Abstract
Transcription of the cytosine deaminase (codBA) operon of Escherichia coli is regulated by nitrogen, with about three times more codBA expression in cells grown in nitrogen-limiting medium than in nitrogen-excess medium. β-Galactosidase expression from codBp-lacZ operon fusions showed that the nitrogen assimilation control protein NAC was necessary for this regulation. In vitro transcription from the codBA promoter with purified RNA polymerase was stimulated by the addition of purified NAC, confirming that no other factors are required. Gel mobility shifts and DNase I footprints showed that NAC binds to a site centered at position −59 relative to the start site of transcription and that mutants that cannot bind NAC there cannot activate transcription. When a longer promoter region (positions −120 to +67) was used, a double footprint was seen with a second 26-bp footprint separated from the first by a hypersensitive site. When a shorter fragment was used (positions −83 to +67), only the primary footprint was seen. Nevertheless, both the shorter and longer fragments showed NAC-mediated regulation in vivo. Cytosine deaminase expression in Klebsiella pneumoniae was also regulated by nitrogen in a NAC-dependent manner. K. pneumoniae differs from E. coli in having two cytosine deaminase genes, an intervening open reading frame between the codB and codA orthologs, and a different response to hypoxanthine which increased cod expression in K. pneumoniae but decreased it in E. coli.
Escherichia coli can utilize cytosine as its sole source of nitrogen by using cytosine deaminase to cleave cytosine to ammonia (a source of nitrogen) and uracil (a source of pyrimidines). Cytosine deaminase formation is subject to a complex regulation that responds to purines, pyrimidines, and the nitrogen supply of the medium (1). A mutant that expressed a constitutively active Ntr system constitutively expressed cytosine deaminase. This was taken as evidence that the Ntr system of E. coli is involved in the regulation of cytosine deaminase formation (1).
The Ntr system is a complex regulatory cascade that senses the quality of the available nitrogen supply and ultimately results in the activation of a transcriptional activator, NTRC, when the nitrogen source is growth rate limiting (20). Many of the genes in the Ntr regulon are directly regulated by the NTRC-mediated activation of RNA polymerase bearing the unusual sigma factor σ54. But some genes in the Ntr regulon are regulated indirectly, and their transcription is activated by the nitrogen assimilation control protein NAC, which activates RNA polymerase bearing the more common sigma factor σ70 (4).
When NAC activates transcription, it binds to a consensus sequence often abbreviated ATA-N9-TAT (26). A search of available DNA sequence data for sequences resembling this site uncovered many such sequences within the E. coli genome (12), one of which was in the promoter region of codBA, the operon that encodes cytosine deaminase (codA) and a cytosine permease (codB). When a nac mutant of E. coli was tested for growth with cytosine as the sole nitrogen source, the nac mutant grew more slowly than the wild type (23). The presence of a NAC site in the codBA promoter region and the slow growth phenotype of a nac mutant when cytosine was the sole nitrogen course suggested that NAC was involved in the nitrogen regulation of codBA.
An analysis using microarrays to measure steady-state mRNA levels in response to physiological and genetic signals that affect nitrogen regulation has been recently reported (28). These data show that codBA expression is indeed regulated by nitrogen, that this response requires the Ntr system, and that the response also requires NAC. The experiments reported here were designed to determine whether NAC directly regulates the codBA operon or whether NAC might act indirectly.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Descriptions and genotypes of the bacterial strains used here are listed in Table 1. All E. coli strains were derived from E. coli strain K-12. All Klebsiella pneumoniae strains were derived from strain W70. This strain was formerly known as Klebsiella aerogenes; however, the species K. aerogenes has been subsumed into the species K. pneumoniae.
TABLE 1.
List of strains
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| E. colia | ||
| DH5α | Δ(cod-lac) | 16 |
| W3110 | Wild type | R. Matthews |
| YMC10 | Δ(cod-lac)U169 | 2 |
| YMC15 | Δ(cod-lac)U169 glnL2302 | 6 |
| EB3264 | DH5α/pCB630 | This work |
| EB3364 | W3110 nac-28 | 22 |
| EB3846 | W3110/pPC36 | 17 |
| EB3870 | YMC10 nac-28/pPC36/pCB911 | This work |
| EB3872 | YMC10 nac-28/pPC36/pCB910 | This work |
| EB3874 | YMC15/pCB910 | This work |
| EB3875 | YMC15 nac-28/pCB910 | This work |
| EB3876 | YMC10/pCB910 | This work |
| EB3877 | YMC10 nac-28/pCB910 | This work |
| EB3878 | YMC15/pCB911 | This work |
| EB3879 | YMC15 nac-28/pCB911 | This work |
| EB3880 | YMC10/pCB911 | This work |
| EB3881 | YMC10 nac-28/pCB911 | This work |
| EB5550 | YMC10/pCB1375 | This work |
| EB5551 | YMC10 nac-28/pCB1375 | This work |
| K. pneumoniaeb | ||
| KC2668 | Wild type | 17 |
| KC2725 | KC2668 nac-203::Tn5-131 | 17 |
All E. coli strains are derived from E. coli strain K-12.
All K. pneumoniae strains are derived from K. aerogenes (now called K. pneumoniae) strain W70.
Enzyme assays.
β-Galactosidase was measured as described previously (27) except that the assay was performed at 30°C. For cytosine deaminase assays, cells were grown to mid-log phase, collected by centrifugation, and washed once with cold assay buffer (50 mM HEPES, pH 7.8). The cells were resuspended in 1/10 of their original volume of assay buffer and disrupted by sonic oscillation. Cellular debris was removed by centrifugation. The resulting cell extract contained about 1 mg of total protein/ml, determined by the method of Lowry (19). The reaction mixture contained 0.4 ml of assay buffer containing 50 mM cytosine equilibrated to 37°C. The reaction was initiated by the addition of 50 μl of the cell extract, samples were removed at 0.5, 10, 20, and 30 min, and the ammonia released from cytosine was measured as described previously (11). Specific activities are reported in nanomoles of ammonia released per minute per milligram of total protein.
In vitro transcription.
In vitro transcription from the codBA promoter in plasmid pCB592 was carried out essentially as described by Maquat and Reznikoff (21) by using RNA polymerase with σ70 (purchased from Epicentre Technologies) and NAC (purified essentially as described by Goss and Bender [12]). [α-32P]UTP was included in the reaction mixture, and the resulting radioactive transcripts were separated by polyacrylamide gel electrophoresis and visualized by autoradiography.
Gel mobility shift assays.
DNA fragments were isolated and labeled with [α-32P]dATP by using the Klenow fragment (Roche) according to the manufacturer's instructions. Labeled DNA fragments were incubated with increasing concentrations of purified NAC protein with 50 mM Tris, pH 7.5, and 20 nM poly(d · IC) in a total volume of 5 μl. The binding mixtures were incubated for 20 min at 30°C, and after this incubation period, 1 μl of DNA loading buffer (2 mg of bromophenol blue, 2 mg of xylene cyanol, 40 mM Tris [pH 8.4], 4 mM EDTA [pH 8.0], 25% glycerol) was added. Each reaction was loaded onto a Tris-borate-EDTA 5% polyacrylamide gel, and the gel was run, treated, and exposed to film as previously described (26).
DNase I footprinting.
Plasmid DNA was linearized by digestion with EcoRI or HindIII, and the resulting 3′ ends were labeled with the Klenow fragment of DNA polymerase I. The labeled DNA was digested with a second restriction enzyme to generate two end-labeled fragments, one less than 20 bp and the other carrying the promoter of interest. A 7-μl sample containing about 0.2 pmol of DNA (about 500,000 cpm) was mixed with purified NAC and allowed to incubate for 30 min at room temperature. Digestion was initiated by the addition of 10−3 U of DNase I and terminated 2 min later by the addition of 7 μl of formamide, and samples were incubated at 80°C for 5 min. Samples were prepared for electrophoresis, and the digested fragments were separated by electrophoresis in polyacrylamide gels containing 7 M urea. The results were visualized by autoradiography. DNase I digestion patterns were aligned with the DNA sequence by comparison with the G and/or A+G sequencing ladders that were run on the same gels.
Construction of plasmids.
The plasmid pRAC82 (a kind gift of Jan Neuhard) carries a HaeIII fragment of E. coli DNA cloned into the SmaI site in pUC19. The full-length codBA promoter region, extending from positions −120 to +67 relative to the start site of transcription, was subcloned as an EcoRI-BamHI fragment into the lacZ expression vector pRJ800 (3), resulting in plasmid pCB1375. Plasmid pCB910 contains a shorter fragment extending from positions −83 to +67 that was generated by PCR. Plasmid pCB911 was constructed exactly as was pCB910, except that the upstream primer contained an A-to-C change in the first nucleotide of the NAC-binding sequence. Thus, plasmid pCB911 is identical to pCB910 except that pCB910 carries the sequence ATA-N9-TAT and pCB911 carries CTA-N9-TAT. Plasmids pCB816 and pCB864 (carrying the longer and shorter promoter regions, respectively) were used for DNase I footprinting. Plasmid pCB816 carries an EcoRI-HindIII fragment from positions −120 to +67 cloned into pBC KS+. Plasmid pCB864 carries the region from positions −83 to +67 cloned as an EcoRI-BamHI fragment in pBC KS+. Thus, pCB816 and pCB864 are in opposite orientations in pBC KS+. Plasmid pCB592 carries the full-length codBA promoter region (positions −120 to +67) cloned upstream from the strong transcription terminator in pTE103 (9).
A cytosine deaminase gene from K. pneumoniae was initially cloned by using the in vivo method based on phage Mu and described by Groisman and Casadaban (15). The resulting plasmid, pCB619, contained about 5 kb of K. pneumoniae DNA inserted into pEG5005. Plasmid pCB619 allowed strain YMC10 (Δcod-lac) to grow with cytosine as the sole nitrogen source. A 7.4-kb HindIII fragment from pCB619 (containing the kanamycin resistance element from pEG5005 as well as the codA gene from K. pneumoniae) was subcloned into pGB2 (7), resulting in pCB628. A 6.6kb SacI-EcoRI fragment from pCB628 was then subcloned into pBluescript (pBS KS−), resulting in pCB630.
RESULTS
NAC-induced sensitivity to fluorocytosine.
Wild-type strains of E. coli are sensitive to the cytosine analog fluorocytosine because this compound is metabolized to the toxic 5-fluorouracil by cytosine deaminase (24). However, codBA mutants are resistant to this compound. As an initial estimate of NAC participation in codBA regulation, disks containing fluorocytosine were placed on lawns of wild-type and nac strains growing with various nitrogen sources. As expected, nitrogen-limiting medium resulted in larger zones of growth inhibition for the wild-type strain than nitrogen-excess medium (Table 2). In contrast, strains carrying nac-28, a null mutation of the nac gene, showed the same small zone of inhibition whether growing on nitrogen-limiting or nitrogen-excess medium.
TABLE 2.
Sensitivity to 5-fluorocytosine
| Strain | Relevant genotype | Mediuma | Zone of clearingb (mm) |
|---|---|---|---|
| W3110 | Wild type (E. coli) | GNArg | 21 |
| W3110 | Wild type (E. coli) | GArg | 71 |
| EB3366 | nac-28 | GNArg | 21 |
| EB3366 | nac-28 | GArg | 23 |
| EB3846c | Wild type/pPC36 (inducible NACK) | GN | 37 |
| EB3846 | Wild type/pPC36 (inducible NACK) | GN IPTG | >75 |
| KC2668 | Wild type (K. pneumoniae) | GN | 12 |
| KC2668 | Wild type (K. pneumoniae) | GGlt | 61 |
| KC2725 | nac-203::Tn5-131 | GGlt | 14 |
| W3110 | Wild type (E. coli) | GNArg+Hpxt | 6 |
| W3110 | Wild type (E. coli) | GArg+Hpxt | 10 |
| KC2668 | Wild type (K. pneumoniae) | GN+Hpxt | 25 |
| KC2668 | Wild type (K. pneumoniae) | GGlt+Hpxt | 75 |
Growth medium contained the following: G, 0.4% (wt/vol) glucose; N, 0.2% (wt/vol) ammonium sulfate; Arg, 0.2% (wt/vol) l-arginine hydrochloride; IPTG, 0.5 mM IPTG; Glt, 0.2% (wt/vol) monosodium glutamate; Hpxt, 50 μg of hypoxanthine/ml.
Small disks were saturated with a solution of 5-fluorocytosine at 50 μg/ml for E. coli strains and 600 μg/ml for K. pneumoniae strains.
Plasmid pPC36 carries an IPTG-inducible gene that encodes NAC from K. pneumoniae (NACK).
The NAC protein from K. pneumoniae is very similar to NAC from E. coli and can functionally replace E. coli NAC in all assays tested (23). When strain EB3846 (W3110 with an isopropyl-β-d-thiogalactopyranoside [IPTG]-inducible nac gene from K. pneumoniae) was induced with IPTG, the strain was sensitive to fluorocytosine even when grown on nitrogen-excess medium. Thus, NAC was necessary for codBA-mediated sensitivity to fluorocytosine. Neither NTRC nor nitrogen limitation was required if NAC was provided.
Cytosine deaminase in K. pneumoniae.
Our laboratory has a longstanding interest in nitrogen regulation in K. pneumoniae where nitrogen-regulated genes are more numerous and often more tightly regulated than those of E. coli. Therefore, we examined the regulation of cytosine deaminase expression in K. pneumoniae. Wild-type K. pneumoniae strain KC2668 was much more sensitive to fluorocytosine under nitrogen-limiting conditions than under nitrogen excess (Table 2). Moreover, a nac mutation abolished the increased sensitivity (Table 2, strain KC2725), suggesting that K. pneumoniae has a NAC-regulated cytosine deaminase.
Surprisingly, E. coli and K. pneumoniae differed in their response to the presence of hypoxanthine. In E. coli, the presence of hypoxanthine in the medium reduced codBA expression because codBA is under the control of the purR repressor protein in addition to control by NAC (1). As a result, hypoxanthine reduced the sensitivity of E. coli to fluorocytosine (Table 2, strain W3110). In contrast, hypoxanthine increased the sensitivity of K. pneumoniae to fluorocytosine (Table 2, strain KC2668).
NAC-dependent activation of codBA expression in vivo.
The data in Table 3 show that cytosine deaminase formation is derepressed about threefold by nitrogen-limited growth in E. coli (strain W3110). This confirms the earlier data of Anderson et al. (1) by using a different assay. This derepression was abolished in the nac mutant, strain EB3366. In strain EB3846, which carries the IPTG-inducible nac gene on pPC36, the addition of IPTG led to a threefold induction of cytosine deaminase even in nitrogen-excess medium. Thus, NAC was necessary for the derepression of cytosine deaminase formation.
TABLE 3.
Cytosine deaminase activity and β-galactosidase expression from codB-lacZ fusions
| Strain | Genotype or description | Growth mediuma | Cytosine deaminaseb | β-Galactosidaseb |
|---|---|---|---|---|
| W3110 | Wild type | GNArg | 50 | |
| W3110 | Wild type | GArg | 165 | |
| EB3366 | nac-28 | GNArg | 43 | |
| EB3366 | nac-28 | GArg | 52 | |
| EB3846c | nac+/pPC36 (NACK inducible) | GN | 78 | |
| EB3846 | nac+/pPC36 (NACK inducible) | GN IPTG | 211 | |
| EB5550 | Δ(cod-lac)/pCB1375 | GNArg | 1360 | |
| EB5550 | Δ(cod-lac)/pCB1375 | GArg | 7470 | |
| EB5551 | Δ(cod-lac) nac-28/pCB1375 | GNArg | 1530 | |
| EB5551 | Δ(cod-lac) nac-28/pCB1375 | GArg | 1420 | |
| EB3876 | Δ(cod-lac)/pCB910 | GArg | 4830 | |
| EB3877 | Δ(cod-lac) nac-28/pCB910 | GArg | 1932 | |
| EB3874d | Δ(cod-lac) glnL2302/pCB910 | GN | 4291 | |
| EB3875 | Δ(cod-lac) glnL2302 nac-28/pCB910 | GN | 1430 | |
| EB3872 | Δ(cod-lac) nac-28/pPC36/pCB910 | GN | 1432 | |
| EB3872 | Δ(cod-lac) nac-28/pPC36/pCB910 | GN IPTG | 4152 | |
| EB3880 | Δ(cod-lac)/pCB911 | GArg | 1110 | |
| EB3881 | Δ(cod-lac) nac-28/pCB911 | GArg | 1233 | |
| EB3878 | Δ(cod-lac) glnL2302/pCB911 | GN | 1267 | |
| EB3879 | Δ(cod-lac) glnL2302 nac-28/pCB911 | GN | 1176 | |
| EB3870 | Δ(cod-lac) nac-28/pPC36/pCB911 | GN | 939 | |
| EB3870 | Δ(cod-lac) nac-28/pPC36/pCB911 | GN IPTG | 1241 | |
| EB3264e | Δ(cod-lac)/pCB630 (codAK) | GNArg | 311 | |
| EB3264 | Δ(cod-lac)/pCB630 (codAK) | GArg | 1545 |
W4 minimal medium (14) was supplemented with the following: G, 0.4% (wt/vol) glucose; N, 0.2% (wt/vol) ammonium sulfate; Arg, 0.2% l-arginine hydrochloride; IPTG, 0.5 mM IPTG.
β-Galactosidase and cytosine deaminase assays are reported as the specific activity and are the averages from three or more experiments.
Plasmid pPC36 carries an IPTG-inducible gene which encodes NAC from K. pneumoniae.
Plasmid pCB910 carries a short fragment of the codBA promoter region; pCB911 carries the same region but with a mutation in the NAC-binding site. The glnL2302 mutation causes high-level expression of the Ntr system, even in the presence of ammonia.
Plasmid pCB630 carries the K. pneumoniae codA gene.
To confirm that this derepression reflected a transcriptional control of codBA expression, we took advantage of three plasmids. Plasmid pCB1375 contains codBA promoter DNA from positions −120 to +67 relative to the start of transcription. (The key features of the codBA promoter region are illustrated in Fig. 1). Plasmid pCB910 contained the region from positions −83 to +67, and plasmid pCB911 was identical to pCB910 except that the conserved ATA of the recognized NAC consensus was changed to CTA (an A-to-C change at position −66). This change should abolish NAC binding (26).
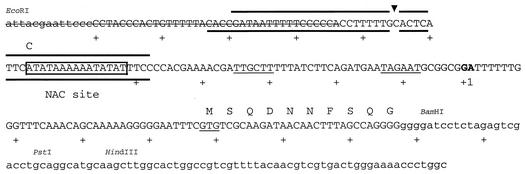
FIG. 1.
DNA sequence of the codBA promoter region showing key features. The DNA sequence is as found in pRAC82. Small letters represent vector DNA; capital letters represent E. coli DNA. Boxed nucleotides are the NAC consensus sequence. Over- and underscoring show the extent of the DNase I footprint of NAC on the top and bottom strands of this fragment (Fig. 3 and data not shown). The arrowhead indicates the strong hypersensitive site. The letters with a line through them represent the segment that was deleted (replaced) in the shorter promoter in pCB910 and pCB911. The bold letters at position +1 are the start sites of transcription (8). Underlined sequences are putative −10 and −35 regions, and GTG is the start site of the codB-coding sequence. The C above the A at position −66 indicates the nucleotide changed in the construction of pCB911 from pCB910.
The longer cod-lac fusion in pCB1375 showed a fivefold derepression in response to nitrogen limitation (Table 3, strain EB5550), and this derepression was not seen in the congenic strain carrying the nac-28 mutation (Table 3, strain EB5551). The shorter cod-lac fusion in pCB910 showed a threefold response to NAC (the same as seen for chromosomally encoded cytosine deaminase) in three separate comparisons. First, a wild-type strain carrying pCB910 had high-level expression of the cod-lac fusion in response to nitrogen limitation (Table 3, strain EB3876). A congenic strain carrying the nac-28 mutation had almost threefold less (strain EB3877). Second, a strain that had a constitutively active Ntr system caused by the glnL2303 mutation had high-level expression of the cod-lac fusion even in nitrogen-rich medium (Table 3, strain EB3874); again, a congenic strain carrying the nac-28 mutation had about threefold less (strain EB3875). Finally, a strain carrying the nac-28 mutation and an IPTG-inducible nac gene (on plasmid pPC36) had high-level expression of the cod-lac fusion when IPTG was added, even though the cells were grown in nitrogen-rich medium (Table 3, strain EB3872). The same strain grown without IPTG induction had about threefold less. Thus, NAC was necessary for the derepression of a cod-lac transcriptional fusion in vivo and the NAC-mediated control of cytosine deaminase formation functioned at the level of transcription.
Plasmid pCB911 with its defective NAC site was used in the same three tests as pCB910. Under nitrogen-limiting conditions, a wild-type strain showed no more cod-lac expression than a strain carrying the nac-28 mutation (Table 3, strains EB3880 and EB3881). The Ntr constitutive strain showed no more cod-lac expression than the nac-28 strain (Table 3, strains EB3878 and EB3879). Finally, even IPTG induction of the nac gene on pPC36 resulted in only a slight (about 30%) increase in cod-lac expression. Thus, the ATA-N9-TAT sequence thought to be important for NAC binding was necessary for the NAC-mediated activation of codBA transcription in vivo.
NAC binds to the codBA promoter.
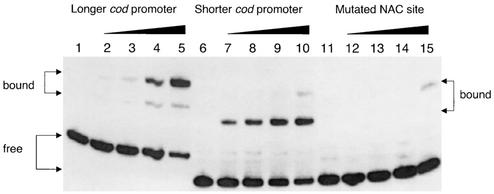
The importance of the ATA-N9-TAT sequence for NAC binding was confirmed by gel mobility shift analysis and by DNase I footprinting. Figure 2 shows that a DNA fragment containing the mutant codBA promoter from pCB911 was defective in NAC binding, whereas comparable wild-type fragments from pRAC82 and pCB910 did bind NAC. However, the fragments from pRAC82 and pCB910 showed different patterns of mobility shift by NAC. In both cases, two specific shifts were detected. When the longer fragment (positions −120 to +67) was used, the slower-migrating band predominated. When the shorter fragment was used, the faster-migrating band predominated. Curiously, the only shift detected with the mutant fragment was the slower-migrating one, which, though weak, was about as strong as the comparable band in the fragment from pCB910 (Fig. 2, lanes 10 and 15).
FIG. 2.
Gel mobility shift assay of the interaction of NAC with the codBA promoter region. Each lane contains about 100,000 cpm of 32P-end-labeled DNA. Lanes 1 through 5 (longer promoter), the EcoRI-HindIII fragment from pRAC82 containing promoter DNA from positions −120 to +67; lanes 6 through 10 (shorter promoter), an EcoRI-BamHI fragment from pCB910 containing promoter DNA from positions −83 to +67; lanes 11 through 15 (mutant promoter), an EcoRI-BamHI fragment from pCB911 containing the same promoter DNA (positions −83 to +67) but with an A-to-C mutation in the NAC-binding site. In each set of five, NAC was absent from the first lane and the remaining four samples were incubated with 7, 26, 66, or 132 ng of NAC before electrophoresis.
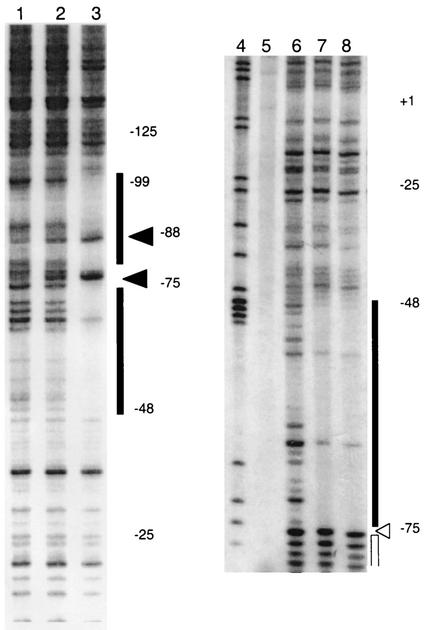
The DNase I footprints of NAC bound to the longer promoter (positions −120 to +67) showed strong protection of a 26- to 27-bp region from positions −48 to −75 with the NAC consensus ATA-N9-TAT at its center (Fig. 3, lanes 1 through 3). On the promoter-distal side of this protected region was a site with increased DNase I sensitivity followed by an extended footprint of about 25 to 28 bp. When the shorter promoter (with DNA upstream from position −83 replaced by vector DNA) was analyzed, the core footprint from positions −48 to −75 centered on ATA-N9-TAT was again observed (Fig. 3, lanes 4 through 8). The locations of the DNase I footprints and the DNA replacement are illustrated in Fig. 1. Neither the hypersensitive site nor the extended footprint were seen with the short promoter. Thus, the short promoter (from positions −83 to +67) bound NAC at the consensus site, protected a 26-bp region as seen elsewhere (12), and caused a modest gel mobility shift with a small fraction shifted to the more slowly migrating species. The long promoter (positions −120 to +67) gave two adjacent footprints of similar size (roughly 26 bp) separated by a hypersensitive site. In mobility shift assays, the longer promoter showed only a trace amount of the modest shift, with the majority of the shifted target belonging to the more slowly migrating species.
FIG. 3.
DNase I protection assay of the codBA promoter. Lanes 1 through 3 show the top strand of pCB816 (labeled at the HindIII site), which contained the longer promoter fragment. Lanes 6 through 8 show the DNase I footprint of NAC on pCB864 (labeled at the HindIII site) which contained the shorter promoter fragment. Lane 4 contained the G ladder, and lane 5 contained the no-DNase I control. Lanes 1, 2, and 3 contained 0, 0.1, and 0.5 μg of NAC, respectively. Lanes 6, 7, and 8 contained 0, 0.4, and 0.8 μg of NAC, respectively. Protected regions are indicated with the black bar, and hypersensitive sites are marked with black arrows. The open bar and arrowhead indicate the protected region and hypersensitive site seen in the longer codBA promoter construct, respectively. For comparison, the protected sequences are indicated in Fig. 1.
In vitro transcription of codBA.
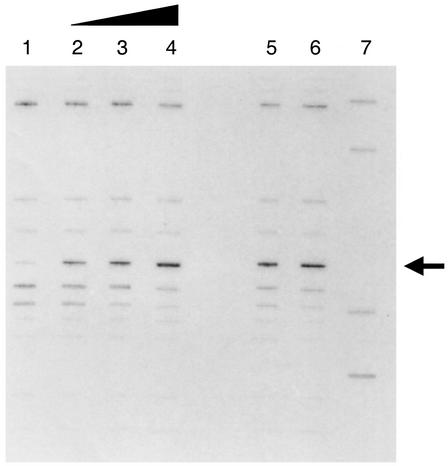
To confirm that NAC activation of codBA expression was direct and required no other factors, we analyzed the ability of NAC to activate transcription from the codBA promoter in vitro by using E. coli RNA polymerase in a purified system. The codBA promoter was cloned upstream of a strong transcriptional terminator such that transcription from codBp would yield a discrete RNA molecule. Figure 4 shows that RNA polymerase alone can generate a transcript of the expected size and that the addition of purified NAC increased the amount of this transcript at least threefold. This shows clearly that NAC alone is sufficient to activate codBA transcription by binding to a site centered at position −59.
FIG. 4.
In vitro transcription from the codBA promoter. The DNA template was supercoiled plasmid pCB592. The reactions in lanes 1 through 6 contained purified RNA polymerase from E. coli. Lane 1 contained no NAC. Lanes 2 through 4 contained 0.1, 0.2, and 1.0 μg of NAC derived from K. pneumoniae, respectively. Lanes 5 and 6 contained 1 μg of NAC (from K. pneumoniae and E. coli, respectively) prepared as an MBP-NAC fusion protein and cleaved with factor Xa to release active NAC immediately before addition to the reaction mixture. Lane 7 contained labeled DNA size standards of 210, 245, 400, and 510 bp. The arrow indicates the runoff transcript from the codB promoter.
The NAC used in this experiment was purified from K. pneumoniae. The E. coli NAC protein is insoluble, and we have not yet succeeded in purifying it in quantity (22). To show that the E. coli NAC can activate codBA transcription, we used a fusion of the E. coli maltose-binding protein (MBP) to the N terminus of NAC. The two proteins were joined by a linker that is readily cleaved by factor Xa protease. Although incubation of this fusion protein with factor Xa liberated intact MBP and NAC, the NAC that is released was rapidly degraded by factor Xa. Therefore, we incubated the MBP-NAC fusion protein (and a comparable fusion of MBP to NAC from K. pneumoniae) with factor Xa for a brief period and immediately added the mixture to the RNA polymerase and codBA DNA reaction. As can be seen in Fig. 4 (lanes 5 and 6), the E. coli NAC was as effective in activating codBA transcription as the K. pneumoniae NAC was.
Two cytosine deaminase genes in K. pneumoniae.
We sought a clone of the gene for cytosine deaminase from K. pneumoniae for comparative study. The in vivo cloning procedure of Groisman and Casadaban (15) yielded plasmid pCB619 (moderate copy number). The E. coli Δcod-lac strain, YMC10, carrying this plasmid (strain EB3246) was able to grow with cytosine as the sole nitrogen source and was sensitive to fluorocytosine. A DNA fragment from pCB619 was subcloned into a low-copy-number plasmid (pCB628) and a high-copy-number plasmid (pCB630) (see Materials and Methods). Plasmid pCB628 allowed the E. coli Δcod-lac strain DH5α to grow with cytosine as sole nitrogen source. Plasmid pCB630 conferred cytosine growth and fluorocytosine sensitivity on strain EB3264 (DH5α/pCB630), but it also made this strain unable to grow on minimal medium unless supplemented with cytosine. Enzyme assays confirmed that strain EB3264 produced a cytosine deaminating activity and that nitrogen limitation led to a fivefold derepression of this cytosine deaminase (Table 3). Thus the cloned fragment contained all the information to produce cytosine deaminase whose formation was regulated by nitrogen.
The DNA sequence of two contiguous BamHI fragments from pCB630 was determined, representing about 5.8 kb of DNA. This sequence was compared to the genomic sequence of K. pneumoniae strain MGH78578 that was determined by the Genome Sequencing Center at Washington University at St. Louis (http://www.genome.wustl.edu) and was identical except for 1 nucleotide. This 5.8-kb region contained a number of open reading frames (ORFs), of which three were of particular interest. These three ORFs span a continuous sequence of 3,657 bp such that the termination codon of each ORF (TGA in each case) overlapped the initiation codon of the next ORF. When the deduced amino acid sequences of these three ORFs were compared to the sequences of deduced E. coli proteins, the first ORF showed similarity to the codB (cytosine permease) protein and the third ORF showed strong similarity to the codA (cytosine deaminase) protein. The deduced amino acid sequence of the intervening ORF (predicted to be 411 amino acids long) showed no strong similarity to any E. coli protein. Thus, the organization of this putative operon differs from the codBA operon of E. coli.
Surprisingly, when the codA gene from E. coli was compared to the genomic sequence of K. pneumoniae strain MGH78578, a second unlinked gene that was even more similar to E. coli codA than was the ortholog on pCB630 was found. Perhaps this may explain another peculiar difference between E. coli and K. pneumoniae.
DISCUSSION
The data presented here confirm previous observations that cytosine deaminase formation is regulated by the Ntr system acting through the NAC protein (1, 28). The present study also shows that the activation of codBA transcription by NAC is direct. However, the activation of codBA transcription by NAC is unusual in several respects.
Many of the previous examples of promoters strongly activated by NAC have a NAC-binding site centered at −64 relative to the start site of transcription (4). An analysis of NAC-mediated activation by using the lacZ promoter region confirmed that the strongest activation occurred when the NAC site was centered at position −64 (25). However, significant activation (about threefold) was observed when the NAC site was centered at position −42, −52, or −54. Consistent with these artificial constructs, we found that the alanine utilization operon (dadAB) from K. pneumoniae is activated about threefold and has a NAC site centered at position −44 (17). However, no activation of the lacZ promoter was seen when the NAC-binding site of the artificial constructs was moved 5 bp from these sites to position −47, −49, or −59 (25). Yet NAC can activate codBA transcription from a site centered at position −59. Such a location should put the NAC binding on the opposite side of the DNA helix from all other known NAC activation sites.
The location of the NAC-binding site at cod is not its only unusual feature; the architecture of the site is also unusual. The consensus sequence for NAC-activated promoters is ATA-N9-TAT. At these sites, NAC generates a simple footprint of about 26 bp with the consensus sequence located at the center. Occasionally, a slight increase in DNase I sensitivity is seen just at the promoter-proximal edge of the footprint but it is always very slight at best. The consensus-binding site for NAC-repressible promoters (gdhA and nac) includes ATA-N9-GAT (13). At these sites, NAC has a complex footprint consisting of a core footprint of 26-bp that includes the ATA-N9-GAT sequence, a region of strong DNase I hypersensitivity, and an extended footprint of similar size covering a nucleotide sequence that bears no recognizable similarity to the known consensus sequences for NAC binding. At nac, the extended footprint is strong and has distinct endpoints (10). At gdhA, the extended footprint is weaker and its endpoint is less distinct (13). In addition, gel mobility shift assays at gdhA show two retarded species in contrast to the single species seen at activatable promoters (18). The extended footprints seen in DNase I footprints and the slower-migrating bands seen in gel shifts at gdhA and codBA suggest the presence of a second NAC dimer (possibly NAC tetramers) bound to the DNA. Thus, the footprints and gel mobility shift pattern of NAC at codBA resemble those of NAC-repressible promoters more closely than other NAC-activatable promoters. The significance of this observation is unclear, especially since replacement of part of the extended footprint with vector DNA sequences abolished the complex footprint and gel mobility shift patterns without abolishing the NAC-mediated activation.
One other feature of the NAC site at codBA is its extreme AT richness. The codBA promoter region (positions −120 to +67) includes five runs of T5 or T6 as well as a run of A6 that lies in the center of the NAC-binding site. In fact, the 15 bp consensus region for NAC binding at codBA contains only A's and T's.
The identification of two putative cytosine deaminase genes in K. pneumoniae was unexpected. The gene that was cloned in pCB630 certainly encodes a cytosine deaminase. First, it allows a Δcod-lac deletion of E. coli to use cytosine as its sole nitrogen source. Second, it confers sensitivity to fluorocytosine on strains that carry it. And third, cell extracts of strains that carry this gene contain an activity that can release ammonia from cytosine. We have no direct evidence that the other codA ortholog is an authentic cytosine deaminase gene, but its strong similarity to the E. coli codA gene makes it likely.
The codBA operon of E. coli is part of the “insertion bubble” that distinguishes the lacZYA region of E. coli from other enteric bacteria (5). Thus, one may ask where the codA genes of K. pneumoniae lie. The ORFs on pCB630 give no clue to this question. However, a comparison of the genes on the same DNA segment of the K. pneumoniae genomic sequence strongly suggests that the codA ortholog on pCB630 lies at a position analogous to min 13 of the E. coli genetic map and that the other codA ortholog lies at min 75. About 15 kb from the codA ortholog found on pCB630 is a sequence of ORFs that are orthologous to E. coli fepB, entC, entE, entA, cstA, and ybdH (in that order and transcribed convergently toward the 3′ end of codA). That places this ortholog near min 13. The other codA ortholog appears to be neatly inserted between the nirB gene (part of the nitrite reductase operon) and an unidentified ORF (orf383). In E. coli, orf393 and nirB are separated by 262 bp and are transcribed in the same direction. In K. pneumoniae, the codA ortholog is 39 bp downstream from orf393, 239 bp upstream from nirB, and transcribed in the direction opposite both of them. This places the other codA ortholog at min 75, near the crp gene.
The regulation of cytosine deaminase formation in E. coli is quite complex. This is expected because the deamination of cytosine can lead to an imbalance in pyrimidine pools. The threefold increase in cytosine deaminase formation mediated by NAC has a profound effect on fluorocytosine sensitivity. The 10-fold increase seen when the high-copy-number clone pCB630 was present seems to have led to such rapid degradation of endogenous cytosine that the cells were unable to synthesize enough cytosine to allow growth and could grow only when an exogenous source was provided. In other words, even small changes in expression can have serious consequences and therefore, tight regulation is necessary.
Acknowledgments
We are grateful to J. Neuhard for providing plasmid pRAC82, to B. K. Janes for his help in preparing the manuscript and for providing plasmid pCB1375, and to T. J. Goss for his advice about in vitro transcription and NAC purification.
This work was supported by Public Health Service grant GM47156 from the National Institutes of Health to R.A.B.
REFERENCES
- 1.Anderson, L., M. Kilstrup, and J. Neuhard. 1989. Pyrimidine, purine and nitrogen control of cytosine deaminase synthesis in Escherichia coli K 12. Involvement of the glnLG and purR genes in the regulation of codA expression. Arch. Microbiol. 152:115-118. [DOI] [PubMed] [Google Scholar]
- 2.Backman, K., Y. M. Chen, and B. Magasanik. 1981. Physical and genetic characterization of the glnA-glnG region of the Escherichia coli chromosome. Proc. Natl. Acad. Sci. USA 78:3743-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball, C. A., R. Osuna, K. C. Ferguson, and R. C. Johnson. 1992. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J. Bacteriol. 174:8043-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, R. A. 1991. The role of the NAC protein in the nitrogen regulation of Klebsiella aerogenes. Mol. Microbiol. 11:2575-2580. [DOI] [PubMed] [Google Scholar]
- 5.Buvinger, W. E., K. A. Lampel, R. J. Bojanowski, and M. Riley. 1984. Location and analysis of nucleotide sequences at one end of a putative lac transposon in the Escherichia coli chromosome. J. Bacteriol. 159:618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y. M., K. Backman, and B. Magasanik. 1982. Characterization of a gene, glnL, the product of which is involved in the regulation of nitrogen utilization in Escherichia coli. J. Bacteriol. 150:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchward, G., D. Belin, and Y. Nagamine. 1984. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene 31:165-171. [DOI] [PubMed] [Google Scholar]
- 8.Danielson, S., M. Kilstrup, K. Barilla, B. Jochimsen, and J. Neuhard. 1992. Characterization of the Escherichia coli codBA operon encoding cytosine permease and cytosine deaminase. Mol. Microbiol. 6:1335-1344. [DOI] [PubMed] [Google Scholar]
- 9.Elliott, T., and E. P. Geiduschek. 1984. Defining a bacteriophage T4 late promoter: absence of a “-35” region. Cell 36:211-219. [DOI] [PubMed] [Google Scholar]
- 10.Feng, J., T. J. Goss, R. A. Bender, and A. J. Ninfa. 1995. Repression of the Klebsiella aerogenes nac promoter. J. Bacteriol. 177:5535-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich, C. G., and G. Mitrenga. 1981. Utilization of aliphatic amides and formation of two different amidases by Alcaligenes eutrophus. J. Gen. Microbiol. 125:367-374. [Google Scholar]
- 12.Goss, T. J., and R. A. Bender. 1995. The nitrogen assimilation control protein, NAC, is a DNA binding transcription activator in Klebsiella aerogenes. J. Bacteriol. 177:3546-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goss, T. J., B. K. Janes, and R. A. Bender. 2002. Repression of glutamate dehydrogenase formation in Klebsiella aerogenes requires two binding sites for the nitrogen assimilation control protein, NAC. J. Bacteriol. 184:6966-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goss, T. J., A. Perez-Matos, and R. A. Bender. 2001. Roles of glutamate synthase, gltBD, and gltF in nitrogen metabolism of Escherichia coli and Klebsiella aerogenes. J. Bacteriol. 183:6607-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groisman, E. A., and M. J. Casadaban. 1986. Mini-Mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J. Bacteriol. 168:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 17.Janes, B. K., and R. A. Bender. 1998. Alanine catabolism in Klebsiella aerogenes: molecular characterization of the dadAB operon and its regulation by the nitrogen assimilation control protein. J. Bacteriol. 180:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janes, B. K., C. J. Rosario, and R. A. Bender. 2003. Isolation of a negative control mutant of the nitrogen assimilation control protein, NAC, in Klebsiella aerogenes. J. Bacteriol. 185:688-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 20.Magasanik, B. 1996. Regulation of nitrogen utilization, p. 1344-1356. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington D.C.
- 21.Maquat, L. E., and W. Reznikoff. 1978. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J. Mol. Biol. 125:467-490. [DOI] [PubMed] [Google Scholar]
- 22.Muse, W. B. 1996. Ph.D. thesis. University of Michigan, Ann Arbor.
- 23.Muse, W. B., and R. A. Bender. 1998. The nac (nitrogen assimilation control) gene from Escherichia coli. J. Bacteriol. 180:1166-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhard, J., and J. Ingraham. 1968. Mutants of Salmonella typhimurium requiring cytidine for growth. J. Bacteriol. 95:2431-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pomposiello, P. J., and R. A. Bender. 1995. Activation of the Escherichia coli lacZ promoter by the Klebsiella aerogenes nitrogen assimilation control protein (NAC), a LysR family transcription factor. J. Bacteriol. 177:4820-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomposiello, P. J., B. K. Janes, and R. A. Bender. 1998. Two roles for the DNA recognition site of the Klebsiella aerogenes nitrogen assimilation control protein. J. Bacteriol. 180:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prival, M. J., and B. Magasanik. 1971. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J. Biol. Chem. 246:6288-6296. [PubMed] [Google Scholar]
- 28.Zimmer, D. P., E. Soupene, H. L. Lee, V. F. Wendisch, A. B. Khodursky, B. J. Peter, R. A. Bender, and S. Kustu. 2000. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc. Natl. Acad. Sci. USA 97:14674-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]