos-Gallego et al, 2019 | Pig model of non-diabetic heart failure | EMPA | After modeling, 10 mg/kg/day was administered orally for 2 months | CMRI and echocardiography | LVEDV↓; LVESV↓; LVM↓; LVEF↑; GLS↑ | (38) |
| Yurista et al, 2019 | Rat model of MI induced by coronary artery ligation | EMPA | Oral doses of 30 mg/kg/day were started 2 days before surgery (early stage) or 2 weeks after surgery (late stage) | Echocardiography | LVEF↑; LVM↓ | (39) |
| Lahnwong et al, 2020 | Acute myocardial ischemia/reperfusion injury rat model | DAPA | 1 mg/kg was administered before ischemia, during ischemia and at the beginning of reperfusion | Echocardiography | LVEF↑ | (43) |
| Kräker et al, 2020 | A novel rodent model of heart failure induced by combined hypertension and diabetes | EMPA | 10 mg/kg/day for 4 weeks after modeling | Echocardiography | LVEF↑; LVFS↑; GLS↑ | (44) |
| Santos-Gallego et al, 2021 | A 2-h balloon occlusion of the proximal left anterior descending branch induced diastolic dysfunction in non-diabetic heart failure model pigs | EMPA | 10 days after MI, 7 mg/day for 2 months | CMRI and echocardiography | E/e′↓; EDPVR↓; LVEDP↓ | (45) |
| Lin et al, 2021 | Myocardial dysfunction induced by mitral regurgitation in rats | DAPA | Two weeks after surgery, 10 mg/kg/day for 6 weeks | Echocardiography | Ves↓; Ved↓; LVIDd↓; LVEF↑; LVFS↑; +dP/dt↑ | (46) |
| Zhang et al, 2021 | Angiotensin II-induced cardiac fibrosis in rats | DAPA | Oral administration of 5 mg/kg/day for 4 weeks | Echocardiography | LVEF↓; LVFS↓; e′↑; E/e′↓; GLS↓ | (178) |
Ves, end-systolic volume; Ved, end-diastolic volume; +dP/dt, maximal velocity of pressure rise; -dP/dt, maximal velocity of pressure fall; LVIDd, left ventricular internal dimension at end-diastole; EDPVR, end-diastolic pressure volume relationship; LVEDP, left ventricular end-diastolic pressure; SGLT2is, sodium-glucose cotransporter type 2 inhibitors; CMRI, cardiac magnetic resonance imaging; GLS, global longitudinal strain; EMPA, Empagliflozin; DAPA, Dapagliflozin.
3. Effects of SGLT2is on myocardial hypertrophy in cardiomyocytes and cardiac fibrosis in cardiac fibroblasts
Pathological cardiac remodeling causes hypertrophy of cardiomyocytes and the proliferation of non-cardiomyocytes in numerous cardiovascular diseases, including hypertension, diabetic cardiomyopathy, aortic stenosis, MI, pathological stimulation, cardiomyocyte hypertrophy and cardiac fibrosis (3). The characteristics of cardiac hypertrophy are abnormal size and function of myocardial cells, often manifested as increased ventricular mass, myocardial cell volume and expression of fetal genes, such as atrial natriuretic peptide, brain natriuretic peptide and β-myosin heavy chain (49). Myocardial fibrosis, the excessive deposition of extracellular matrix, is closely associated with the severity of myocardial fibrosis. Type I collagen is the most abundant structural protein (50,51). Myocardial fibroblasts are the main cellular effectors that lead to cardiac fibrosis. Pathological stimuli can reduce the number of cardiomyocytes, which in turn stimulates inflammation. In order to compensate for the loss of cardiomyocytes, cardiac fibroblasts proliferate and differentiate into myofibroblasts, leading to scar formation (52).
Cardiac hypertrophy and cardiac fibrosis are the major pathological processes in cardiac remodeling and are closely related to the prognosis of cardiovascular diseases, making them the primary intervention targets for heart failure (53,54). Several studies have demonstrated that SGLT2is attenuates or inhibits cardiomyocyte hypertrophy and cardiac fibrosis by regulating multiple signaling pathways in numerous models, such as transverse aortic constriction (TAC), left coronary artery ligation MI and diabetes (39,40,55–63).
4. Role of SGLT2is in cellular pathophysiological processes
Apoptosis
Apoptosis is a type of programmed cell death that serves a key role in embryonic development and tissue homeostasis (64). Apoptosis is mediated by death receptors, also known as extrinsic apoptotic pathways and mitochondria, also called intrinsic apoptotic pathways, both of which can activate cysteine-dependent proteases (caspases) (65). Apoptosis serves a crucial role in the development of the heart and is associated with the occurrence and development of numerous cardiovascular diseases. Studies have reported that apoptosis is a pathological feature of MI and heart failure, and that the inhibition of apoptosis can prevent and treat post-MI remodeling and heart failure (66).
Further studies have reported that SGLT2is reduces cardiac remodeling and improves cardiac function by inhibiting the apoptosis pathways. EMPA inhibits cardiomyocyte apoptosis and improves cardiac remodeling in early MI in non-diabetic mice (67).
In mice with autoimmune myocarditis induced by α-myosin-heavy chain peptides, CANA markedly reduces the Bax/Bcl-2 ratio and the level of cleaved caspase-3 protein, followed by inhibition of apoptosis, which was reported to improve myocarditis (68). In cardiac I/R rats, DAPA-induced pre-ischemia upregulated the levels of anti-apoptotic protein Bcl-2 to protect cardiomyocytes from apoptosis, thereby alleviating cardiac mitochondrial dysfunction by reducing reactive oxygen species (ROS) production (43). DAPA mediates the cardioprotective effect in diabetic rats by activating the phosphorylation of Akt, JAK2 and MAPK signaling cascades, increasing the erythropoietin levels and reducing apoptosis (69). DAPA also normalizes mitochondrial fission and reduces cardiomyocyte apoptosis by activating the phosphoglycerate mutase member 5 (PGAM5)/dynamin-related protein 1 (Drp1) signaling pathway, thereby improving cardiac remodeling after acute MI (70). However, this study did not use an agonist for the PGAM5/Drp1 pathway, so the relationship between DAPA and the PGAM5/Drp1 signaling pathway could not be assessed.
A recent study reported that in Doxorubicin-induced cardiac dysfunction, DAPA decreased the cardiac expression of Bax and cleaved caspase-3, but increased the expression of Bcl-2, as well as signal transducer and activator of transcription 3 (STAT3) that was subsequently inhibited by Doxorubicin (71). These findings indicate that DAPA activates the expression of sirtuin1 (SIRT1), which inhibits the protein kinase RNA-like endoplasmic reticulum (ER) kinase (PERK)-eukaryotic translation initiation factor 2α (eIF2α)-C/EBP homologous protein signaling pathway of the ER stress response in angiotensin II (Ang II)-treated cardiomyocytes to reduce cardiomyocyte apoptosis and improve TAC-induced cardiac remodeling in mice (72). Therefore, it may be suggested that SGLT2is improves damaged cardiac function by continual myocardial cell apoptosis.
Many of the mechanisms are effectuated through the mitochondrial pathway. However, only a small number of studies have assessed the role of SGLT2is in myocardial cell apoptosis induced by the death receptor pathway. Hence, this mechanism should be evaluated to understand the anti-apoptotic mechanism of SGLT2is.
Autophagy
Autophagy is a process that degrades and recirculates damaged organelles, misfolded proteins and other macromolecules through lysosomal-dependent pathways to maintain cell homeostasis and function (73). Previous studies have shown a crucial role of basal autophagy in cardiac development and in the maintenance of normal cardiac function (74–77). However, insufficient or excessive autophagy can affect the development of pathological cardiac remodeling (78–80). Furthermore, the activation of autophagy leads to the death of cardiomyocytes in MI and I/R injury and has been shown to have a dual effect in numerous research models, which may be related to the Beclin 1 (BECN1) or AMPK-mammalian target of rapamycin (mTOR) pathways (81–83). Several studies have demonstrated that SGLT2is exerts cardioprotective effects through the activation or inhibition of autophagy.
In mouse models of coronary artery ligation-induced diabetic and non-diabetic MI, EMPA-treated mice demonstrated a significant decrease in cardiomyocyte death due to excessive autophagy, which reduced autophagic flux by targeting the Na+/H+ exchanger 1 (NHE1) on cardiomyocytes (63). EMPA exerts myocardial protective effects through mitochondrial autophagy and the novel BECN1-Toll-like receptor (TLR)9-SIRT3 axis (84). Furthermore, EMPA exerts cardioprotective effects in non-diabetic mice with MI with acute hyperglycemia by suppressing beclin 1 (BCN1)-dependent autophagy rather than targeting NHE1 in cardiomyocytes (85). Previous studies have demonstrated that BCN1 promotes the crosstalk between apoptosis and autophagy (86). In another study, EMPA was reported to inhibit ER stress-induced autophagy by inhibiting the PERK/activating transcription factor 4/BCN1 signaling pathway, thereby alleviating myocardial I/R injury and cardiomyocyte apoptosis (87). Furthermore, overexpression of p62 and light chain 3II/I activates autophagy when EMPA is administered. It reduces cardiac lipid toxicity in Zucker diabetic fatty (ZDF) rats (88).
Likewise, DAPA represses cardiac remodeling and hypoxia-induced apoptosis in heart failure through the activation of autophagy via the AMPK/mTOR pathway (89). It also protects against myocardial I/R injury by limiting NLR family pyrin domain containing 3 (NLRP3) inflammatory vesicle activation and regulating autophagy (90). The dose of DAPA administered in this study was 40 mg/kg/day, which is 20X higher compared with the allometric-adapted dose used in human clinical trials. It cannot be ruled out that the final result is related to high doses. SGLT2is exert cardioprotective effects through the activation and inhibition of autophagy via the interference of varied pathological conditions and detection time. SGLT2is regulate autophagy through the AMPK pathway, ER stress and inflammasomes. These pathways also regulate the processes of cell apoptosis, inflammation and angiogenesis. However, the association between these pathological processes and autophagy or the precise mechanisms of SGLT2is are yet to be elucidated.
Ferroptosis
Ferroptosis is a form of programmed cell death different from cell apoptosis, cell necrosis and cell autophagy. It is mediated by iron-dependent lipid peroxides and characterized by reduced intracellular glutathione (GSH) expression, reduced GSH peroxidase 4 (GPX4) activity and the accumulation of ROS and lipid peroxides (91–93). Several studies have shown the role of ferroptosis in numerous cardiovascular diseases, such as cardiomyopathy, MI, myocardial I/R injury, atherosclerosis and heart failure (94–98). Furthermore, SGLT2is exert cardioprotective effects through the ferroptosis pathway. In model rats, CANA can treat heart failure with preserved ejection fraction (HFpEF) by reducing iron intake and iron overload, reducing lipid peroxidation, increasing GSH production and inhibiting oxidative stress to regulate ferroptosis (99).
Furthermore, advanced glycation end-products inhibit the expression of solute carrier family 7 member 11 and ferritin in diabetic cardiomyopathy and reduce GSH levels. This elevates lipid peroxidation levels and ferroptosis, which in turn triggers cardiac inflammation and cardiac remodeling, including cardiomyocyte hypertrophy, pro-fibrotic response, fibrosis and ultimately cardiac dysfunction (100). Another study reported that CANA may reduce ferroptosis and improve myocardial oxidative stress in diabetic cardiomyopathy mice by regulating iron metabolism and the systemic cystine-glutamate antiporter (Xc−)/GSH/GPX4 axis (101). However, the relationship and specific mechanism of CANA in regulating iron metabolism and the Xc−/GSH/GPX4 axis require further evaluation. In addition, CANA has been reported to inhibit inflammation and ferroptosis through the activation of the AMPK pathway, thereby reducing lipotoxicity in cardiomyocytes (102).
Furthermore, EMPA prevents DNA oxidation and ferroptosis in trastuzumab-induced C57BL/6J mice, which attenuates cardiotoxicity (103). Likewise, DAPA suppresses the MAPK signaling pathway in a model of myocardial I/R injury, reducing ferroptosis and exerting protective benefits on the heart (104).
Inflammation
Inflammation is a leading factor affecting cardiac remodeling and the progression of heart failure (105,106). Toll-like receptors (TLRs), a family of transmembrane receptors, are recognized by danger-associated molecular patterns (DAMPs) in MI and activate nuclear factor-B (NF-κB), which in turn activates a cascade of inflammatory mediators, including cell adhesion molecules, chemokines, and inflammatory cytokines (107,108). Furthermore, the inflammasome, which are polymeric protein structures, form molecular platforms that are activated when cells are infected or stressed, stimulates the inflammatory response by activating several inflammatory cytokines, such as IL-1 and IL-18 (109). Thus, targeting specific cytokines, growth factors or inflammatory pathways could alleviate adverse cardiac remodeling.
Proinflammatory cytokines: TNF-α, IL-1 and IL-6
The activation of multiple pro-inflammatory cytokines, such as TNF-α, IL-1 and IL-6, mediates cardiac remodeling through their effects on cardiomyocytes, fibroblasts and immune cells (110). These cytokines can induce cardiomyocyte hypertrophy and apoptosis (111–113). Pro-inflammatory cytokines enhance the activity of matrix metalloproteinases and decrease the production of extracellular matrix (ECM) components in fibroblasts, which causes the ECM to degrade (114–116). Thus, pro-inflammatory cytokines serve a role in pathological cardiac remodeling. Furthermore, the pleiotropic anti-inflammatory factor IL-10 decreases the expression of TNF-α, IL-1 and IL-6 to reduce cardiac inflammation (117,118).
Furthermore, SGLT2is downregulates pro-inflammatory cytokines and improves cardiac function in cardiovascular diseases. DAPA decreased the levels of inflammatory cytokines IL-6 and TNF-α in HFpEF pigs administered deoxycorticosterone acetate and Ang II to construct an ejection fraction-preserving heart failure model (41). Likewise, EMPA decreased TNF-α and IL-6 levels in patients with HfpEF and ZDF obese rats, reduced inflammation and enhanced myocardial function (119). Furthermore, EMPA markedly decreased the level of TNF-α and reduced myocardial fibrosis in hypertensive heart failure rats (42). DAPA decreased the levels of the pro-inflammatory cytokines IL-1, IL-6 and TNF-α in viral myocarditis mice infected with Coxsackievirus B3. DAPA facilitated macrophage polarization through STAT3-related pathways to reduce myocarditis (120). Furthermore, DAPA improves cardiac hypertrophy in streptozocin-induced type 2 diabetic rats by inhibiting the nuclear translocation of NF-κB and reducing the expression of calpain-1 in cardiomyocytes, decreasing IL-6 and TNF-α levels and upregulating IL-10 levels (58). In addition, DAPA regulates malondialdehyde, TNF-α and ROS levels by blocking the C-X3-C motif chemokine ligand 1/receptor 1 axis and NF-κB activity, thereby reducing lipopolysaccharide-induced inflammation and oxidative stress (121). However, this study was performed in vitro using H9C2 cells and requires validation in patients.
NLRP3 inflammasome
The NLRP3 inflammasome accelerates the process of fibrosis by stimulating the production of proinflammatory cytokines IL-1β and IL-18 (122). Several factors, including MI, stress, obesity, diabetes and metabolic syndrome, activate the NLRP3 inflammasome and promote inflammation (123–125). DAPA exerts anti-inflammatory effects on the development of diabetic cardiomyopathy in type 2 diabetic mice by decreasing the expression of NLRP3 inflammasome, IL-1β, IL-6 and TNF-α (126,127). Likewise, EMPA inhibits cardiac fibrosis and inflammation in non-diabetic mice treated with Doxorubicin via the NLRP3 and MyD88 signaling pathways and inhibition of NLRP3 and NF-κB inhibits the pro-inflammatory cytokine storms in Doxorubicin-treated cardiomyocytes (128). Furthermore, DAPA decreases p38-dependent TLR4 expression to prevent NLRP3 activation, which then enhances cardiac function in Doxorubicin-induced dilated cardiomyopathy (129). Finally, CANA reduces type 17 T-helper cell infiltration and protects cardiomyocytes from apoptosis by inhibiting the NLRP3 inflammasome pathway, which reduces myocarditis-induced cardiac inflammation (68).
Macrophages also serve a role in the inflammatory response during cardiac remodeling (130). SGLT2is reduce cardiac fibrosis by regulating macrophage M2 polarization in infarcted rat hearts via the STAT3 signaling pathway (131). Inflammatory and NF-κB signaling pathways are triggered in patients with arrhythmogenic cardiomyopathy (ACM) (132). DAPA reduces cardiac fibrosis and inflammation in ACM mice by reversing hypoxia-inducible factor (HIF)-2α signaling, inhibiting the NF-κB signaling pathway (133). Accumulating evidence indicates that the role of SGLT2is in controlling inflammation is associated with fat reduction, which is efficacious in epicardial adipose tissue (134). Although the aforementioned studies have reported that SGLT2is serve a myocardial-protective role through anti-inflammatory mechanisms, another study on EMPA reported conflicting results; EMPA did not show any effect on the NLRP3 inflammasome pathway or interleukin-1β levels (135). The present review concluded that the effectiveness of SGLT2is in inhibiting inflammation is indeterminate, and more comprehensive information is essential to draw further conclusions.
Oxidative stress and mitochondrial dysfunction
Oxidative stress is a redox imbalance caused by the excessive production of ROS and/or an impaired antioxidant response (136). The primary ROS sources in the heart are mitochondria, NADPH oxidase (NOX), xanthine oxidase (XO) and uncoupled nitric oxide synthase (NOS) (137). A large number of heart cells can be affected by NOX via redox signal transduction. NOX regulates redox-sensitive target proteins to limit the production of ROS (138). Under physiological circumstances, normal ROS signaling controls the growth and maturation of cardiomyocytes, the processing of cardiac calcium, excitatory systolic coupling and vascular tone (139). However, oxidative stress effectuated by a sharp rise in ROS causes cardiac hypertrophy, fibrosis, apoptosis and contractile failure under pathological circumstances (140). Furthermore, oxidative stress is considered a key factor in the development of pathological cardiac remodeling and heart failure, as this disrupts mitochondrial activity by inducing oxidative damage to mitochondrial DNA, RNA, lipids and proteins. Oxidative stress also impairs myocardial cell systolic function by inducing mitochondria-associated oxidative modifications of excitation-contraction-coupled core proteins (141). Several studies have shown that the cardiac benefits of SGLT2is are reduced oxidative stress in vivo and ameliorated mitochondrial dysfunction through multiple signaling pathways.
EMPA improves mitochondrial function by inhibiting mitochondrial fission in type 2 diabetic hearts, as demonstrated by an increase in the expression of mitochondrial fusion-related proteins mitofusin-1 and optic atrophy 1 and the inhibition of DRP1 expression in type 2 diabetic db/db mice and H9C2 cardiomyocytes. In the present study, oxidative stress was reduced by increasing the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream genetic targets (59).
DAPA protects cardiomyocytes from hyperglycemia-induced damage by inhibiting NOX-mediated oxidative stress (142), whereas treatment with EMPA reduces LV hypertrophy and fibrosis after TAC and MI in non-diabetic mouse models and Sprague Dawley (SD) rats with coronary artery ligation-induced oxidative stress. Hypertrophy and fibrosis were improved by upregulating mitochondrial biogenesis, enhancing mitochondrial oxidative phosphorylation, reducing ROS production, attenuating apoptosis and increasing autophagy (39,143). EMPA treatment in diet-induced obese mice reduced cardiac fat accumulation and mitochondrial injury, improved myocardial hypertrophy and cardiac fibrosis and reduced cardiac dysfunction. This effect may be reduced by Sestrin2-mediated AMPK-mTOR signaling and Nrf2/heme oxygenase 1-mediated oxidative stress responses (144). In addition, EMPA inhibited high-fructose diet-induced cardiac dysfunction in type 2 diabetic SD rats by attenuating mitochondria-driven oxidative stress (145). DAPA reduces oxidative stress, mitochondrial dysfunction, fibrosis, hypertrophy and inflammation in Doxorubicin-stimulated rats via inhibition of PI3K/AKT/Nrf2 signaling (61). This study used only male and no female animals, while females may be more sensitive to Doxorubicin and mimic the clinical state.
Energy metabolism
Modifications in myocardial energy metabolism contribute to the development of pathological cardiac remodeling (146). It is often manifested by a switch of the heart back to the fetal genetic program and a shift in preference of metabolic substrate from fatty acids to glucose (147,148). Cardiac remodeling is associated with reduced lipid oxidation capacity and increased glucose dependence (149). Although the conversion of myocardial metabolic substrates from fatty acids to glucose lowers oxygen consumption, it can be detrimental to cardiac performance and aggravate heart failure because of insufficient energy production (150,151). Furthermore, preserving fatty acid oxidation during stress overload prevents the effects of glucose on cardiac remodeling (152,153). Thus, promoting the use of fatty acids and other metabolic substrates to regulate energy metabolism may be a promising therapeutic strategy for heart failure and to improve cardiac remodeling (154).
EMPA reduces excessive glycolysis in TAC-induced cardiac overload mice by binding to glucose transporter (GLUT) proteins, such as GLUT1 and GLUT4, which increases the expression of CD36, restores fatty acid uptake and improves mitochondrial oxidative phosphorylation. The reduced glucose uptake may also lead to an impaired pentose phosphate pathway, which in turn activates AMP-activated protein kinases and blocks mTOR complex 1 (mTORC1) to reduce cardiac hypertrophy (57).
Contrastingly, EMPA has been shown to improve diabetic cardiac remodeling in diabetic cardiomyopathic rats by reducing fatty acid and increasing glucose metabolism (155), although this may be related to increased fatty acids in diabetic heart disease, which leads to lipid toxicity and insulin resistance (146,156), hence the contrasting results reported. EMPA significantly elevated cardiac metabolism and cardiac ATP production in coronary artery-ligated non-diabetic male SD rats by increasing ketone body bioavailability and myocardial oxidation of glucose and fatty acids (39). However, this study did not provide direct evidence that EMPA-treated hearts were associated with increased ketone body oxidation, nor did it quantify the relationship between ketone body oxidation and increased myocardial ATP levels.
Similarly, EMPA altered the myocardial fuel metabolic substrates from glucose to ketone bodies, free fatty acids and branched-chain amino acids in non-diabetic pigs induced by 2 h of proximal balloon occlusion of the left anterior descending branch. This improved myocardial energy, enhanced LV systolic function and improved unfavorable LV remodeling (38).
Angiogenesis
Angiogenesis is the physiological and pathological process of forming new microvessels from pre-existing capillaries in response to hypoxia. Angiogenesis involves endothelial cell proliferation, migration, differentiation, tube formation and regulation of angiogenic factors (157–159). The development of cardiac remodeling is significantly influenced by microvascular density (157,160). Several studies have demonstrated that promoting angiogenesis increases the density of microvessels and arteriolar, thus reducing cardiac remodeling (161–165).
Previous studies have shown that EMPA promotes myocardial microcirculatory perfusion and cardiac function by reducing AMPK-mediated mitochondrial fission and oxidative stress and stabilizing F-actin (166). Studies in a mouse model of diabetes-related hindlimb ischemia found that DAPA promotes vascular endothelial cell proliferation and migration through the prolyl hydroxylase domain protein 2/HIF-1α axis, the secretion of multiple angiogenic factors, the formation of neovascularization and increases in blood perfusion (167). EMPA improves systolic dysfunction during LV pressure overload in mice by activating the AKT/endothelial NOS (eNOS)/NO pathway to prevent endothelial apoptosis and maintain capillarization (168). In the event of myocardial I/R injury in non-diabetic mice, EMPA inhibits the DNA-dependent protein kinase catalytic subunit/fission 1 protein/mitochondrial fission pathway, protecting the microvascular system (169). However, the microvascular function in vivo is difficult to evaluate. In this study, only electron microscopy was used to observe the structural changes of microvessels in mice treated with EMPA, which is insufficient. However, coronary blood flow reserve can also be used. Another study demonstrated that DAPA reduces cardiac endothelial dysfunction and microvascular injury by inhibition of the XO/sarco(endo) plasmic reticulum calcium ATPase 2/calmodulin-dependent kinase II/coffilin pathway in I/R injury mice (170). EMPA also improves endothelial cell dysfunction induced by a mutant aldehyde dehydrogenase 2 unable to metabolize acetaldehyde by inhibiting NHE1 and activating the AKT kinase and eNOS pathways (171). EMPA attenuates cardiac microvascular I/R injury through the activation of the AMP-activated protein kinase α1 (AMPKα1)/UNC-52-like kinase 1/FUN14 domain containing 1/mitophagy pathway (172).
5. Molecular mechanisms of SGLT2is in pathological cardiac remodeling
TGF-β1/Smad2/3 pathway
The development of cardiac fibrosis is regulated by members of the TGF-β family, particularly TGF-β1, which activates Smad-dependent or non-Smad-mediated signaling pathways (173). TGF-β1 is a key cytokine mediating the conversion of cardiac fibroblasts into myofibroblasts that is regulated by numerous substances (174). Previous studies have shown that EMPA significantly decreases TGF-β1/Smad2 levels and upregulates the expression of the negative feedback regulator Smad7 to alleviate cardiac oxidative stress and fibrosis in diabetic mice (175). Furthermore, the antifibrotic activity of Smad7 on TGF-β and epidermal growth factor receptor 2 reduces myofibroblast activation and the production of structural and matrix proteins (176). Early administration of EMPA during MI reduces myocardial fibrosis and inhibits the TGF-1/Smad3 fibrotic pathway (177). This study explored the effects of EMPA on early cardiac physiology and fibrosis after myocardial infarction. Only samples taken after 4 weeks of administration were examined, and earlier samples were not evaluated, so the results may differ. DAPA reduces TGF-β1 levels and increases the expression of the negative feedback regulator Smad7 in Ang II-induced cardiac remodeling (178).
Reportedly, the activation of AMPKα inhibits the TGF-β/Smad pathway (179,180). DAPA protects against diabetic cardiomyopathy and myocardial fibrosis by inhibiting endothelial-interstitial transformation and fibroblast activation in the AMPKα/TGF-β/Smad signaling pathway (181). Furthermore, DAPA reduces myocardial fibrosis by inhibiting TGF-β1/Smad signaling pathways in normoglycemic chronic heart failure rabbits (182).
MAPK pathway
MAPK is a class of highly conserved serine/threonine protein kinases regulated by a cascade of tertiary phosphorylation activation (183,184). MAPK is divided into four subgroups: Extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38 MAPK and ERK5 (185,186). Under pathological conditions, the MAPK signaling pathway is activated by numerous extracellular stimulation signals and is essential for cell proliferation, differentiation, apoptosis and stress response (184,187). These findings suggested that the MAPK signaling pathway regulates cardiac remodeling due to multiple pathologies (188–191).
Furthermore, TAC activated ERK1/2, p38 and JNK in mice and treatment with DAPA inhibited the expression of JNK and p38 to reduce cardiac remodeling (56). Likewise, DAPA attenuated palmitic acid-induced cell hypertrophy and apoptosis and improved cardiac dysfunction and remodeling in high-fat diet-induced obese mice. This protective effect both in vivo and in vitro is mediated by the NHE1/MAPK signaling pathway (192), and whether DAPA exerts cardio-protective effects through NHE1 requires further evaluation. Furthermore, this suggests that the protective effect of EMPA on the heart may be mediated through stimulation of the ERK1/2 signaling pathway in I/R injury (69).
mTOR and Akt
mTOR, a class of atypical serine/threonine protein kinases, is a member of the phosphatidylinositol 3-kinase (PI3K)-related protein kinase family. The interaction of mTOR with different proteins forms two macromolecular complexes with different structures and functions, mTORC1 and mTORC2 (193). mTOR also integrates multiple extracellular signals, such as nutrient levels, energy and growth factors, and serves a role in cell growth, proliferation, survival, protein synthesis, autophagy and metabolism (194,195). Several studies have shown its crucial role in the physiological and pathological processes of the heart (196–201). Furthermore, the Akt and AMPK pathways are regulators of mTORC1, with AMPK negatively regulating the mTOR signaling pathway (202).
Another study reported that Ertugliflozin reduces LV fibrosis in mice with cardiac hypertrophy by activating the AMPK/mTOR pathway and inhibiting its downstream targets p70S6K and 4E-BP1 (203). This target mediates translation to promote mTORC1 synthesis and causes mTORC1-induced myocardial hypertrophy (204). Likewise, EMPA modulates autophagy in cardiomyocytes to ameliorate sunitinib-induced cardiac dysfunction, an effect mediated by the activation of sunitinib-inhibited AMPK and reducing Sunitinib-activated mTOR levels (37). AMPK/mTOR is one of the main pathways regulating autophagy, which can be regulated by direct phosphorylation of UNC-51-like kinases 1 (205). Furthermore, EMPA improves obesity-related cardiac dysfunction by increasing the AMPK level and endothelial nitric oxide synthase phosphorylation, and inhibiting Akt and mTOR phosphorylation (144). Previous research has demonstrated that the heart can be protected by inhibiting the PI3K/AKT/mTOR pathway (206). CANA alleviates cardiomyocyte lipotoxicity in diabetic cardiomyopathy mouse models by blocking the mTOR/HIF-1 pathway (207). Likewise, CANA is an SGLT1i/SGLT2is and its impact on the mTOR signaling pathway should be excluded from SGLT1 interference.
Other molecular signaling pathways
Serum and glucocorticoid-induced protein kinase 1 (SGK1) are the main mediators of cardiac remodeling through the activation of epithelial sodium channel (ENaC) proteins responsible for promoting fibrosis and upregulating NHE1 activity (208,209). DAPA attenuates LVDD and myocardial fibrosis by modulating SGK1 signaling and ENaC protein (210). Due to the use of pigs in this study, the sample size was small and the results had statistical limitations. The JAK/STAT signaling pathway is a promoter of fibroblast activation and ischemic-induced cardiac dysfunction (211,212). CANA attenuates fibrosis by reducing JAK/STAT signaling, activating AMPK and through antioxidant signaling (213). Characteristics of diabetic cardiomyopathy include decreased cyclic guanosine monophosphate (cGMP) levels and altered soluble guanylate cyclase enzyme (sGC)-cGMP- dependent protein kinase (PKG) signaling, which regulate systolic and diastolic dysfunction under diabetic conditions (214,215). Furthermore, EMPA improves cardiac function by preventing oxidative stress-induced injury via the sGC/cGMP/PKG pathway (216).
6. Conclusion
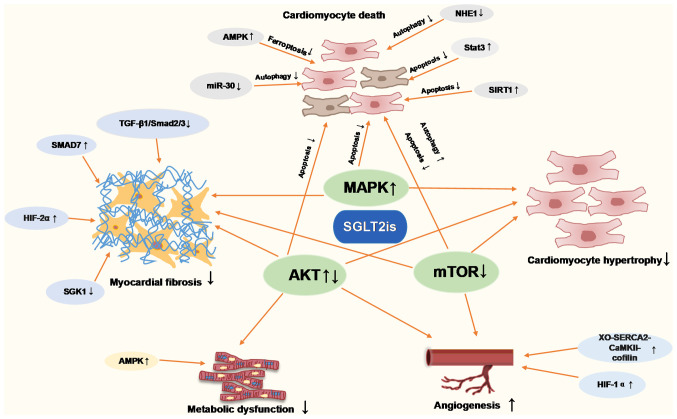
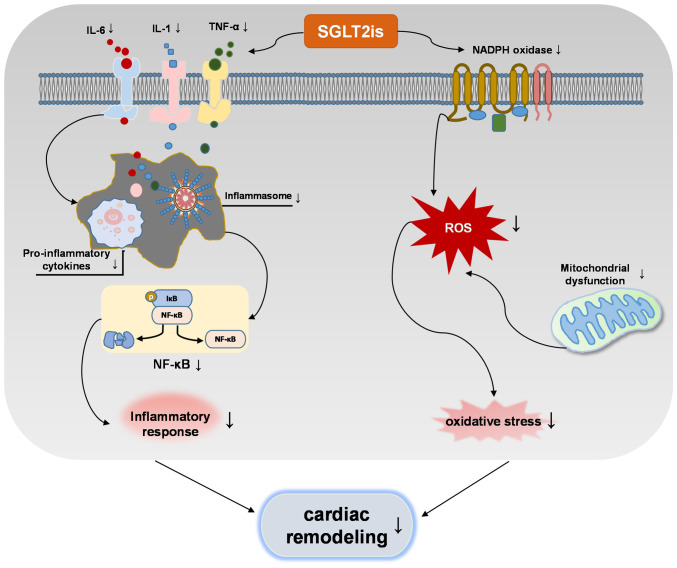
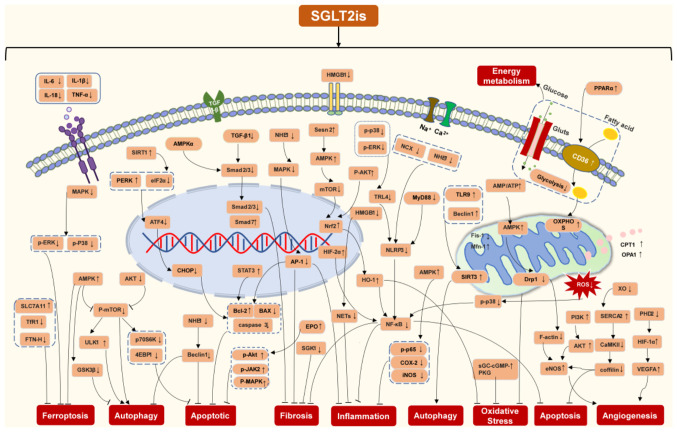
The present review provided a comprehensive summary of the molecular mechanisms through which SGLT2is attenuate pathological cardiac remodeling in animal and in vitro cellular models. The molecular pathways of SGLT2is in cardiac remodeling in terms of cardiac hypertrophy, cardiac fibrosis, inflammation, apoptosis, autophagy, ferroptosis, oxidative stress and energy metabolism, were summarized in Fig. 2. Thus, which supports the potential use of SGLT2i as a therapeutic which can inhibit numerous mechanisms of cardiac remodeling, such as MI, I/R and diabetic cardiomyopathy. SGLT2is are directly or indirectly involved in regulating molecular pathways of cardiac remodeling. Of note, the interaction between inflammation and oxidative stress increases the production of ROS and pro-inflammatory mediators, and SGLT2is inhibit this interaction to regulate cardiac remodeling (Fig. 3). Based on this summary, it is speculated that SGLT2is exert inhibitory effects on cardiac remodeling (Fig. 4).
Figure 2.
Role of SGLT2is in inhibiting pathological cardiac remodeling. SGLT2is, sodium-glucose cotransporter type 2 inhibitors; AMPK, AMP-activated protein kinase; NHE1, Na+/H+ exchanger 1; SIRT1, sirtuin-1; XO, xanthine oxidase; SERCA2, sarco(endo)plasmic reticulum calcium-ATPase 2; CaMKII, calmodulin-dependent kinase II; HIF1-α, hypoxia-inducible factor 1-α; SGK1, serum and glucocorticoid-induced protein kinase 1.
Figure 3.
Indirect effect of SGLT2is on pathological cardiac remodeling by inhibiting the crosstalk between inflammation and oxidative stress. SGLT2is, sodium-glucose cotransporter type 2 inhibitors; ROS, reactive oxygen species; IκB, inhibitor of NF-κB.
Figure 4.
Schematic of the regulatory mechanisms of SGLT2is in pathological cardiac remodeling. SGLT2is, sodium-glucose cotransporter type 2 inhibitors; HMGB1, high mobility group box 1; TNF-α, tumor necrosis factor-α; ROS, reactive oxygen species; AMPK, AMP-activated protein; Nrf2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor-B; ERK, extracellular signal-regulated kinase; PI3K, phosphatidylinositol 3-kinase; CD36, cluster of differentiation 36; SGK1, serum and glucocorticoid-induced protein kinase 1; NHE1, Na+/H+ exchanger 1; SIRT1, sirtuin-1; XO, xanthine oxidase; SERCA2, sarco(endo)plasmic reticulum calcium-ATPase 2; CaMKII, calmodulin-dependent kinase II; HIF1-α, hypoxia-inducible factor 1-α; sGC, soluble guanylate cyclase enzyme; cGMP, cyclic guanosine monophosphate; PKG, cGMP-dependent protein kinase; eIF2, eukaryotic initiation factor 2; PERK, protein kinase RNA-like ER kinase; CHOP, C/EBP homologous protein; ATF4, activating transcription factor 4; ULK1, UNC-52-like kinase 1; GSK3β, glycogen synthase kinase 3β; p70S6K, 70 kDa ribosomal protein S6 kinase; 4EBP1, 4E-binding protein 1; SLC7A11, solute carrier family 7a member 11; PPARα, peroxisome proliferator-activated receptor α; NCX, sodium-calcium exchangers; TFR1, transferrin receptor 1; FTN-H, ferritin heavy-chain; AP-1, activator protein-1; EPO, erythropoietin; NETs, neutrophil extracellular traps; HO-1, heme oxygenase-1; TRL4, toll-like receptor 4; MyD88, myeloid differentiation primary response 88; NLRP3, NLR family pyrin domain containing 3; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; OXPHOS, oxidative phosphorylation; Drp1, dynamin-related protein 1; eNOS, endothelial nitric oxide synthase; Fis-1, fission 1; MFN-1, mitofusin 1; CPT1, carnitine O-palmitoyltransferase 1; Opa1, optic atrophy 1; PHD2, prolyl hydroxylase 2; VEGFA, vascular endothelial growth factor A.
To date, the effect of SGLT2is on cardiac remodeling has been evaluated by several approaches, but studies on how it functions in the heart require further evaluation. In addition, the epigenetic mechanisms of SGLT2is in cardiac remodeling have not been reported. In recent years, the impact of epigenetics on disease development has received significant attention and studies on cardiac diseases suggest that the epigenetic mechanisms of SGLT2is require further assessment in future studies.
Regarding diabetic and non-diabetic pathological cardiac remodeling, few studies have simultaneously compared whether both occur through the same mechanism. SGLT2is have been clinically approved for use in non-diabetic heart failure, while in diabetic heart disease, their role may be influenced by SGLT2 targets. Thus, exploring the mechanism of action of SGLT2is in non-diabetic cardiac remodeling may provide a basis for clinical application in the heart.
The present review emphasizes that SGLT2is are not only effective in controlling blood sugar in diabetes but can also mitigate heart damage, suggesting their dual use in managing both conditions.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 82104156).
Availability of data and materials
Not applicable.
Authors' contributions
BC, YF and JG conceived, designed and planned the study. All authors collected and read the literature. BC and JG were responsible for the literature review and preparing the first draft of the manuscript. HY, XW and JG revised the manuscript. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.Yang D, Liu HQ, Liu FY, Tang N, Guo Z, Ma SQ, An P, Wang MY, Wu HM, Yang Z, et al. The roles of noncardiomyocytes in cardiac remodeling. Int J Biol Sci. 2020;16:2414–2429. doi: 10.7150/ijbs.47180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu QQ, Xiao Y, Yuan Y, Ma ZG, Liao HH, Liu C, Zhu JX, Yang Z, Deng W, Tang QZ. Mechanisms contributing to cardiac remodelling. Clin Sci (Lond) 2017;131:2319–2345. doi: 10.1042/CS20171167. [DOI] [PubMed] [Google Scholar]
- 4.Gao J, Xu W, Wang J, Wang K, Li P. The role and molecular mechanism of non-coding RNAs in pathological cardiac remodeling. Int J Mol Sci. 2017;18:608. doi: 10.3390/ijms18030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang LS, Liu Y, Chen Y, Ren JL, Zhang YR, Yu YR, Jia MZ, Ning ZP, Du J, Tang CS, Qi YF. Intermedin alleviates pathological cardiac remodeling by upregulating klotho. Pharmacol Res. 2020;159:104926. doi: 10.1016/j.phrs.2020.104926. [DOI] [PubMed] [Google Scholar]
- 6.Takefuji M, Wirth A, Lukasova M, Takefuji S, Boettger T, Braun T, Althoff T, Offermanns S, Wettschureck N. G(13)-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure. Circulation. 2012;126:1972–1982. doi: 10.1161/CIRCULATIONAHA.112.109256. [DOI] [PubMed] [Google Scholar]
- 7.McCarroll CS, He W, Foote K, Bradley A, McGlynn K, Vidler F, Nixon C, Nather K, Fattah C, Riddell A, et al. Runx1 deficiency protects against adverse cardiac remodeling after myocardial infarction. Circulation. 2018;137:57–70. doi: 10.1161/CIRCULATIONAHA.117.028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 9.Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: Beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19:98. doi: 10.1186/s12933-020-01071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV, RECORD Study Team Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox T, De Block C, Schwartzbard AZ, Newman JD. Diabetic agents, from metformin to SGLT2 inhibitors and GLP1 receptor agonists: JACC focus seminar. J Am Coll Cardiol. 2020;75:1956–1974. doi: 10.1016/j.jacc.2020.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 14.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 15.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 16.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 17.Braunwald E. Gliflozins in the management of cardiovascular disease. N Engl J Med. 2022;386:2024–2034. doi: 10.1056/NEJMra2115011. [DOI] [PubMed] [Google Scholar]
- 18.Van Steenbergen A, Balteau M, Ginion A, Ferté L, Battault S, Ravenstein CM, Balligand JL, Daskalopoulos EP, Gilon P, Despa F, et al. Sodium-myoinositol cotransporter-1, SMIT1, mediates the production of reactive oxygen species induced by hyperglycemia in the heart. Sci Rep. 2017;7:41166. doi: 10.1038/srep41166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie MR, Fisher M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 20.Tripolt NJ, Kolesnik E, Pferschy PN, Verheyen N, Ablasser K, Sailer S, Alber H, Berger R, Kaulfersch C, Leitner K, et al. Impact of EMpagliflozin on cardiac function and biomarkers of heart failure in patients with acute MYocardial infarction-the EMMY trial. Am Heart J. 2020;221:39–47. doi: 10.1016/j.ahj.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 21.von Lewinski D, Tripolt NJ, Sourij H, Pferschy PN, Oulhaj A, Alber H, Gwechenberger M, Martinek M, Seidl S, Moertl D, et al. Ertugliflozin to reduce arrhythmic burden in ICD/CRT patients (ERASe-trial)-a phase III study. Am Heart J. 2022;246:152–160. doi: 10.1016/j.ahj.2022.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF) Circulation. 2021;143:516–525. doi: 10.1161/CIRCULATIONAHA.120.052186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aimo A, Vergaro G, González A, Barison A, Lupón J, Delgado V, Richards AM, de Boer RA, Thum T, Arfsten H, et al. Cardiac remodelling-part 2: Clinical, imaging and laboratory findings. A review from the study group on biomarkers of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2022;24:944–958. doi: 10.1002/ejhf.2522. [DOI] [PubMed] [Google Scholar]
- 24.Singh JSS, Fathi A, Vickneson K, Mordi I, Mohan M, Houston JG, Pearson ER, Struthers AD, Lang CC. Research into the effect of SGLT2 inhibition on left ventricular remodelling in patients with heart failure and diabetes mellitus (REFORM) trial rationale and design. Cardiovasc Diabetol. 2016;15:97. doi: 10.1186/s12933-016-0419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–255. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: The EMPA-HEART cardiolink-6 randomized clinical trial. Circulation. 2019;140:1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
- 27.Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Tuxen CD, Möller S, Gustafsson F, et al. Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: A substudy of the empire HF randomized clinical trial. JAMA Cardiol. 2021;6:836–840. doi: 10.1001/jamacardio.2020.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang IC, Cho GY, Yoon YE, Park JJ, Park JB, Lee SP, Kim HK, Kim YJ, Sohn DW. Different effects of SGLT2 inhibitors according to the presence and types of heart failure in type 2 diabetic patients. Cardiovasc Diabetol. 2020;19:69. doi: 10.1186/s12933-020-01042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, Hirata KI. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17:132. doi: 10.1186/s12933-018-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: The DAPA-LVH trial. Eur Heart J. 2020;41:3421–3432. doi: 10.1093/eurheartj/ehaa419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan NSR, Yeap BB, Fegan PG, Green G, Rankin JM, Dwivedi G. Empagliflozin and left ventricular diastolic function following an acute coronary syndrome in patients with type 2 diabetes. Int J Cardiovasc Imaging. 2021;37:517–527. doi: 10.1007/s10554-020-02034-w. [DOI] [PubMed] [Google Scholar]
- 32.von Lewinski D, Kolesnik E, Tripolt NJ, Pferschy PN, Benedikt M, Wallner M, Alber H, Berger R, Lichtenauer M, Saely CH, et al. Empagliflozin in acute myocardial infarction: The EMMY trial. Eur Heart J. 2022;43:4421–4432. doi: 10.1093/eurheartj/ehac494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ersbøll M, Jürgens M, Hasbak P, Kjær A, Wolsk E, Zerahn B, Brandt-Jacobsen NH, Gæde P, Rossing P, Faber J, et al. Effect of empagliflozin on myocardial structure and function in patients with type 2 diabetes at high cardiovascular risk: The SIMPLE randomized clinical trial. Int J Cardiovasc Imaging. 2022;38:579–587. doi: 10.1007/s10554-021-02443-5. [DOI] [PubMed] [Google Scholar]
- 34.Palmiero G, Cesaro A, Galiero R, Loffredo G, Caturano A, Vetrano E, Rinaldi L, Salvatore T, Ruggiero R, Rosaria Di Palo M, et al. Impact of gliflozins on cardiac remodeling in patients with type 2 diabetes mellitus & reduced ejection fraction heart failure: A pilot prospective study. GLISCAR study. Diabetes Res Clin Pract. 2023;200:110686. doi: 10.1016/j.diabres.2023.110686. [DOI] [PubMed] [Google Scholar]
- 35.Russo V, Malvezzi Caracciolo D'Aquino M, Caturano A, Scognamiglio G, Pezzullo E, Fabiani D, Del Giudice C, Carbone A, Bottino R, Caso V, et al. Improvement of global longitudinal strain and myocardial work in type 2 diabetes patients on sodium-glucose cotransporter 2 inhibitors therapy. J Cardiovasc Pharmacol. 2023;82:196–200. doi: 10.1097/FJC.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 36.Nagueh SF. Left ventricular diastolic function: Understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging. 2020;13:228–244. doi: 10.1016/j.jcmg.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 37.Ren C, Sun K, Zhang Y, Hu Y, Hu B, Zhao J, He Z, Ding R, Wang W, Liang C. Sodium-glucose CoTransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK-mTOR signaling pathway-mediated autophagy. Front Pharmacol. 2021;12:664181. doi: 10.3389/fphar.2021.664181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 39.Yurista SR, Silljé HHW, Oberdorf-Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21:862–873. doi: 10.1002/ejhf.1473. [DOI] [PubMed] [Google Scholar]
- 40.Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Barron B, Mayoux E, Rector RS, Whaley-Connell A, DeMarco VG. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16:9. doi: 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang N, Feng B, Ma X, Sun K, Xu G, Zhou Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2019;18:107. doi: 10.1186/s12933-019-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, Dai ZK, Chen WY, Chen YF, Lee AS. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019;18:45. doi: 10.1186/s12933-019-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. 2020;19:91. doi: 10.1186/s12933-020-01066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kräker K, Herse F, Golic M, Reichhart N, Crespo-Garcia S, Strauß O, Grune J, Kintscher U, Ebrahim M, Bader M, et al. Effects of empagliflozin and target-organ damage in a novel rodent model of heart failure induced by combined hypertension and diabetes. Sci Rep. 2020;10:14061. doi: 10.1038/s41598-020-70708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Garcia-Ropero A, Ishikawa K, Watanabe S, Picatoste B, Vargas-Delgado AP, Flores-Umanzor EJ, Sanz J, et al. Empagliflozin ameliorates diastolic dysfunction and left ventricular fibrosis/stiffness in nondiabetic heart failure: A multimodality study. JACC Cardiovasc Imaging. 2021;14:393–407. doi: 10.1016/j.jcmg.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 46.Lin YW, Chen CY, Shih JY, Cheng BC, Chang CP, Lin MT, Ho CH, Chen ZC, Fisch S, Chang WT. Dapagliflozin improves cardiac hemodynamics and mitigates arrhythmogenesis in mitral regurgitation-induced myocardial dysfunction. J Am Heart Assoc. 2021;10:e019274. doi: 10.1161/JAHA.120.019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dini FL, Galeotti GG, Terlizzese G, Fabiani I, Pugliese NR, Rovai I. Left ventricular mass and thickness: Why does it matter? Heart Fail Clin. 2019;15:159–166. doi: 10.1016/j.hfc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17:73. doi: 10.1186/s12933-018-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 50.Querejeta R, López B, González A, Sánchez E, Larman M, Martínez Ubago JL, Díez J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 51.Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Travers JG, Tharp CA, Rubino M, McKinsey TA. Therapeutic targets for cardiac fibrosis: From old school to next-gen. J Clin Invest. 2022;132:e148554. doi: 10.1172/JCI148554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aluja D, Delgado-Tomás S, Ruiz-Meana M, Barrabés JA, Inserte J. Calpains as potential therapeutic targets for myocardial hypertrophy. Int J Mol Sci. 2022;23:4103. doi: 10.3390/ijms23084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yerra VG, Batchu SN, Kabir G, Advani SL, Liu Y, Siddiqi FS, Connelly KA, Advani A. Empagliflozin disrupts a Tnfrsf12a-mediated feed forward loop that promotes left ventricular hypertrophy. Cardiovasc Drugs Ther. 2022;36:619–632. doi: 10.1007/s10557-021-07190-2. [DOI] [PubMed] [Google Scholar]
- 56.Shi L, Zhu D, Wang S, Jiang A, Li F. Dapagliflozin attenuates cardiac remodeling in mice model of cardiac pressure overload. Am J Hypertens. 2019;32:452–459. doi: 10.1093/ajh/hpz016. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Lu Q, Qiu Y, do Carmo JM, Wang Z, da Silva AA, Mouton A, Omoto ACM, Hall ME, Li J, Hall JE. Direct Cardiac actions of the sodium glucose co-transporter 2 inhibitor empagliflozin improve myocardial oxidative phosphorylation and attenuate pressure-overload heart failure. J Am Heart Assoc. 2021;10:e018298. doi: 10.1161/JAHA.120.018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L, Luo H, Liang Y, Tang J, Shu Y. Dapagliflozin ameliorates STZ-induced cardiac hypertrophy in type 2 diabetic rats by inhibiting the calpain-1 expression and nuclear transfer of NF-kappaB. Comput Math Methods Med. 2022;2022:3293054. doi: 10.1155/2022/3293054. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Wang J, Huang X, Liu H, Chen Y, Li P, Liu L, Li J, Ren Y, Huang J, Xiong E, et al. Empagliflozin ameliorates diabetic cardiomyopathy via attenuating oxidative stress and improving mitochondrial function. Oxid Med Cell Longev. 2022;2022:1122494. doi: 10.1155/2022/1122494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura T, Nakamura K, Miyoshi T, Yoshida M, Akazawa K, Saito Y, Akagi S, Ohno Y, Kondo M, Miura D, et al. Inhibitory effects of tofogliflozin on cardiac hypertrophy in dahl salt-sensitive and salt-resistant rats fed a high-fat diet. Int Heart J. 2019;60:728–735. doi: 10.1536/ihj.18-392. [DOI] [PubMed] [Google Scholar]
- 61.Hsieh PL, Chu PM, Cheng HC, Huang YT, Chou WC, Tsai KL, Chan SH. Dapagliflozin mitigates doxorubicin-caused myocardium damage by regulating AKT-mediated oxidative stress, cardiac remodeling, and inflammation. Int J Mol Sci. 2022;23:10146. doi: 10.3390/ijms231710146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asensio Lopez MDC, Lax A, Hernandez Vicente A, Saura Guillen E, Hernandez-Martinez A, Fernandez Del Palacio MJ, Bayes-Genis A, Pascual Figal DA. Empagliflozin improves post-infarction cardiac remodeling through GTP enzyme cyclohydrolase 1 and irrespective of diabetes status. Sci Rep. 2020;10:13553. doi: 10.1038/s41598-020-70454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, Xu S, Xu Y, Hwa J, Li J, et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. 2022;13:336–359. doi: 10.1007/s13238-020-00809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 65.Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev. 2019;99:1765–1817. doi: 10.1152/physrev.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acute myocardial infarction and heart failure: Role of apoptosis. Int J Biochem Cell Biol. 2006;38:1834–1840. doi: 10.1016/j.biocel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Wu M, Xu J, Xu B, Kang L. Empagliflozin prevents from early cardiac injury post myocardial infarction in non-diabetic mice. Eur J Pharm Sci. 2021;161:105788. doi: 10.1016/j.ejps.2021.105788. [DOI] [PubMed] [Google Scholar]
- 68.Long Q, Li L, Yang H, Lu Y, Yang H, Zhu Y, Tang Y, Liu C, Yuan J. SGLT2 inhibitor, canagliflozin, ameliorates cardiac inflammation in experimental autoimmune myocarditis. Int Immunopharmacol. 2022;110:109024. doi: 10.1016/j.intimp.2022.109024. [DOI] [PubMed] [Google Scholar]
- 69.El-Sayed N, Mostafa YM, AboGresha NM, Ahmed AAM, Mahmoud IZ, El-Sayed NM. Dapagliflozin attenuates diabetic cardiomyopathy through erythropoietin up-regulation of AKT/JAK/MAPK pathways in streptozotocin-induced diabetic rats. Chem Biol Interact. 2021;347:109617. doi: 10.1016/j.cbi.2021.109617. [DOI] [PubMed] [Google Scholar]
- 70.Fan ZG, Xu Y, Chen X, Ji MY, Ma GS. Appropriate dose of dapagliflozin improves cardiac outcomes by normalizing mitochondrial fission and reducing cardiomyocyte apoptosis after acute myocardial infarction. Drug Des Devel Ther. 2022;16:2017–2030. doi: 10.2147/DDDT.S371506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang WT, Shih JY, Lin YW, Chen ZC, Kan WC, Lin TH, Hong CS. Dapagliflozin protects against doxorubicin-induced cardiotoxicity by restoring STAT3. Arch Toxicol. 2022;96:2021–2032. doi: 10.1007/s00204-022-03298-y. [DOI] [PubMed] [Google Scholar]
- 72.Ren FF, Xie ZY, Jiang YN, Guan X, Chen QY, Lai TF, Li L. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin. 2022;43:1721–1732. doi: 10.1038/s41401-021-00805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zein L, Fulda S, Kögel D, van Wijk SJL. Organelle-specific mechanisms of drug-induced autophagy-dependent cell death. Matrix Biol. 2021;100-101:54–64. doi: 10.1016/j.matbio.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Liu J, Huang Y, Chang JYF, Liu L, McKeehan WL, Martin JF, Wang F. FRS2α-mediated FGF signals suppress premature differentiation of cardiac stem cells through regulating autophagy activity. Circ Res. 2012;110:e29–e39. doi: 10.1161/CIRCRESAHA.111.255950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morales PE, Arias-Durán C, Ávalos-Guajardo Y, Aedo G, Verdejo HE, Parra V, Lavandero S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Aspects Med. 2020;71:100822. doi: 10.1016/j.mam.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 2015;116:456–467. doi: 10.1161/CIRCRESAHA.114.303788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 78.Munasinghe PE, Riu F, Dixit P, Edamatsu M, Saxena P, Hamer NS, Galvin IF, Bunton RW, Lequeux S, Jones G, et al. Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. Int J Cardiol. 2016;202:13–20. doi: 10.1016/j.ijcard.2015.08.111. [DOI] [PubMed] [Google Scholar]
- 79.Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation. 2016;133:1249–1263. doi: 10.1161/CIRCULATIONAHA.115.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishida K, Otsu K. Autophagy during cardiac remodeling. J Mol Cell Cardiol. 2016;95:11–18. doi: 10.1016/j.yjmcc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Nah J, Zhai P, Huang CY, Fernández ÁF, Mareedu S, Levine B, Sadoshima J. Upregulation of Rubicon promotes autosis during myocardial ischemia/reperfusion injury. J Clin Invest. 2020;130:2978–2991. doi: 10.1172/JCI132366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ikeda S, Zablocki D, Sadoshima J. The role of autophagy in death of cardiomyocytes. J Mol Cell Cardiol. 2022;165:1–8. doi: 10.1016/j.yjmcc.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gatica D, Chiong M, Lavandero S, Klionsky DJ. The role of autophagy in cardiovascular pathology. Cardiovasc Res. 2022;118:934–950. doi: 10.1093/cvr/cvab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang CY, Chen CC, Lin MH, Su HT, Ho MY, Yeh JK, Tsai ML, Hsieh IC, Wen MS. TLR9 binding to Beclin 1 and mitochondrial SIRT3 by a sodium-glucose co-transporter 2 inhibitor protects the heart from doxorubicin toxicity. Biology (Basel) 2020;9:369. doi: 10.3390/biology9110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng R, Jiang K, Chen F, Miao Y, Lu Y, Su F, Liang J, Qian J, Wang D, Xiang Y, Shen L. Novel cardioprotective mechanism for empagliflozin in nondiabetic myocardial infarction with acute hyperglycemia. Biomed Pharmacother. 2022;154:113606. doi: 10.1016/j.biopha.2022.113606. [DOI] [PubMed] [Google Scholar]
- 86.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang CC, Li Y, Qian XQ, Zhao H, Wang D, Zuo GX, Wang K. Empagliflozin alleviates myocardial I/R injury and cardiomyocyte apoptosis via inhibiting ER stress-induced autophagy and the PERK/ATF4/Beclin1 pathway. J Drug Target. 2022;30:858–872. doi: 10.1080/1061186X.2022.2064479. [DOI] [PubMed] [Google Scholar]
- 88.Aragón-Herrera A, Feijóo-Bandín S, Otero Santiago M, Barral L, Campos-Toimil M, Gil-Longo J, Costa Pereira TM, Garcia-Caballero T, Rodriguez-Segade S, Rodriguez J, et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol. 2019;170:113677. doi: 10.1016/j.bcp.2019.113677. [DOI] [PubMed] [Google Scholar]
- 89.Ma H, Ma Y. Dapagliflozin inhibits ventricular remodeling in heart failure rats by activating autophagy through AMPK/mTOR pathway. Comput Math Methods Med. 2022;2022:6260202. doi: 10.1155/2022/6260202. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Yu YW, Que JQ, Liu S, Huang KY, Qian L, Weng YB, Rong FN, Wang L, Zhou YY, Xue YJ, Ji KT. Sodium-glucose co-transporter-2 inhibitor of dapagliflozin attenuates myocardial ischemia/reperfusion injury by limiting NLRP3 inflammasome activation and modulating autophagy. Front Cardiovasc Med. 2022;8:768214. doi: 10.3389/fcvm.2021.768214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qin Y, Qiao Y, Wang D, Tang C, Yan G. Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications. Biomed Pharmacother. 2021;141:111872. doi: 10.1016/j.biopha.2021.111872. [DOI] [PubMed] [Google Scholar]
- 92.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang X, Song Y, Wei L, Guo J, Xu W, Li M. The emerging roles of ferroptosis in organ fibrosis and its potential therapeutic effect. Int Immunopharmacol. 2023;116:109812. doi: 10.1016/j.intimp.2023.109812. [DOI] [PubMed] [Google Scholar]
- 94.Feng Y, Madungwe NB, Imam Aliagan AD, Tombo N, Bopassa JC. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem Biophys Res Commun. 2019;520:606–611. doi: 10.1016/j.bbrc.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M, Woo JD, Aoyagi H, Marh KS, Kitaoka H, Matsui T. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314:H659–H668. doi: 10.1152/ajpheart.00452.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bai T, Li M, Liu Y, Qiao Z, Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 98.Chen X, Xu S, Zhao C, Liu B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun. 2019;516:37–43. doi: 10.1016/j.bbrc.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 99.Ma S, He LL, Zhang GR, Zuo QJ, Wang ZL, Zhai JL, Zhang TT, Wang Y, Ma HJ, Guo YF. Canagliflozin mitigates ferroptosis and ameliorates heart failure in rats with preserved ejection fraction. Naunyn Schmiedebergs Arch Pharmacol. 2022;395:945–962. doi: 10.1007/s00210-022-02243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, Wang Q, Tan Y, Keller BB, Tong Q, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12:708–722. doi: 10.1016/j.apsb.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du S, Shi H, Xiong L, Wang P, Shi Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front Endocrinol (Lausanne) 2022;13:1011669. doi: 10.3389/fendo.2022.1011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang W, Lu J, Wang Y, Sun P, Gao T, Xu N, Zhang Y, Xie W. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway. Int J Mol Sci. 2023;24:858. doi: 10.3390/ijms24010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Min J, Wu L, Liu Y, Song G, Deng Q, Jin W, Yu W, Abudureyimu M, Pei Z, Ren J. Empagliflozin attenuates trastuzumab-induced cardiotoxicity through suppression of DNA damage and ferroptosis. Life Sci. 2023;312:121207. doi: 10.1016/j.lfs.2022.121207. [DOI] [PubMed] [Google Scholar]
- 104.Chen W, Zhang Y, Wang Z, Tan M, Lin J, Qian X, Li H, Jiang T. Dapagliflozin alleviates myocardial ischemia/reperfusion injury by reducing ferroptosis via MAPK signaling inhibition. Front Pharmacol. 2023;14:1078205. doi: 10.3389/fphar.2023.1078205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M, Giannoni A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27:494–510. doi: 10.1177/2047487319870344. [DOI] [PubMed] [Google Scholar]
- 106.Halade GV, Lee DH. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine. 2022;79:103992. doi: 10.1016/j.ebiom.2022.103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DPV. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–283. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 109.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 110.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131:1019–1030. doi: 10.1161/CIRCULATIONAHA.114.008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest. 2007;117:2692–2701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric NH. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res. 1999;84:21–33. doi: 10.1161/01.RES.84.1.21. [DOI] [PubMed] [Google Scholar]
- 113.Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 115.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol. 2013;191:4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chou CH, Hung CS, Liao CW, Wei LH, Chen CW, Shun CT, Wen WF, Wan CH, Wu XM, Chang YY, et al. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc Res. 2018;114:690–702. doi: 10.1093/cvr/cvy013. [DOI] [PubMed] [Google Scholar]
- 117.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szalay G, Sauter M, Hald J, Weinzierl A, Kandolf R, Klingel K. Sustained nitric oxide synthesis contributes to immunopathology in ongoing myocarditis attributable to interleukin-10 disorders. Am J Pathol. 2006;169:2085–2093. doi: 10.2353/ajpath.2006.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kolijn D, Pabel S, Tian Y, Lódi M, Herwig M, Carrizzo A, Zhazykbayeva S, Kovács A, Fülöp GÁ, Falcão-Pires I, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res. 2021;117:495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- 120.Yan P, Song X, Tran J, Zhou R, Cao X, Zhao G, Yuan H. Dapagliflozin alleviates coxsackievirus B3-induced acute viral myocarditis by regulating the macrophage polarization through Stat3-related pathways. Inflammation. 2022;45:2078–2090. doi: 10.1007/s10753-022-01677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Faridvand Y, Nemati M, Zamani-Gharehchamani E, Nejabati HR, Zamani ARN, Nozari S, Safaie N, Nouri M, Jodati A. Dapagliflozin protects H9c2 cells against injury induced by lipopolysaccharide via suppression of CX3CL1/CX3CR1 axis and NF-κB activity. Curr Mol Pharmacol. 2022;15:862–869. doi: 10.2174/1874467214666211008142347. [DOI] [PubMed] [Google Scholar]
- 122.Pinar AA, Scott TE, Huuskes BM, Tapia Cáceres FE, Kemp-Harper BK, Samuel CS. Targeting the NLRP3 inflammasome to treat cardiovascular fibrosis. Pharmacol Ther. 2020;209:107511. doi: 10.1016/j.pharmthera.2020.107511. [DOI] [PubMed] [Google Scholar]
- 123.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 124.Suetomi T, Willeford A, Brand CS, Cho Y, Ross RS, Miyamoto S, Brown JH. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation. 2018;138:2530–2544. doi: 10.1161/CIRCULATIONAHA.118.034621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sokolova M, Ranheim T, Louwe MC, Halvorsen B, Yndestad A, Aukrust P. NLRP3 inflammasome: A novel player in metabolically induced inflammation-potential influence on the myocardium. J Cardiovasc Pharmacol. 2019;74:276–284. doi: 10.1097/FJC.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 126.Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31:119–132. doi: 10.1007/s10557-017-6725-2. [DOI] [PubMed] [Google Scholar]
- 127.Chen H, Tran D, Yang HC, Nylander S, Birnbaum Y, Ye Y. Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: An AMPK-mTOR interplay. Cardiovasc Drugs Ther. 2020;34:443–461. doi: 10.1007/s10557-020-06978-y. [DOI] [PubMed] [Google Scholar]
- 128.Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, Paccone A, Altucci L, Conte M, Canale ML, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20:150. doi: 10.1186/s12933-021-01346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu J, Xu J, Tan X, Li D, Yao D, Xu B, Lei Y. Dapagliflozin protects against dilated cardiomyopathy progression by targeting NLRP3 inflammasome activation. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:1461–1470. doi: 10.1007/s00210-023-02409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pullen AB, Jadapalli JK, Rhourri-Frih B, Halade GV. Re-evaluating the causes and consequences of non-resolving inflammation in chronic cardiovascular disease. Heart Fail Rev. 2020;25:381–391. doi: 10.1007/s10741-019-09817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. doi: 10.1016/j.freeradbiomed.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 132.Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A, Amat-Alarcon N, Andersen P, Judge DP, Tung L, Saffitz JE. Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation. 2019;140:1491–1505. doi: 10.1161/CIRCULATIONAHA.119.040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang Z, Li T, Xian J, Chen J, Huang Y, Zhang Q, Lin X, Lu H, Lin Y. SGLT2 inhibitor dapagliflozin attenuates cardiac fibrosis and inflammation by reverting the HIF-2alpha signaling pathway in arrhythmogenic cardiomyopathy. FASEB J. 2022;36:e22410. doi: 10.1096/fj.202200243R. [DOI] [PubMed] [Google Scholar]
- 134.Salvatore T, Galiero R, Caturano A, Vetrano E, Rinaldi L, Coviello F, Di Martino A, Albanese G, Colantuoni S, Medicamento G, et al. Dysregulated epicardial adipose tissue as a risk factor and potential therapeutic target of heart failure with preserved ejection fraction in diabetes. Biomolecules. 2022;12:176. doi: 10.3390/biom12020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gordon M, Meagher P, Connelly KA. Effect of empagliflozin and liraglutide on the nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3 inflammasome in a rodent model of type 2 diabetes mellitus. Can J Diabetes. 2021;45:553–556. doi: 10.1016/j.jcjd.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 136.Michaeloudes C, Abubakar-Waziri H, Lakhdar R, Raby K, Dixey P, Adcock IM, Mumby S, Bhavsar PK, Chung KF. Molecular mechanisms of oxidative stress in asthma. Mol Aspects Med. 2022;85:101026. doi: 10.1016/j.mam.2021.101026. [DOI] [PubMed] [Google Scholar]
- 137.Weissman D, Maack C. Redox signaling in heart failure and therapeutic implications. Free Radic Biol Med. 2021;171:345–364. doi: 10.1016/j.freeradbiomed.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 138.Zhang M, Perino A, Ghigo A, Hirsch E, Shah AM. NADPH oxidases in heart failure: Poachers or gamekeepers? Antioxid Redox Signal. 2013;18:1024–1041. doi: 10.1089/ars.2012.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111:1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 140.van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: Past, present and future. Eur J Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li A, Zheng N, Ding X. Mitochondrial abnormalities: A hub in metabolic syndrome-related cardiac dysfunction caused by oxidative stress. Heart Fail Rev. 2022;27:1387–1394. doi: 10.1007/s10741-021-10109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xing YJ, Liu BH, Wan SJ, Cheng Y, Zhou SM, Sun Y, Yao XM, Hua Q, Meng XJ, Cheng JH, et al. A SGLT2 inhibitor dapagliflozin alleviates diabetic cardiomyopathy by suppressing high glucose-induced oxidative stress in vivo and in vitro. Front Pharmacol. 2021;12:708177. doi: 10.3389/fphar.2021.708177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li X, Flynn ER, do Carmo JM, Wang Z, da Silva AA, Mouton AJ, Omoto ACM, Hall ME, Hall JE. Direct cardiac actions of sodium-glucose cotransporter 2 inhibition improve mitochondrial function and attenuate oxidative stress in pressure overload-induced heart failure. Front Cardiovasc Med. 2022;9:859253. doi: 10.3389/fcvm.2022.859253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun X, Han F, Lu Q, Li X, Ren D, Zhang J, Han Y, Xiang YK, Li J. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes. 2020;69:1292–1305. doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- 145.Bugga P, Mohammed SA, Alam MJ, Katare P, Meghwani H, Maulik SK, Arava S, Banerjee SK. Empagliflozin prohibits high-fructose diet-induced cardiac dysfunction in rats via attenuation of mitochondria-driven oxidative stress. Life Sci. 2022;307:120862. doi: 10.1016/j.lfs.2022.120862. [DOI] [PubMed] [Google Scholar]
- 146.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 147.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: A suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci. 2010;1188:191–198. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2013;55:31–41. doi: 10.1016/j.yjmcc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 151.De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33:860–871. doi: 10.1016/j.cjca.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 152.Kolwicz SC, Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012;111:728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi YS, de Mattos ABM, Shao D, Li T, Nabben M, Kim M, Wang W, Tian R, Kolwicz SC., Jr Preservation of myocardial fatty acid oxidation prevents diastolic dysfunction in mice subjected to angiotensin II infusion. J Mol Cell Cardiol. 2016;100:64–71. doi: 10.1016/j.yjmcc.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail. 2008;14:82–88. doi: 10.1016/j.cardfail.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trang NN, Chung CC, Lee TW, Cheng WL, Kao YH, Huang SY, Lee TI, Chen YJ. Empagliflozin and liraglutide differentially modulate cardiac metabolism in diabetic cardiomyopathy in rats. Int J Mol Sci. 2021;22:1177. doi: 10.3390/ijms22031177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lai J, Chen C. The role of epoxyeicosatrienoic acids in cardiac remodeling. Front Physiol. 2021;12:642470. doi: 10.3389/fphys.2021.642470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shi W, Xin Q, Yuan R, Yuan Y, Cong W, Chen K. Neovascularization: The main mechanism of MSCs in ischemic heart disease therapy. Front Cardiovasc Med. 2021;8:633300. doi: 10.3389/fcvm.2021.633300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shu HY, Peng YZ, Hang WJ, Zhang M, Shen L, Wang DW, Zhou N. Trimetazidine enhances myocardial angiogenesis in pressure overload-induced cardiac hypertrophy mice through directly activating Akt and promoting the binding of HSF1 to VEGF-A promoter. Acta Pharmacol Sin. 2022;43:2550–2561. doi: 10.1038/s41401-022-00877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ni Y, Deng J, Bai H, Liu C, Liu X, Wang X. CaMKII inhibitor KN-93 impaired angiogenesis and aggravated cardiac remodelling and heart failure via inhibiting NOX2/mtROS/p-VEGFR2 and STAT3 pathways. J Cell Mol Med. 2022;26:312–325. doi: 10.1111/jcmm.17081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blom JN, Wang X, Lu X, Kim MY, Wang G, Feng Q. Inhibition of intraflagellar transport protein-88 promotes epithelial-to-mesenchymal transition and reduces cardiac remodeling post-myocardial infarction. Eur J Pharmacol. 2022;933:175287. doi: 10.1016/j.ejphar.2022.175287. [DOI] [PubMed] [Google Scholar]
- 163.Wei T, Huang G, Gao J, Huang C, Sun M, Wu J, Bu J, Shen W. Sirtuin 3 deficiency accelerates hypertensive cardiac remodeling by impairing angiogenesis. J Am Heart Assoc. 2017;6:e006114. doi: 10.1161/JAHA.117.006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gogiraju R, Hubert A, Fahrer J, Straub BK, Brandt M, Wenzel P, Münzel T, Konstantinides S, Hasenfuss G, Schäfer K. Endothelial leptin receptor deletion promotes cardiac autophagy and angiogenesis following pressure overload by suppressing Akt/mTOR signaling. Circ Heart Fail. 2019;12:e005622. doi: 10.1161/CIRCHEARTFAILURE.118.005622. [DOI] [PubMed] [Google Scholar]
- 165.Gu J, Wang S, Guo H, Tan Y, Liang Y, Feng A, Liu Q, Damodaran C, Zhang Z, Keller BB, et al. Inhibition of p53 prevents diabetic cardiomyopathy by preventing early-stage apoptosis and cell senescence, reduced glycolysis, and impaired angiogenesis. Cell Death Dis. 2018;9:82. doi: 10.1038/s41419-017-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nugrahaningrum DA, Marcelina O, Liu C, Wu S, Kasim V. Dapagliflozin promotes neovascularization by improving paracrine function of skeletal muscle cells in diabetic hindlimb ischemia mice through PHD2/HIF-1α axis. Front Pharmacol. 2020;11:1104. doi: 10.3389/fphar.2020.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakao M, Shimizu I, Katsuumi G, Yoshida Y, Suda M, Hayashi Y, Ikegami R, Hsiao YT, Okuda S, Soga T, Minamino T. Empagliflozin maintains capillarization and improves cardiac function in a murine model of left ventricular pressure overload. Sci Rep. 2021;11:18384. doi: 10.1038/s41598-021-97787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zou R, Shi W, Qiu J, Zhou N, Du N, Zhou H, Chen X, Ma L. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc Diabetol. 2022;21:106. doi: 10.1186/s12933-022-01532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma L, Zou R, Shi W, Zhou N, Chen S, Zhou H, Chen X, Wu Y. SGLT2 inhibitor dapagliflozin reduces endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury through normalizing the XO-SERCA2-CaMKII-coffilin pathways. Theranostics. 2022;12:5034–5050. doi: 10.7150/thno.75121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guo H, Yu X, Liu Y, Paik DT, Justesen JM, Chandy M, Jahng JWS, Zhang T, Wu W, Rwere F, et al. SGLT2 inhibitor ameliorates endothelial dysfunction associated with the common ALDH2 alcohol flushing variant. Sci Transl Med. 2023;15:eabp9952. doi: 10.1126/scitranslmed.abp9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cai C, Guo Z, Chang X, Li Z, Wu F, He J, Cao T, Wang K, Shi N, Zhou H, et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKalpha1/ULK1/FUNDC1/mitophagy pathway. Redox Biol. 2022;52:102288. doi: 10.1016/j.redox.2022.102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hanna A, Frangogiannis NG. The role of the TGF-beta superfamily in myocardial infarction. Front Cardiovasc Med. 2019;6:140. doi: 10.3389/fcvm.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tarbit E, Singh I, Peart JN, Rose'Meyer RB. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail Rev. 2019;24:1–15. doi: 10.1007/s10741-018-9720-1. [DOI] [PubMed] [Google Scholar]
- 175.Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18:15. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Humeres C, Shinde AV, Hanna A, Alex L, Hernández SC, Li R, Chen B, Conway SJ, Frangogiannis NG. Smad7 effects on TGF-β and ErbB2 restrain myofibroblast activation and protect from postinfarction heart failure. J Clin Invest. 2022;132:e146926. doi: 10.1172/JCI146926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Daud E, Ertracht O, Bandel N, Moady G, Shehadeh M, Reuveni T, Atar S. The impact of empagliflozin on cardiac physiology and fibrosis early after myocardial infarction in non-diabetic rats. Cardiovasc Diabetol. 2021;20:132. doi: 10.1186/s12933-021-01322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang Y, Lin X, Chu Y, Chen X, Du H, Zhang H, Xu C, Xie H, Ruan Q, Lin J, et al. Dapagliflozin: a sodium-glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFβ1/Smad signaling. Cardiovasc Diabetol. 2021;20:121. doi: 10.1186/s12933-021-01312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qi H, Liu Y, Li S, Chen Y, Li L, Cao Y, E M, Shi P, Song C, Li B, Sun H. Activation of AMPK attenuated cardiac fibrosis by inhibiting CDK2 via p21/p27 and miR-29 family pathways in rats. Mol Ther Nucleic Acids. 2017;8:277–290. doi: 10.1016/j.omtn.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hinson JT, Chopra A, Lowe A, Sheng CC, Gupta RM, Kuppusamy R, O'Sullivan J, Rowe G, Wakimoto H, Gorham J, et al. Integrative analysis of PRKAG2 cardiomyopathy iPS and microtissue models identifies AMPK as a regulator of metabolism, survival, and fibrosis. Cell Rep. 2016;17:3292–3304. doi: 10.1016/j.celrep.2016.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tian J, Zhang M, Suo M, Liu D, Wang X, Liu M, Pan J, Jin T, An F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J Cell Mol Med. 2021;25:7642–7659. doi: 10.1111/jcmm.16601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen X, Yang Q, Bai W, Yao W, Liu L, Xing Y, Meng C, Qi P, Dang Y, Qi X. Dapagliflozin attenuates myocardial fibrosis by inhibiting the TGF-β1/Smad signaling pathway in a normoglycemic rabbit model of chronic heart failure. Front Pharmacol. 2022;13:873108. doi: 10.3389/fphar.2022.873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hepworth EMW, Hinton SD. Pseudophosphatases as regulators of MAPK signaling. Int J Mol Sci. 2021;22:12595. doi: 10.3390/ijms222212595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu Z, Sun J, Tong Q, Lin Q, Qian L, Park Y, Zheng Y. The role of ERK1/2 in the development of diabetic cardiomyopathy. Int J Mol Sci. 2016;17:2001. doi: 10.3390/ijms17122001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G, Wang Y, Fu C, Jiang Y, He C, Wei Q. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7:78. doi: 10.1038/s41392-022-00925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 188.Streicher JM, Ren S, Herschman H, Wang Y. MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ Res. 2010;106:1434–1443. doi: 10.1161/CIRCRESAHA.109.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu X, Chen K, Zhuang Y, Huang Y, Sui Y, Zhang Y, Lv L, Zhang G. Paeoniflorin improves pressure overload-induced cardiac remodeling by modulating the MAPK signaling pathway in spontaneously hypertensive rats. Biomed Pharmacother. 2019;111:695–704. doi: 10.1016/j.biopha.2018.12.090. [DOI] [PubMed] [Google Scholar]
- 190.Ren J, Zhang S, Kovacs A, Wang Y, Muslin AJ. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38:617–623. doi: 10.1016/j.yjmcc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 191.Yang N, Zou C, Luo W, Xu D, Wang M, Wang Y, Wu G, Shan P, Liang G. Sclareol attenuates angiotensin II-induced cardiac remodeling and inflammation via inhibiting MAPK signaling. Phytother Res. 2023;37:578–591. doi: 10.1002/ptr.7635. [DOI] [PubMed] [Google Scholar]
- 192.Lin K, Yang N, Luo W, Qian JF, Zhu WW, Ye SJ, Yuan CX, Xu DY, Liang G, Huang WJ, Shan PR. Direct cardio-protection of dapagliflozin against obesity-related cardiomyopathy via NHE1/MAPK signaling. Acta Pharmacol Sin. 2022;43:2624–2635. doi: 10.1038/s41401-022-00885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 196.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 199.Das A, Salloum FN, Filippone SM, Durrant DE, Rokosh G, Bolli R, Kukreja RC. Inhibition of mammalian target of rapamycin protects against reperfusion injury in diabetic heart through STAT3 signaling. Basic Res Cardiol. 2015;110:31. doi: 10.1007/s00395-015-0486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhao X, Lu S, Nie J, Hu X, Luo W, Wu X, Liu H, Feng Q, Chang Z, Liu Y, et al. Phosphoinositide-dependent kinase 1 and mTORC2 synergistically maintain postnatal heart growth and heart function in mice. Mol Cell Biol. 2014;34:1966–1975. doi: 10.1128/MCB.00144-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xia Y, Wen HY, Young ME, Guthrie PH, Taegtmeyer H, Kellems RE. Mammalian target of rapamycin and protein kinase A signaling mediate the cardiac transcriptional response to glutamine. J Biol Chem. 2003;278:13143–13150. doi: 10.1074/jbc.M208500200. [DOI] [PubMed] [Google Scholar]
- 202.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114:549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Moellmann J, Mann PA, Kappel BA, Kahles F, Klinkhammer BM, Boor P, Kramann R, Ghesquiere B, Lebherz C, Marx N, Lehrke M. The sodium-glucose co-transporter-2 inhibitor ertugliflozin modifies the signature of cardiac substrate metabolism and reduces cardiac mTOR signalling, endoplasmic reticulum stress and apoptosis. Diabetes Obes Metab. 2022;24:2263–2272. doi: 10.1111/dom.14814. [DOI] [PubMed] [Google Scholar]
- 204.Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen L, Liu P, Feng X, Ma C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med. 2017;21:3178–3189. doi: 10.1111/jcmm.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sun P, Wang Y, Ding Y, Luo J, Zhong J, Xu N, Zhang Y, Xie W. Canagliflozin attenuates lipotoxicity in cardiomyocytes and protects diabetic mouse hearts by inhibiting the mTOR/HIF-1α pathway. iScience. 2021;24:102521. doi: 10.1016/j.isci.2021.102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Das S, Aiba T, Rosenberg M, Hessler K, Xiao C, Quintero PA, Ottaviano FG, Knight AC, Graham EL, Bostrom P, et al. Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation. 2012;126:2208–2219. doi: 10.1161/CIRCULATIONAHA.112.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lang F, Shumilina E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J. 2013;27:3–12. doi: 10.1096/fj.12-218230. [DOI] [PubMed] [Google Scholar]
- 210.Lee SG, Kim D, Lee JJ, Lee HJ, Moon RK, Lee YJ, Lee SJ, Lee OH, Kim C, Oh J, et al. Dapagliflozin attenuates diabetes-induced diastolic dysfunction and cardiac fibrosis by regulating SGK1 signaling. BMC Med. 2022;20:309. doi: 10.1186/s12916-022-02485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, Siddiqui MA. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation. 2001;104:325–329. doi: 10.1161/01.CIR.104.3.325. [DOI] [PubMed] [Google Scholar]
- 212.Chakraborty D, Šumová B, Mallano T, Chen CW, Distler A, Bergmann C, Ludolph I, Horch RE, Gelse K, Ramming A, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8:1130. doi: 10.1038/s41467-017-01236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sabe SA, Xu CM, Sabra M, Harris DD, Malhotra A, Aboulgheit A, Stanley M, Abid MR, Sellke FW. Canagliflozin improves myocardial perfusion, fibrosis, and function in a swine model of chronic myocardial ischemia. J Am Heart Assoc. 2023;12:e028623. doi: 10.1161/JAHA.122.028623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yu LM, Di WC, Dong X, Li Z, Zhang Y, Xue XD, Xu YL, Zhang J, Xiao X, Han JS, et al. Melatonin protects diabetic heart against ischemia-reperfusion injury, role of membrane receptor-dependent cGMP-PKG activation. Biochim Biophys Acta Mol Basis Dis. 2018;1864:563–578. doi: 10.1016/j.bbadis.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 215.Mátyás C, Németh BT, Oláh A, Hidi L, Birtalan E, Kellermayer D, Ruppert M, Korkmaz-Icöz S, Kökény G, Horváth EM, et al. The soluble guanylate cyclase activator cinaciguat prevents cardiac dysfunction in a rat model of type-1 diabetes mellitus. Cardiovasc Diabetol. 2015;14:145. doi: 10.1186/s12933-015-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xue M, Li T, Wang Y, Chang Y, Cheng Y, Lu Y, Liu X, Xu L, Li X, Yu X, et al. Empagliflozin prevents cardiomyopathy via sGC-cGMP-PKG pathway in type 2 diabetes mice. Clin Sci (Lond) 2019;133:1705–1720. doi: 10.1042/CS20190585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.