Abstract
Platelet endothelial adhesion molecule-1 (PECAM-1) is a part of intercellular junctions and triggers intracellular signaling cascades upon homophilic binding. The intracellular domain of PECAM-1 is tyrosine phosphorylated upon homophilic engagement. However, it remains unclear which tyrosine kinase phosphorylates PECAM-1. We sought to isolate tyrosine kinases responsible for PECAM-1 phosphorylation and identified Fer as a candidate, based on expression cloning. Fer kinase specifically phosphorylated PECAM-1 at the immunoreceptor tyrosine-based inhibitory motif. Notably, Fer induced tyrosine phosphorylation of SHP-2, which is known to bind to the immunoreceptor tyrosine-based inhibitory motif of PECAM-1, and Fer also induced tyrosine phosphorylation of Gab1 (Grb2-associated binder-1). Engagement-dependent PECAM-1 phosphorylation was inhibited by the overexpression of a kinase-inactive mutant of Fer, suggesting that Fer is responsible for the tyrosine phosphorylation upon PECAM-1 engagement. Furthermore, by using green fluorescent protein-tagged Fer and a time-lapse fluorescent microscope, we found that Fer localized at microtubules in polarized and motile vascular endothelial cells. Fer was dynamically associated with growing microtubules in the direction of cell-cell contacts, where p120catenin, which is known to associate with Fer, colocalized with PECAM-1. These results suggest that Fer localized on microtubules may play an important role in phosphorylation of PECAM-1, possibly through its association with p120catenin at nascent cell-cell contacts.
INTRODUCTION
Platelet endothelial adhesion molecule-1 (PECAM-1) belongs to the immunoglobulin superfamily of cell adhesion molecules and is expressed on endothelial cells, platelets, leukocytes, and monocytes (Newman et al., 1990). It consists of six extracellular immunoglobulin domains, a transmembrane domain, and an intracellular domain, which is tyrosine phosphorylated upon cellular activation (reviewed in Newman, 1999).
PECAM-1 functions not only as an intercellular adhesion-stabilizing molecule but also as an intracellular signal-triggering molecule by providing phosphotyrosines that bind to Src homology 2 (SH2)-containing molecules (Lu et al., 1997; Masuda et al., 1997; Jackson et al., 1997b). Phosphorylation of PECAM-1 on Tyr663 and Tyr686 (the aa numbers in this study follow the number first reported in Newman et al., 1990), each of which constitute an immunoreceptor tyrosine-based inhibitory motif (ITIM) (Newman, 1999), provides a docking site for the SH2-domain–containing protein phosphatase, SHP-2. PECAM-1 phosphorylation and subsequent association with SHP-2 have been demonstrated in many different cell types and in response to various activating stimuli (Sagawa et al., 1997; Jackson et al., 1997a,b; Cao et al., 1998; Newton-Nash and Newman, 1999). We have demonstrated that PECAM-1 becomes tyrosine phosphorylated and binds SHP-2 in vascular endothelial cells exposed to fluid shear stress or osmotic shock (Harada et al., 1995; Masuda et al., 1997; Osawa et al., 1997,2002).
The protein tyrosine phosphatase SHP-2 consists of amino-terminal tandem SH2 domains and a carboxy terminal phosphatase domain (Feng, 1999). It is involved in a variety of growth factor-mediated signaling events (Kazlauskas et al., 1993; Deb et al., 1998; Myers et al., 1998; Maroun et al., 2000). The two SH2 domains of SHP-2 bind to a bisphosphoryl tyrosine-based activation motif, found in Grb2-associated binder-1 (Gab1) and insulin receptor substrate-1, which fully potentiates the catalytic activity of SHP-2 (Cunnick et al., 2001). In addition, an interaction between SHP-2 and Gab1 is required for epidermal growth factor receptor- or hepatocyte growth factor receptor-mediated activation of extracellular signal-regulated kinase (ERK) (Cunnick et al., 2000; Schaeper et al., 2000). We recently demonstrated that PECAM-1 is required for activation of ERK in vascular endothelial cells exposed to shear stress and osmotic shock and that both SHP-2 and Gab1 are recruited to the cell-cell border where PECAM-1 is phosphorylated in these cells (Osawa et al., 2002).
A role for members of the Src family of protein tyrosine kinases in PECAM-1 tyrosine phosphorylation has been proposed on the basis of studies demonstrating that Src family tyrosine kinases could bind to and phosphorylate PECAM-1 in in vitro kinase assays (Lu et al., 1997; Masuda et al., 1997), that members of both Src and Csk families of protein tyrosine kinases could phosphorylate PECAM-1 upon overexpression in COS cells (Cao et al., 1998), and that PECAM-1 tyrosine phosphorylation in stimulated platelets could be blocked by the selective Src family kinase inhibitor PP2 (Cicmil et al., 2000; Ohmori et al., 2001). In vascular endothelial cells exposed to hyperosmotic shock, however, inhibitors of Src family kinases failed to block PECAM-1 tyrosine phosphorylation (unpublished observation in Osawa et al., 2002), suggesting that the kinase responsible for PECAM-1 tyrosine phosphorylation in mechanically stimulated endothelial cells may be different from those that phosphorylate PECAM-1 in other cells and under other conditions.
The nonreceptor protein-tyrosine-kinase Fer was originally isolated as an Fps (Fujinami poultry sarcoma)/Fes (feline sarcoma)-related protein, and it was thus named Fer (Letwin et al., 1988). The Fps/Fes and Fer kinases share high structural homology: an amino-terminal Fps/Fes/Fer and CIP4 homology (FCH) domain followed by three tandem coiled-coil domains, an SH2 domain, and a carboxy-terminal tyrosine kinase domain (Greer, 2002). Fps/Fes is expressed mainly in hematopoietic cells, and to a lesser extent in vascular endothelial cells and neuronal cells, whereas Fer is ubiquitously expressed. The role of Fps/Fes has been extensively studied in cytokine-mediated signaling. In contrast, Fer is able to bind to and phosphorylate adherens junctional proteins, p120catenin (p120ctn) and β-catenin, and promote its dissociation from N-cadherin (Kim and Wong, 1995; Arregui et al., 2000). Thus, Fer is thought to be involved principally in the regulation of cell-cell contacts, especially at the adherens junction (Rosato et al., 1998).
In this study, we sought to identify the PECAM-1 phosphorylating kinase. We demonstrate that Fer is essential for engagement-dependent phosphorylation of PECAM-1 and show for the first time that Fer localizes at the peripheral microtubules in polarizing and migrating vascular endothelial cells. The association between Fer and microtubules seems to be critical to phosphorylation of PECAM-1 by recruiting Fer to p120ctn colocalized with PECAM-1 at nascent cell-cell contacts.
MATERIALS AND METHODS
Reagent and Antibodies
Protein A and G-Agarose were purchased from Calbiochem (La Jolla, CA). Type I collagen used for coating glass-base dishes was from Nitta Gelatin (Osaka, Japan). Anti-green fluorescent protein (GFP), anti-bovine-PECAM-1, and anti-phospho-bovine-PECAM-1 recognizing phospho-Tyr686 (hereafter, PY-PECAM-1) were developed in our laboratory as reported previously (Osawa et al., 2002). Magnetic beads conjugated with anti-human PECAM-1 were obtained from Dynal ASA (Oslo, Norway). Anti-p120ctn, anti-human-PECAM-1, and anti-phosphotyrosine (PY100) were from Cell Signaling Technology (Beverly, MA). Anti-FLAG (M2) and anti-tubulin were from Sigma-Aldrich (St. Louis, MO), and anti-hemagglutinin (HA) was from Roche Diagnostics (Basel, Switzerland). Anti-SHP-2 was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Gab1 was a generous gift from T. Hirano (Osaka University, Osaka, Japan).
Plasmids
Enhanced green fluorescent protein (EGFP)-PECAM-1 was constructed by inserting cDNA encoding bovine PECAM-1 without a stop codon in frame into EGFP-N1 (BD Biosciences Clontech, Palo Alto, CA). EGFP-Fer wild-type (WT) was an expression vector for amino-terminally EGFP-tagged WT Fer. pCXN2-FLAG-WT Fer or Fes was derived from pCAGGS eukaryotic expression vector and expressed FLAG-tagged Fer or Fes (Niwa et al., 1991). A cDNA encoding a kinase-defective (KD) form of Fer containing an Asp to Arg substitution at position 743 (Craig et al., 2001), or a cDNA encoding a form of Fer with a nonfunctional SH2 domain containing an Arg to Gln substitution at position 483 (R483Q) was amplified by polymerase chain reaction (PCR)-based mutagenesis and subcloned into pCXN2-FLAG (Nagashima et al., 2002) and EGFP-C1 (BD Biosciences Clontech). HcRed-p120ctn was constructed by inserting p120ctn cDNA in frame into HcRed-C1 (BD Biosciences Clontech). cDNAs encoding truncated forms of Fer were amplified by PCR and ligated into EGFP-C1. pGEX-cytoplasmic-PECAM-1 was a bacterial expression vector and contained cDNA encoding glutathione S-transferase followed by cytoplasmic domain of PECAM-1 (aa 593–712) in frame. pcDNA-HA-SHP-2 was described previously (Osawa et al., 2002). Either Tyr663 or Tyr686, and both of PECAM-1 were substituted for Phe by PCR-based mutagenesis (hereafter, Y663F, Y686F, and Y663/686F, respectively). Amplified PCR fragments were inserted into carboxy-terminally V5 epitope-tagging plasmids, pcDNA-DEST40, by using Gateway technology (Invitrogen, Carlsbad, CA). EGFP-Fes and its kinase-defective mutant were obtained from S. Yanagi (Kobe University, Kobe, Japan) (Mitsui et al., 2002). All of the DNA fragments amplified by PCR were ligated into pCR-BluntII-TOPO vector or pENTR-D-TOPO vector (Invitrogen), and the sequence was confirmed with an ABI Prism 3700 (Applied Biosystems Japan, Tokyo, Japan).
Virus
pCXN2-FLAG-internal ribosomal entry site (IRES)-EGFP was constructed as reported previously (Nagashima et al., 2002). PCR-amplified KD Fer cDNA was inserted into this vector, expressing both FLAG-tagged KD Fer and IRES-driven EGFP under bicistronic promoter. DNA encoding the promoter to EGFP was subcloned into pShuttle vector, and the expression cassette was transferred to pAdeno-X according to the manufacturer's protocol (BD Biosciences Clontech). We produced a recombinant adenovirus expressing both FLAG-tagged KD Fer and IRES-driven EGFP by transfecting human embryonic kidney (HEK)293 cells with pAdeno-X-KD Fer-IRES-EGFP. A recombinant adenovirus expressing GFP was obtained from H. Kurose (Kyushu University, Fukuoka, Japan).
Bacterial Expression Cloning
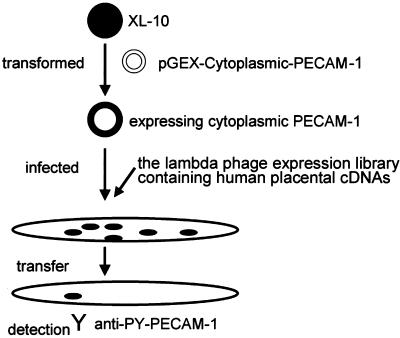
The protocol for expression cloning of PECAM-1 tyrosine phosphorylating kinase is illustrated in Figure 1. The Escherichia coli XL10-Gold was transformed with pGEX-cytoplasmic PECAM-1. Transformed bacteria were cultured overnight, collected by centrifugation at 3,500 × g for 10 min, and resuspended in 10 mM MgSO4. Then, resuspended bacteria were infected with the lambda phage library containing human placental cDNAs (TriplEx2; BD Biosciences Clontech). The protein expression was induced by 20 mM isopropyl β-d-thiogalactoside. The bacteria expressing both cytoplasmic PECAM-1 and library-promoted protein was lifted to the nitrocellulose membrane. The membranes were washed with Tris-buffered saline containing Tween 20 (25 mM Tris-hydrochloride pH 7.5, 150 mM NaCl, 2.5 mM KCl, and 0.05% Tween 20) and incubated with anti-PY-PECAM-1 at 4°C for 24 h. The immunoreaction was detected by peroxidase-conjugated anti-rabbit secondary antibody and visualized by an enhanced chemiluminescence method (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom).
Figure 1.
Schematic illustration of screening for PECAM-1–phosphorylating kinase. E. coli, XL-10 was first transformed by pGEX-cytoplasmic PECAM-1. The transformed bacteria were then infected with a lambda phage expression library containing human placental cDNAs. More than 5 × 105 plaques were transferred to the membrane and examined for the immunoreactivity with anti-PY-PECAM-1.
Cells, Transfection, and Infection
Human aortic endothelial cells (HAECs) were purchased from Cascade Biologics (Portland, OR) and maintained in HuMedia-EG2 (Kurabo, Kurashiki, Japan) supplemented with a growth additive set, as described previously (Nagashima et al., 2002). Bovine aortic endothelial cells (BAECs) were cultured in DF1/2 as described previously (Osawa et al., 1997). HEK293 cells were from the American Type Culture Collection (Manassas, VA) and cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine. HEK293 cells were transfected with pAdeno-X-derived vectors to produce recombinant adenovirus using LipofectAMINE2000 (Invitrogen). HAECs cultured on a collagen-coated 35-mm-diameter glass-base dish (Asahi Techno Glass, Tokyo, Japan) were transfected with 3 μg of plasmid DNA by using LipofectAMINE PLUS reagent (Invitrogen) for 24 h or infected with adenovirus at the appropriate multiplicity of infection for >48 h before the stimulation.
Immunoprecipitation, Immunoblotting, and Cell Staining
Immunoprecipitation, immunoblotting, and immunocytochemistry were performed as reported previously (Nagashima et al., 2002). Briefly, HAECs were washed with Tris-buffered saline containing 1 mM Na3VO4 and lysed in lysis buffer (150 mM NaCl, 20 mM Tris hydrochloride pH 7.5, 1.5 mM MgCl2, 1 mM Na3VO4, 1% Triton X-100, 10 mM NaF, and protease inhibitor cocktail; Roche Diagnostics). Lysates were precleared by centrifugation at 15,000 × g for 10 min, followed by immunoprecipitation by using antibodies indicated in the figures and protein A or G-Agarose (Calbiochem). Immunoprecipitates were subjected to SDS-PAGE and immunoblotting with antibodies as indicated in the figures. Proteins reacting with primary antibodies were visualized by an enhanced chemiluminescence system (Amersham Biosciences UK) for detecting peroxidase-conjugated species-matched secondary antibodies and quantitatively analyzed with an LAS-1000 system (Fuji Film, Tokyo, Japan). HAECs cultured on a collagen-coated glass-base dish and washed with phosphate-buffered saline were fixed by 4% paraformaldehyde at room temperature, followed by permeabilization with 0.1% Triton X-100. Permeabilized cells were incubated with anti-PECAM-1, anti-tubulin, or anti-p120ctn antibody. Proteins reacting with antibodies were detected with Alexa 546 goat anti-mouse IgG (Molecular Probes, Eugene, OR) for visualizing p120ctn and tubulin, and Alexa 488 goat anti-rabbit IgG for PECAM-1. Images for EGFP-tagged Fer and its mutants, PECAM-1, p120ctn, and tubulin were obtained by an Olympus IX71 fluorescent microscope (Olympus, Tokyo, Japan).
Engagement of PECAM-1
HAECs cultured on 60-mm collagen-coated dishes were uninfected or infected with either adenovirus expressing GFP or adenovirus expressing both KD Fer and IRES-driven EGFP for >48 h. HAECs on one dish were incubated with magnet beads conjugated with anti-PECAM-1 at 37°C for 30 min, lysed in lysis buffer, and collected on a magnet, whereas HAECs on another dish were lysed, incubated with beads conjugated with anti-PECAM-1 for 30 min at room temperature, and collected on a magnet. Collected proteins were subjected to SDS-PAGE and immunoblotted with PY100.
Time-Lapse Imaging
For time-lapse imaging, HAECs cultured on a collagen-coated glass-base dish were maintained in DMEM/F-12 (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, 10 mM HEPES, and 1.2 g/l NaHCO3 without phenol red. HAECs transfected with plasmids expressing fluorescence-tagged proteins were imaged on an Olympus IX71 inverted microscope with a 75-W Xenon arc lamp equipped with a cooled charge-coupled device camera, Cool-SNAP-HQ (Roper Scientific, Trenton, NJ), and two filter changers, controlled by MetaMorph 4.6 software (Roper Scientific). Both the GFP image and the HcRed image were obtained through an XF2043 dichroic filter (Omega Optical, Brattleboro, VT) and a set of an S484/15 excitation filter and an S515/30 emission filter (Chroma Technology, Brattleboro, VT) for GFP, and a set of an S555/25 excitation filter and an S630/60 emission filter for HcRed. To monitor the localization of fluorescence-tagged proteins, we obtained a fluorescence image every 20 s. A series of time-lapse images were converted to video format by using MetaMorph 4.6 software.
RESULTS
Isolation and Identification of Fer as a PECAM-1 Phosphorylating Kinase
The cytoplasmic domain of PECAM-1 is phosphorylated upon PECAM-1 engagement and provides two SHP-2 binding sites at phosphorylated Tyr663 and Tyr686 (Osawa et al., 2002). We aimed to identify the PECAM-1 phosphorylating kinase by using a bacterial expression cloning method and an antibody specific for a phosphotyrosine residue at position 686 of PECAM-1 (anti-PY-PECAM-1), as illustrated in Figure 1. E. coli expressing the cytoplasmic domain of PECAM-1 were infected with the lambda phage expression library containing human placental cDNAs, and at least 5 × 105 plaques were screened for immunoreactivity with anti-PY-PECAM-1. Six positive plaques were identified by immunoscreening. Five individual plaques contained the entire coding region of Fer. The other phage carried the cDNA of Src-family kinase, LynB. Src tyrosine kinase was not isolated by this screening, although previous studies have shown that Src phosphorylates PECAM-1 (Lu et al., 1997).
PECAM-1 Is Phosphorylated by Fer and, To a Lesser Extent, by Fes
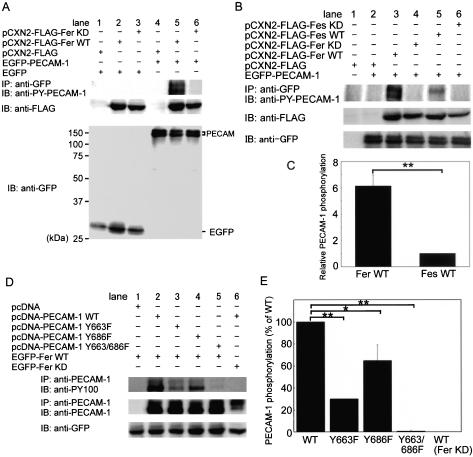
To test whether Fer can phosphorylate PECAM-1 in vivo, we examined whether cotransfection of PECAM-1 with WT or KD Fer results in PECAM-1 tyrosine phosphorylation as detected with anti-PY-PECAM-1 in 293T cells. Carboxy-terminally EGFP-tagged PECAM-1 was phosphorylated by WT Fer (Figure 2A, lane 5) but not KD Fer (Figure 2A, lane 6), whereas EGFP alone used as a negative control was not phosphorylated by either WT Fer or KD Fer, as determined by immunoprecipitation with anti-GFP followed by immunoblotting with anti-phosphotyrosine antibody (our unpublished data).
Figure 2.
PECAM-1 is phosphorylated by Fer and, to a lesser extent, by Fes in vivo. (A) Lysate of 293T cells transfected with the plasmids indicated at the top were either immunoprecipitated (IP), followed by SDS-PAGE and immunoblotting (IB) with the antibodies as indicated, or directly subjected to SDS-PAGE and IB with the antibodies as indicated on the left. EGFP-N1 indicated as EGFP and pCXN2-FLAG were used as a negative control for EGFP-PECAM-1 and pCXN2-FLAG-Fer, respectively. The result is a representative of more than three independent experiments. Note that PECAM-1 is phosphorylated in 293T cells expressing WT Fer, as shown in lane 5. (B) Phosphorylation of PECAM-1 by Fer and Fes tyrosine kinases was analyzed as in A. Note that PECAM-1 is phosphorylated by Fer and, to a lesser extent, by Fes, as shown in lanes 3 and 5, respectively. (C) Quantitative analysis for PECAM-1 phosphorylation by Fer and Fes was performed. The relative intensity of phosphorylated PECAM-1 by Fer to that by Fes, as quantified by the use of LAS-1000 system (Fuji Film), is indicated as relative PECAM-1 phosphorylation. The data are expressed as averages with the SD. A significant difference between two groups determined by t test is indicated with a double asterisk (p < 0.01). (D) Cell lysate of 293T transfected with the plasmids as indicated at the top were used for examining the phosphorylation of Tyr663 and/or Tyr686 as analyzed in A. Note that Y663/686F was not phosphorylated by the overexpression of Fer as shown in lane 5. (E) Phosphorylation of Tyr663 and/or Tyr686 was quantitatively analyzed from the results obtained by the four independent experiments. The intensity of mutant PECAM-1 phosphorylated by Fer was compared with that of WT PECAM-1. The last column indicates that C-terminally V5 epitope-tagged PECAM-1 is not phosphorylated by KD Fer. Quantitative analysis was similarly performed as in C. A significant difference between two groups determined by t test is indicated with an asterisk (p < 0.05) and double asterisk (p < 0.01).
We performed a similar experiment by using Fes, a tyrosine kinase closely related to Fer. WT Fes phosphorylated PECAM-1 in 293T cells, albeit to a lesser extent than WT Fer (Figure 2B, lanes 3 and 5). The phosphorylation of PECAM-1 by either Fer or Fes was quantified as in Figure 2C. The difference in phosphorylation could be explained by the difference in the substrate specificity or localization between Fer and Fes.
Bovine PECAM-1 contains six tyrosine residues in its cytoplasmic domain of which only the two ITIM tyrosine residues, Tyr663 and Tyr686, have been reported to be phosphorylated (Jackson et al., 1997a; Cao et al., 1998). To examine which tyrosine residues are phosphorylated by Fer, we used the carboxy-terminally V5 epitope-tagged PECAM-1 mutants Y663F, Y686F, and Y663/686F as a substrate, and we used anti-phosphotyrosine antibody (PY100) instead of anti-PY-PECAM-1 (Figure 2D). 293T cells were transfected with each of these mutant-expressing vectors and a Fer-expression vector. Y663/686F was not phosphorylated (Figure 2D, lane 5), whereas WT (Figure 2D, lane 2), Y663F (Figure 2D, lane 3), and Y686F (Figure 2D, lane 4) forms of PECAM-1 were all tyrosine phosphorylated by WT Fer. Quantification of phosphorylation of both WT and mutant forms of PECAM-1 by Fer revealed that the levels of phosphorylation of Y663F and Y686F mutant forms of PECAM-1 were decreased by 70 and 30%, respectively. These results indicate that Fer phosphorylates both Tyr663 and Tyr686 but that the former is the preferred site.
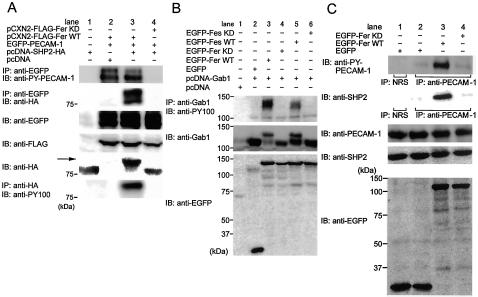
Fer not Only Phosphorylates PECAM-1 to Induce the Association of SHP-2 with PECAM-1 but also Induces the Phosphorylation of SHP-2 and Gab1
The ITIM motif of PECAM-1 is known to be a docking site for SHP-2. We have previously shown that phosphorylated PECAM-1 associates with SHP-2 in BAECs upon PECAM-1 engagement (Osawa et al., 2002). Thus, we tested whether the PECAM-1 phosphorylation mediated by Fer determines the association between PECAM-1 and SHP-2. HA-tagged SHP-2 was coimmunoprecipitated with EGFP-tagged PECAM-1 phosphorylated by Fer (Figure 3A, lane 3), whereas it was not in KD-Fer transfected 293T cells (Figure 3A, lane 4). In addition, we noticed that HA-tagged SHP-2 was detected as an upward-shifted band on an immunoblot, as indicated by the arrow in Figure 3A, which suggested that phosphorylation of SHP-2 might be induced by Fer. We proceeded to examine whether the upward-shifted band was tyrosine phosphorylated SHP-2. Immunoprecipitates by anti-HA antibody were detected by anti-phosphotyrosine antibody (PY100), indicating that SHP-2 was tyrosine phosphorylated by WT Fer (Figure 3A, bottom, lane 3). We also observed that SHP-2 coimmunoprecipitated with EGFP-PECAM-1 was tyrosine phosphorylated (our unpublished data). We have demonstrated that mechanical stress-promoting PECAM-1 phosphorylation induces the translocation of Gab1 to cell-cell contacts with SHP-2 (Osawa et al., 1997). Gab1 contains the bisphosphoryl tyrosine-based activation motif as a binding site for SHP-2. We examined whether the phosphorylation of Gab1 was induced by Fer (Figure 3B). Gab1 in lysates from cells transfected with WT Fer and WT Fes was detected as upward-shifted bands on an immunoblot similar to SHP-2 (Figure 3B, middle, lanes 3 and 5). These upward-shifted bands represented tyrosine phosphorylated Gab1, as demonstrated by immunoprecipitation by anti-Gab1 followed by anti-phosphotyrosine immunoblotting (Figure 3B, top, lanes 3 and 5). WT Fes, to a lesser extent than WT Fer, induced tyrosine phosphorylation of Gab1 (Figure 3B, lane 5). These results suggest that Fer not only functions as a tyrosine kinase for PECAM-1 but also that Fer modulates the downstream signaling of PECAM-1 by inducing phosphorylation of SHP-2 and Gab1.
Figure 3.
Fer not only phosphorylates PECAM-1, resulting in an increased association of SHP-2 and PECAM-1, but also induces the phosphorylation of SHP-2 and Gab1. (A) 293T cells were transfected with the plasmids, as indicated at the top. Cell lysates were subjected to immunoprecipitation (IP) followed by SDS-PAGE and immunoblotting (IB) or IB, as indicated on the left. Note that HA-tagged SHP-2 is detected in the immunoprecipitates with PECAM-1 precipitated by anti-GFP, as shown in lane 3. Furthermore, SHP-2 is detected as an upper-shifted band on an immunoblot with anti-HA, and immunoprecipitates by anti-HA antibody were detected by anti-phosphotyrosine antibody (PY100), indicating that SHP-2 is phosphorylated by Fer. (B) 293T cells were transfected with the plasmids, as indicated at the top. Cell lysates were analyzed by IB with antibodies indicated on the left. Note that Gab1 on the immunoblot with anti-Gab1 was detected as an upper-shifted band and the immunoprecipitates by anti-Gab1 antibody was detected by PY100, indicating that Fer induces the phosphorylation of Gab1. (C) BAECs cultured at 90% confluence were transfected with the plasmids, as indicated at the top. Cell lysates were immunoprecipitated (IP) with either normal rabbit serum (NRS) used as a negative control or anti-PECAM-1, as indicated beneath the panels, followed by SDS-PAGE and immunoblotting with the antibodies, as indicated on the left. Remaining lysates were subjected to SDS-PAGE and immunoblot probed with the antibodies indicated on the left. Note that more PECAM-1 is phosphorylated, that the amount of SHP-2 associating with PECAM-1 is increased, and that PECAM-1 is phosphorylated even without overexpression of Fer (lane 2), presumably depending on the cell-cell contact because of the confluence.
SHP-2 Binds PECAM-1 Phosphorylated by Fer in BAECs
PECAM-1 phosphorylated upon mechanical stress recruits SHP-2 in BAECs (Osawa et al., 2002). To test whether endogenous SHP-2 binds to PECAM-1 phosphorylated by Fer, we examined the association in BAECs. BAECs cultured at subconfluence were transfected with plasmids expressing Fer. PECAM-1 was phosphorylated and associated with SHP-2 in mock-transfected cells (Figure 3C, lane 2), likely as a result of cell contact-induced homophilic binding of PECAM-1. Notably, the more PECAM-1 was phosphorylated by Fer, the more SHP-2 was associated with PECAM-1 in Fer-transfected cells (Figure 3C, lane 3). We could not detect an upward-shifted band of endogenous SHP-2 due to the low transfection efficiency in BAECs.
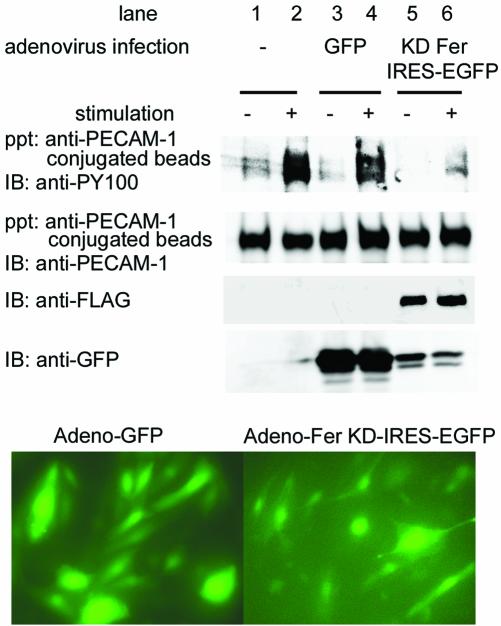
Fer Is Indispensable for Phosphorylation of PECAM-1 upon Engagement
To confirm the requirement of Fer for PECAM-1 engagement-dependent phosphorylation, we used the application of anti-PECAM-1–coated beads to HAECs to mimic homophilic PECAM-1 binding. To determine whether Fer is required for anti-PECAM-1 antibody-induced PECAM-1 phosphorylation, we evaluated the effect of expressing KD Fer, which presumably functions as a dominant negative inhibitor of endogenous Fer activity, on PECAM-1 phosphorylation. HAECs were infected with either adenovirus expressing GFP or adenovirus expressing FLAG-tagged KD Fer and IRES-driven EGFP for >48 h, when >90% of the cells were infected, as determined by the expression of GFP (Figure 4, bottom). PECAM-1 became phosphorylated in cells uninfected and in cells infected with GFP-expressing adenovirus upon incubation with anti-PECAM-1 beads (Figure 4, lanes 2 and 4), whereas PECAM-1 was not phosphorylated in cells infected with adenovirus expressing both KD-Fer and IRES-driven EGFP (Figure 4, lane 6). These results indicate that Fer is required for anti-PECAM-1 antibody-induced phosphorylation of PECAM-1.
Figure 4.
Engagement-dependent phosphorylation of PECAM-1 mimicked by applying PECAM-1 activating antibody. HAECs were uninfected (lanes 1 and 2) or infected with either adenovirus expressing GFP (lanes 3 and 4) or adenovirus expressing both KD Fer and IRES-driven EGFP (lanes 5 and 6). Cell lysates were collected on anti-human-PECAM-1 antibody-conjugated beads without stimulation (–) or after stimulation (+), as described in MATERIALS and METHODS. Proteins bound to the beads were eluted with SDS-sample buffer, subjected to SDS-PAGE, and immunoblot probed with antibodies, as indicated on the left. Cell lysates were analyzed for expression of FLAG-tagged KD Fer or GFP as indicated at the left. Bottom, infection efficiency of adenovirus, as shown by GFP-positive cells. The result is a representative of more than three independent experiments.
Fer Localizes to Microtubules in Vascular Endothelial Cells
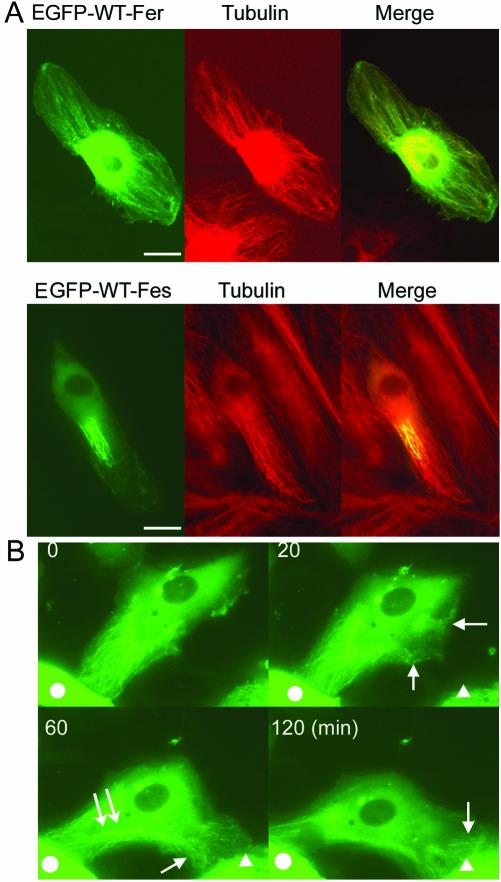
Fer contains an FCH domain, which is thought to be a microtubule-targeting motif (Aspenstrom, 1997; Tian et al., 2000). Previously, Fer and Fes were reported to be localized in the nucleus (Hao et al., 1991; Yates et al., 1995). Another report showed Fes in the trans-Golgi network and Fer in the cytoplasm (Zirngibl et al., 2001). Hence, we examined the localization of Fer and Fes by using EGFP-tagged Fer and Fes in HAECs. EGFP-tagged WT Fer partially localized with tubular structures in the protruded zone of the cell. We tested the colocalization of Fer with microtubules by using an anti-tubulin antibody. Indeed, Fer was colocalized with microtubules in protrusions of polarized cells (Figure 5A, top). In sharp contrast, EGFP-tagged WT Fes was partially localized with microtubules, particularly in the central region of microtubules but not on peripheral microtubules (Figure 5A, bottom). This distinct localization of Fer and Fes may affect the phosphorylation efficiency of PECAM-1 at cell-cell contacts in the endothelial cells.
Figure 5.
Fer localizes to the microtubules in the polarizing area of HAECs. (A) HAECs expressing EGFP-tagged Fer (top) or EGFP-tagged Fes (bottom) plated on the collagen-coated glass-base dish were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and incubated with anti-tubulin antibody. Immunoreactive protein was visualized by Alexa546 goat anti-mouse IgG. Images for GFP and Alexa546 were obtained using an Olympus IX71 microscope. An image for EGFP-tagged Fer or Fes (green) and an image for tubulin (red) were superimposed (merge). Bar, 20 μm. (B) A series of time-lapse images for EGFP-tagged Fer are shown. Images were obtained at the indicated time after the beginning of the observation. Arrows indicate EGFP-Fer localized at the microtubules in the polarizing area of HAECs. Double arrows indicate the dispersion of EGFP-tagged Fer from the microtubules in the retracting area (also see Video 1). Neighboring cells are marked by either a circle or a triangle. Time-lapse images were obtained using an Olympus IX71 equipped with a cooled charge-coupled device camera and two filter changers controlled by MetaMorph 4.6. A series of images were converted to a video (Video 1).
Vascular endothelial cells are motile on the collagen-coated dishes and polarize when they migrate. We monitored EGFP-tagged WT Fer in living cells by using a time-lapse microscope. HAECs were transfected with EGFP-tagged WT Fer and cultured on collagen-coated glass-bottom dishes in DMEM with 10% FBS. Under these conditions, HAECs were motile and in perpetual motion until cells reached confluence. At the point when we started monitoring, Fer was localized with microtubules in the polarizing cell in contact with an adjacent cell indicated by the circle in Figure 5B (time 0 min). As the cell began to move toward the cell indicated by the triangle, EGFP-tagged Fer was relocated at newly assembling microtubules, as indicated by the arrow in Figure 5B (time 20 min). EGFP-tagged Fer disappeared from the retracting tail, which lost cell-cell contact with the cell indicated by the circle in Figure 5B and localized at the newly assembling microtubules in the protrusion that contacts another cell as indicated by the triangle in the figure (time 60 and 120 min, respectively). Notably, newly assembled microtubules indicated by EGFP-tagged Fer reached the cell-cell contact points (Figure 5B, and Video 1). These data indicate that the localization of Fer depends on the assembly and disassembly of microtubules and that Fer is dynamically recruited to nascent microtubules in the protruding side from the retracting tail and may function at the leading edge of the cell.
FCH Domain Is Dispensable for Fer Localization on the Microtubules
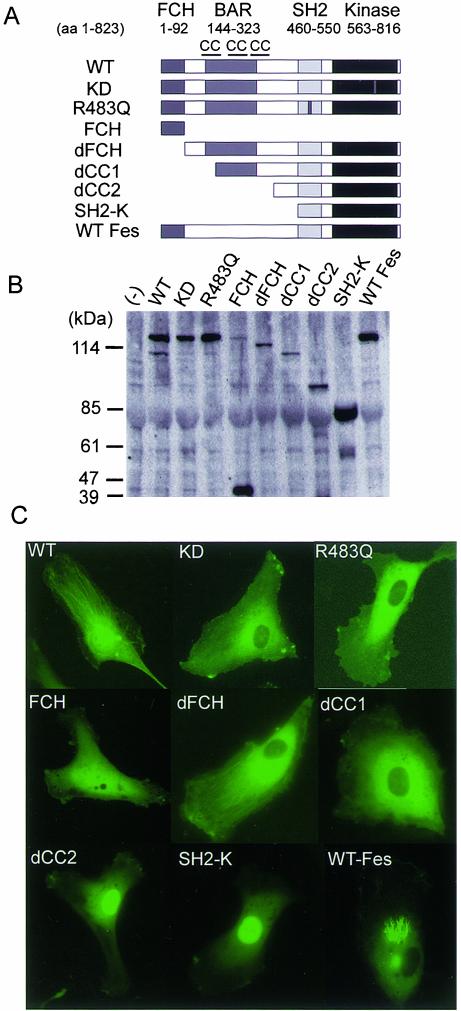
To determine the microtubule-targeting domain of Fer, we constructed a panel of EGFP-tagged truncated and mutated Fer clones (Figure 6A). Recently, a domain conserved among Bin1, Amphiphysin, and a yeast protein, RSV, has been reported and designated as a BAR domain named after these molecules (Ge and Prendergast, 2000); however, its function has not yet been elucidated. Fer contains this domain between the first and the third coiled-coil domain.
Figure 6.
Fer requires coiled-coil, SH2, and kinase domains for localizing to the microtubules in vascular endothelial cells. (A) Fer consists of FCH, BAR flanked by the first and the third coiled-coil domain, SH2, and tyrosine kinase domain, as schematically illustrated. The amino acid number encoding each domain is indicated at the top. The kinase defective mutation (KD) and SH2 mutation (R483Q) of Fer are shown as small dark boxes. dFCH, dCC1, and dCC2 denote deletion of the FCH domain, that of the FCH and the first coiled-coil domain, and that of the FCH and all coiled-coil domain, respectively. SH2-K denotes the deletion mutant of Fer consisting of the SH2 and tyrosine kinase domains. (B) 293T cells were transfected with the plasmids encoding amino-terminally EGFP-tagged DNA, as indicated at the top. Cell lysates were subjected to SDS-PAGE, followed by immunoblot probed with anti-GFP antibody. (C) HAECs were transfected with the plasmids used in B and imaged through an Olympus IX71 fluorescent microscope. Note that WT Fer and dFCH localize on the polarized microtubules, whereas mutants lacking the first coiled-coil domain, KD, or R483Q, do not localize on the microtubules.
To examine whether EGFP-tagged mutants of Fer were correctly constructed, we transfected 293T cells with a panel of plasmids encoding EGFP-tagged mutants. EGFP-tagged molecules were detected at the expected molecular weight, as demonstrated by an immunoblot probed with anti-GFP antibody (Figure 6B).
The FCH domain has been thought to be a microtubule-targeting domain because CIP4 localizes on the microtubules (Tian et al., 2000). Neither EGFP-tagged KD Fer, nonfunctional SH2-mutant (R483Q) of Fer, nor EGFP-tagged FCH of Fer localized on microtubules. Unexpectedly, dFCH did localize on microtubules in polarized cells as did WT Fer. Apparently, deletion mutants lacking the first (dCC1) or all (dCC2) of the coiled-coil domains, or those containing only the SH2 and kinase domain (SH2-K), were not capable of targeting Fer to microtubules (Figure 6). dCC2 and SH2-K localized in the nucleus, probably as a result of a bipartite nuclear localization signal between aa 540 and aa 557 (Ben Dor et al., 1999). These results suggest that the coiled-coil in the BAR domain, the SH2 domain, and the kinase domain, but not FCH domains are required for localization of Fer to microtubules.
PECAM-1 Colocalized at Nascent Cell-Cell Contact with p120ctn
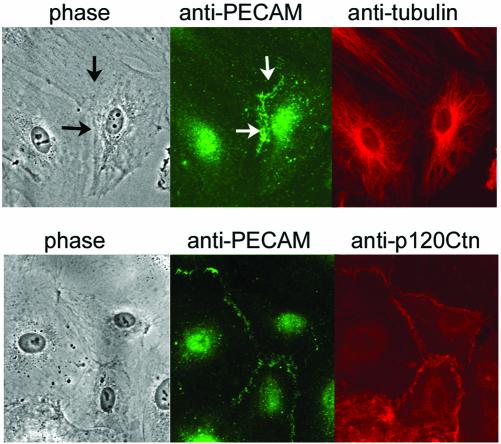
To understand the cellular mechanism by which Fer colocalized with microtubules can phosphorylate PECAM-1, we examined the localization of PECAM-1, microtubules, and p120ctn, because a previous study has demonstrated that Fer binds to p120ctn via the coiled-coil domain and phosphorylates it (Kim and Wong, 1995). PECAM-1 localized at nascent cell-cell contacts, as indicated by the arrows in Figure 7 (top). Notably, microtubules were organized toward cell-cell contacts, as demonstrated by the immunostaining by using an anti-tubulin antibody (Figure 7). p120ctn binds to the conserved cadherin juxtamembrane domain, resulting in localization at cell-cell contacts, in particular at adherens junctions (Anastasiadis and Reynolds, 2000). We further examined the localization of PECAM-1 together with p120ctn and found that p120ctn colocalized with PECAM-1 at cell-cell contacts (Figure 7, bottom).
Figure 7.
Microtubules are organized toward the cell-cell contact, where PECAM-1 colocalizes with p120ctn in HAECs. HAECs cultured on a glass-base dish were permeabilized with 0.1% Triton X-100 and immunostained with both rabbit anti-PECAM-1 antibody and mouse anti-tubulin antibody (top) or mouse anti-p120ctn antibody (bottom). PECAM-1 (green) and tubulin (top, red)/p120ctn (bottom, red) were visualized by fluorophore-labeled species-matched Alexa secondary antibody. PECAM-1 at cell-cell contact is indicated by the arrows (top). Note that the microtubules are organized toward the cell-cell contact, which is revealed by the immunostaining with PECAM-1 (top) and that PECAM-1 colocalizes with p120ctn (bottom). Phase, phase contrast view.
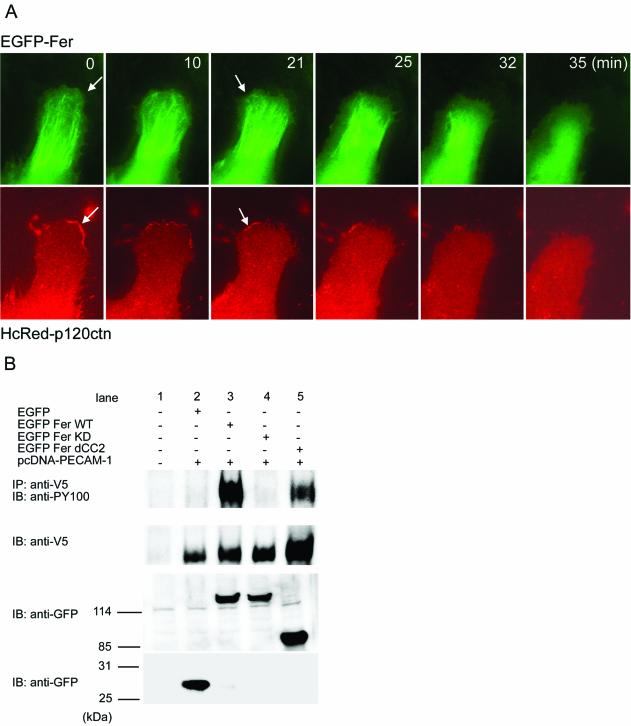
To decipher the localization of Fer to microtubules in living cells, we monitored the accumulation of p120ctn at the cell-cell contact and Fer on the microtubules by EGFP-tagged Fer and HcRed-tagged p120ctn in motile HAECs by using a time-lapse microscope. EGFP-tagged Fer localized to assembling microtubules at the lamellipodia in the protruding zone of the HAEC, as indicated by the arrows in Figure 8. In addition, during the shift from the protruding zone to the retracting zone of HAECs, EGFP-tagged Fer disappeared in the retracting zone (Figure 8, top, and Video 2). In the same cell, HcRed-tagged p120ctn began to accumulate at the cell-cell contacts with adjacent cells (Figure 8, bottom, and Video 3). On the disruption of cell-cell contact, DsRed-tagged 120ctn dispersed from the cell-cell contact. These findings suggest that upon cell-cell contact, p120ctn accumulates at the adherens junction and recruits Fer on the microtubules to adherens junctions. To examine whether p120ctn is involved in phosphorylation of PECAM-1 by Fer, we compared the phosphorylation of PECAM-1 by WT Fer and that by dCC2 lacking coiled-coil domains required for the association with p120ctn. Notably, the efficiency of PECAM-1 phosphorylation was less in cells transfected with dCC2 than in those transfected with WT Fer (Figure 8B, lanes 3 and 5). We observed that autophosphorylation of dCC2 was not perturbed by the removal of coiled-coil domains (our unpublished data). These results suggest that the coiled-coil domain-mediated interaction between Fer and p120ctn may contribute to phosphorylation of PECAM-1 by Fer at cell-cell contacts.
Figure 8.
Fer is recruited to the microtubules, which grow toward the cell-cell contact where p120ctn accumulates. HAECs expressing both EGFP-tagged Fer and HcRed-tagged p120ctn was time-lapse imaged using Olympus IX71 microscope as described in the legend of Figure 5. GFP images and HcRed images at the time after the beginning of the observation are shown. A series of time-lapse images for EGFP-tagged Fer and for HcRed-tagged p120ctn were converted to Video 2 and Video 3, respectively. Arrows point to the EGFP-Fer recruited to the growing microtubules (top). Arrows in the lower panel indicate the accumulating p120ctn at the nascent cell-cell contact. (B) BAECs were transfected with the plasmids indicated at the top. Phosphorylation of carboxy-terminally V5 epitope-tagged PECAM-1 by WT Fer, KD Fer, or dCC2 was analyzed by immunoprecipitation (IP) followed by SDS-PAGE and immunoblotting (IB) by using antibodies indicated at the left. Cell lysates were immunoblotted with antibodies indicated at the left. Note that dCC2 phosphorylates PECAM-1 to a lesser extent than WT Fer.
DISCUSSION
PECAM-1 functions as an adhesive molecule and as a signaling mediator during cell-cell contact (Newman, 1997). Phosphorylation of PECAM-1 is required for triggering intracellular signaling (Newman, 1999). Herein, we have identified Fer tyrosine kinase as a PECAM-1 phosphorylating kinase and as an inducer for SHP-2 and Gab1 phosphorylation. In addition, we have suggested an important role in localization of Fer to microtubules for phosphorylation of PECAM-1.
Bacterial expression cloning enabled us to isolate Fer kinase as a PECAM-1 phosphorylating kinase. This method is advantageous in screening kinases that directly phosphorylate the cytoplasmic domain of PECAM-1. Previous data have shown that Src is capable of phosphorylating and binding to PECAM-1 by in vitro kinase assays and in an overexpression study (Lu et al., 1997; Cao et al., 1998). In our laboratory, phosphorylation of PECAM-1 induced by mechanical stretch was not perturbed in the presence of the Src family kinase inhibitor PP2 (unpublished observation in Osawa et al., 2002). Thus, Fer seems to be the best candidate for a PECAM-1–phosphorylating kinase. This notion is supported by the evidence that Tyr663 of PECAM-1 is a preferred site for Fer (Figure 2E), whereas Tyr686 is a preferred site for Src and Csk families of protein tyrosine kinase (Cao et al., 1998). Because mice harboring inactivating mutation of Fer are viable (Craig et al., 2001), Fes and/or LynB, both of which have been shown to phosphorylate PECAM-1, may compensate for the defects in these mice.
The physiological consequence of PECAM-1 phosphorylation remains unknown. We previously demonstrated that phosphorylated PECAM-1 triggered SHP-2-mediated intracellular signaling, including recruitment of Gab1 and ERK activation in vascular endothelial cells (Masuda et al., 1997; Osawa et al., 2002). In the present study, we have shown that Fer can induce phosphorylation of both SHP-2 and Gab1 (Figure 3). Moreover, Gab1 and SHP-2 cooperatively function to activate ERK and to induce branching morphogenesis, thereby triggering active cell motility in Madin-Darby canine kidney cells (Schaeper et al., 2000). ERK is involved in actomyosin contractility, which is essential for cell migration, by phosphorylating myosin light chains (Cheresh et al., 1999). In addition, cortactin, a substrate of Fer kinase, participates in membrane ruffling by binding to F-actin (Kim and Wong, 1998). A deficiency of cortactin phosphorylation and a migration defect were found in Fer-deficient fibroblasts and in Fer-deficient mast cells, respectively (Craig et al., 2001; Craig and Greer, 2002). Collectively, Fer seems to orchestrate the downstream signaling of PECAM-1, leading to cell migration by phosphorylating signaling molecules regulating cell motility.
Most cytoplasmic protein tyrosine kinases contain conserved domains such as SH2, SH3, and pleckstrin homology besides tyrosine kinase domain (Blume-Jensen and Hunter, 2001). Among protein tyrosine kinases, Fer and Fes are the only family members that contain an FCH domain, the function of which has not yet been defined. Because the FCH domain of CIP4 binds to microtubules (Tian et al., 2000), the amino-terminal FCH of Fer and Fes has been expected to function as a microtubule-targeting domain (Greer, 2002). We found that GFP-tagged Fer and Fes partially colocalized with microtubules and demonstrated that microtubule targeting of Fer was perturbed by deletion of the coiled-coil domain. Intriguingly, the removal of the FCH domain from Fer did not perturb the localization of Fer to microtubules, as shown in Figure 6. These data indicate that the FCH domain is not the sole microtubule-targeting domain. EGFP-tagged Fer localized at both proximal and peripheral microtubules in the polarizing zone toward the leading edge. In contrast, Fes localized at proximal microtubules. The distinct localization of Fer and Fes may account for efficiency of the PECAM-1 phosphorylation. Moreover, the evidence that Fer localized on vesicular structures near proximal microtubules such as GFP-tagged Fes (Figure 5B) coincides with the previous report demonstrating the localization of Fes/Fps to vesicular structures and partial colocalization with several Rab proteins (Zirngibl et al., 2001).
We have demonstrated for the first time that Fer localizes to microtubules, as shown in Figure 5. We developed an antibody against Fer to examine the localization of endogenous Fer in endothelial cells. Endogenous Fer could not be detected on microtubules by using the antibody we developed, raising two possibilities; one is that endogenous Fer that is inactive without any stimulation may not localize to microtubules, and the other is simply that the antibody is not sensitive enough to detect the endogenous Fer. The former possibility is supported by the evidence that overexpressed Fer is autophosphorylated in fibroblasts (Rosato et al., 1998) and our data that EGFP-tagged WT Fer but not KD Fer localized to microtubules. In contrast, Hao et al., 1991 and Yates et al., 1995 reported that Fer and Fes localize in the nucleus. Although our results did not coincide with these previous reports, the localization of Fer may depend on whether PECAM-1 is activated.
Microtubules are polarized in the migrating cells and grow forward to the leading edge (Wittmann and Waterman-Storer, 2001). In vascular endothelial cells, microtubules detected by EGFP-tagged Fer grew toward neighboring cells and reached cell-cell contacts, where PECAM-1 accumulated (Figures 5B and 7). As shown in Figure 8, p120ctn also accumulated at nascent cell-cell contacts toward which microtubules were growing. p120ctn can associate with Fer via its coiled-coil domain (Kim and Wong, 1995). In addition, we found that dCC2 lacking p120ctn association-domain phosphorylated PECAM-1 to a lesser extent than WT (Figure 8B). Thus, an association between Fer and p120ctn at nascent cell-cell contacts seems to contribute to phosphorylation of PECAM-1.
In conclusion, we identified Fer tyrosine kinase as a PECAM-1–phosphorylating kinase and demonstrated for the first time that Fer localizes to microtubules in the polarizing vascular endothelial cells. The microtubule-targeted Fer seems to be essential for phosphorylation of PECAM-1 by p120ctn-mediated recruitment to cell-cell contacts.
Supplementary Material
Acknowledgments
We thank S. Yanagi and A.B. Reynolds for the plasmids, T. Hirano for the plasmids and antibody, H. Kuorse for the virus, M. Matsuda for advice, H.K. Surks for critical reading of the manuscript, and M. Sone and H. Shimamoto for technical assistance. This work was supported in part by grants from the Ministry of Health, Labor, and Welfare Foundation of Japan; the Promotion of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research of Japan; the Ministry of Education, Science, Sports and Culture of Japan; and the Uehara Memorial Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-02-0080. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-02-0080.
Online version of this article contains video material for some figures. Online version is available at www.molbiolcell.org.
References
- Anastasiadis, P.Z., and Reynolds, A.B. (2000). The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113, 1319–1334. [DOI] [PubMed] [Google Scholar]
- Arregui, C., Pathre, P., Lilien, J., and Balsamo, J. (2000). The nonreceptor tyrosine kinase Fer mediates cross-talk between N-cadherin and beta1-integrins. J. Cell Biol. 149, 1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenstrom, P. (1997). A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7, 479–487. [DOI] [PubMed] [Google Scholar]
- Ben Dor, I., Bern, O., Tennenbaum, T., and Nir, U. (1999). Cell cycle-dependent nuclear accumulation of the p94fer tyrosine kinase is regulated by its NH2 terminus and is affected by kinase domain integrity and ATP binding. Cell Growth Differ. 10, 113–129. [PubMed] [Google Scholar]
- Blume-Jensen, P., and Hunter, T. (2001). Oncogenic kinase signalling. Nature 411, 355–365. [DOI] [PubMed] [Google Scholar]
- Cao, M.Y., Huber, M., Beauchemin, N., Famiglietti, J., Albelda, S.M., and Veillette, A. (1998). Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J. Biol. Chem. 273, 15765–15772. [DOI] [PubMed] [Google Scholar]
- Cheresh, D.A., Leng, J., and Klemke, R.L. (1999). Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J. Cell Biol. 146, 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicmil, M., Thomas, J.M., Sage, T., Barry, F.A., Leduc, M., Bon, C., and Gibbins, J.M. (2000). Collagen, convulxin, and thrombin stimulate aggregation-independent tyrosine phosphorylation of CD31 in platelets. Evidence for the involvement of Src family kinases. J. Biol. Chem. 275, 27339–27347. [DOI] [PubMed] [Google Scholar]
- Craig, A.W., and Greer, P.A. (2002). Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Mol. Cell Biol. 22, 6363–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A.W., Zirngibl, R., Williams, K., Cole, L.A., and Greer, P.A. (2001). Mice devoid of Fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell Biol. 21, 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnick, J.M., Dorsey, J.F., Munoz-Antonia, T., Mei, L., and Wu, J. (2000). Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 275, 13842–13848. [DOI] [PubMed] [Google Scholar]
- Cunnick, J.M., Mei, L., Doupnik, C.A., and Wu, J. (2001). Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of SHP2. J. Biol. Chem. 276, 24380–24387. [DOI] [PubMed] [Google Scholar]
- Deb, T.B., Wong, L., Salomon, D.S., Zhou, G., Dixon, J.E., Gutkind, J.S., Thompson, S.A., and Johnson, G.R. (1998). A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J. Biol. Chem. 273, 16643–16646. [DOI] [PubMed] [Google Scholar]
- Feng, G.S. (1999). Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 253, 47–54. [DOI] [PubMed] [Google Scholar]
- Ge, K., and Prendergast, G.C. (2000). Bin2, a functionally nonredundant member of the BAR adaptor gene family. Genomics 67, 210–220. [DOI] [PubMed] [Google Scholar]
- Greer, P. (2002). Closing in on the biological functions of Fps/Fes and Fer. Nat. Rev. Mol. Cell Biol. 3, 278–289. [DOI] [PubMed] [Google Scholar]
- Hao, Q.L., Ferris, D.K., White, G., Heisterkamp, N., and Groffen, J. (1991). Nuclear and cytoplasmic location of the FER tyrosine kinase. Mol. Cell Biol. 11, 1180–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, N., Masuda, M., and Fujiwara, K. (1995). Fluid flow and osmotic stress induce tyrosine phosphorylation of an endothelial cell 128 kDa surface glycoprotein. Biochem. Biophys. Res. Commun. 214, 69–74. [DOI] [PubMed] [Google Scholar]
- Jackson, D.E., Kupcho, K.R., Newman, P.J. (1997a). Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J. Biol. Chem. 272, 24868–24875. [DOI] [PubMed] [Google Scholar]
- Jackson, D.E., Ward, C.M., Wang, R., and Newman, P.J. (1997b). The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J. Biol. Chem. 272, 6986–6993. [DOI] [PubMed] [Google Scholar]
- Kazlauskas, A., Feng, G.S., Pawson, T., and Valius, M. (1993). The 64-kDa protein that associates with the platelet-derived growth factor receptor beta subunit via Tyr-1009 is the SH2-containing phosphotyrosine phosphatase Syp. Proc. Natl. Acad. Sci. USA 90, 6939–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, L., and Wong, T.W. (1995). The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol. Cell Biol. 15, 4553–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, L., and Wong, T.W. (1998). Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J. Biol. Chem. 273, 23542–23548. [DOI] [PubMed] [Google Scholar]
- Letwin, K., Yee, S.P., and Pawson, T. (1988). Novel protein-tyrosine kinase cDNAs related to Fps/Fes and Eph cloned using anti-phosphotyrosine antibody. Oncogene 3, 621–627. [PubMed] [Google Scholar]
- Lu, T.T., Barreuther, M., Davis, S., Madri, J.A. (1997). Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J. Biol. Chem. 272, 14442–14446. [DOI] [PubMed] [Google Scholar]
- Maroun, C.R., Naujokas, M.A., Holgado-Madruga, M., Wong, A.J., and Park, M. (2000). The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell Biol. 20, 8513–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, M., Osawa, M., Shigematsu, H., Harada, N., and Fujiwara, K. (1997). Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 408, 331–336. [DOI] [PubMed] [Google Scholar]
- Mitsui, N., Inatome, R., Takahashi, S., Goshima, Y., Yamamura, H., and Yanagi, S. (2002). Involvement of Fes/Fps tyrosine kinase in semaphorin3A signaling. EMBO J. 21, 3274–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, M.G., Jr., Mendez, R., Shi, P., Pierce, J.H., Rhoads, R., and White, M.F. (1998). The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 273, 26908–26914. [DOI] [PubMed] [Google Scholar]
- Nagashima, K., Endo, A., Ogita, H., Kawana, A., Yamagishi, A., Kitabatake, A., Matsuda, M., and Mochizuki, N. (2002). Adaptor protein Crk is required for ephrin-B1-induced membrane ruffling and focal complex assembly of human aortic endothelial cells. Mol. Biol. Cell 13, 4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, P.J. (1997). The biology of PECAM-1. J. Clin. Invest. 99, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, P.J. (1999). Switched at birth: a new family for PECAM-1. J. Clin. Invest. 103, 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, P.J., Berndt, M.C., Gorski, J., White, G.C., Lyman, S., Paddock, C., and Muller, W.A. (1990). PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 247, 1219–1222. [DOI] [PubMed] [Google Scholar]
- Newton-Nash, D.K., and Newman, P.J. (1999). A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J. Immunol. 163, 682–688. [PubMed] [Google Scholar]
- Niwa, H., Yamamura, K., and Miyazaki, J. (1991). Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199. [DOI] [PubMed] [Google Scholar]
- Ohmori, T., Yatomi, Y., Wu, Y., Osada, M., Satoh, K., and Ozaki, Y. (2001). Wheat germ agglutinin-induced platelet activation via platelet endothelial cell adhesion molecule-1: involvement of rapid phospholipase C gamma 2 activation by Src family kinases. Biochemistry 40, 12992–13001. [DOI] [PubMed] [Google Scholar]
- Osawa, M., Masuda, M., Harada, N., Lopes, R.B., and Fujiwara, K. (1997). Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur. J. Cell Biol. 72, 229–237. [PubMed] [Google Scholar]
- Osawa, M., Masuda, M., Kusano, K., and Fujiwara, K. (2002). Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J. Cell Biol. 158, 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato, R., Veltmaat, J.M., Groffen, J., and Heisterkamp, N. (1998). Involvement of the tyrosine kinase Fer in cell adhesion. Mol. Cell. Biol. 18, 5762–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa, K., Kimura, T., Swieter, M., and Siraganian, R.P. (1997). The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31). J. Biol. Chem. 272, 31086–31091. [DOI] [PubMed] [Google Scholar]
- Schaeper, U., Gehring, N.H., Fuchs, K.P., Sachs, M., Kempkes, B., and Birchmeier, W. (2000). Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149, 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, L., Nelson, D.L., and Stewart, D.M. (2000). Cdc42-interacting protein 4 mediates binding of the Wiskott-Aldrich syndrome protein to microtubules. J. Biol. Chem. 275, 7854–7861. [DOI] [PubMed] [Google Scholar]
- Wittmann, T., and Waterman-Storer, C.M. (2001). Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795–3803. [DOI] [PubMed] [Google Scholar]
- Yates, K.E., Lynch, M.R., Wong, S.G., Slamon, D.J., and Gasson, J.C. (1995). Human c-FES is a nuclear tyrosine kinase. Oncogene 10, 1239–1242. [PubMed] [Google Scholar]
- Zirngibl, R., Schulze, D., Mirski, S.E., Cole, S.P., and Greer, P.A. (2001). Subcellular localization analysis of the closely related Fps/Fes and Fer protein-tyrosine kinases suggests a distinct role for Fps/Fes in vesicular trafficking. Exp. Cell Res. 266, 87–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.