Abstract
Females of many animal species store sperm for taxon-specific periods of time, ranging from a few hours to years. Female sperm storage has important reproductive and evolutionary consequences, yet relatively little is known of its molecular basis. Here, we report the isolation of a loss-of-function mutation of the Drosophila melanogaster Acp29AB gene, which encodes a seminal fluid protein that is transferred from males to females during mating. Using this mutant, we show that Acp29AB is required for the normal maintenance of sperm in storage. Consistent with this role, Acp29AB localizes to female sperm storage organs following mating, although it does not appear to associate tightly with sperm. Acp29AB is a predicted lectin, suggesting that sugar–protein interactions may be important for D. melanogaster sperm storage, much as they are in many mammals. Previous association studies have found an effect of Acp29AB genotype on a male's sperm competitive ability; our findings suggest that effects on sperm storage may underlie these differences in sperm competition. Moreover, Acp29AB's effects on sperm storage and sperm competition may explain previously documented evidence for positive selection on the Acp29AB locus.
THE acts of insemination and fertilization are temporally separate events in many animal species. Rather than traveling immediately to the waiting ovum, sperm are typically held in storage in the mated female, often in specialized regions of her reproductive tract. In most mammals, for example, sperm are stored in an oviductal reservoir for a period of a few hours or days (reviewed in Suarez 2002; Rodriguez-Martinez 2007). Moreover, many insects store sperm in highly specialized storage organs, with sperm surviving for weeks (as in Drosophila; e.g., Bloch Qazi et al. 2003) to many years (as in some social hymenopterans; Hölldobler and Wilson 1990).
Sperm storage has a number of important functional and evolutionary consequences. From a functional perspective, storage of sperm is often a vital component of reproduction: studies in mammals suggest that sperm storage in the oviductal reservoir helps to prevent polyspermy (reviewed in Suarez 2002) and that it may facilitate control over the process of sperm activation (Suarez 2002; Rodriguez-Martinez 2007). In insects, female sperm storage may reduce the number of potentially costly matings required for full female fecundity and allows the fertilization of hundreds or thousands of eggs from one or a few matings. In Drosophila melanogaster, for example, females store ∼700–1000 of the 4000 sperm received in a single mating and use ∼400 for fertilization over a period of ∼2 weeks (reviewed in Neubaum and Wolfner 1999b; Bloch Qazi et al. 2003).
In addition to being important for successful reproduction, the phenomenon of sperm storage can have profound evolutionary consequences. In combination with multiple mating by females (polyandry), sperm storage can generate strong selective pressures on both males and females. If sperm from different males are simultaneously present in the reproductive tract of a single female, then any trait that grants greater fertilization success to the sperm of one male over those of his competitor(s) will be favored by selection. Multiple mating and sperm storage thus create the potential for at least two types of selective regime: sperm competition, whereby sperm from different males present in the same female at the same time compete for ova (e.g., Birkhead and Møller 1998; Parker 1998; Simmons 2001), and cryptic female choice, whereby a female preferentially uses sperm from one male over those from another (Eberhard 1996). Consequently, sperm competition and cryptic female choice are thought to underlie such diverse phenomena as sperm gigantism (e.g., Miller and Pitnick 2002), sperm heteromorphism (e.g., Holman and Snook 2006; Holman et al. 2008), and the rapid evolution of some reproductive proteins (reviewed in Clark et al. 2006; Panhuis et al. 2006).
In D. melanogaster, sperm storage follows a stereotyped physiological progression. During copulation, sperm are transferred to the female along with seminal fluid proteins (and possibly other nonprotein ejaculate components) and form a discrete “sperm mass.” Over a period of ∼1 hr, the sperm mass progresses from the vagina to the anterior end of the uterus, with concomitant expansion and morphological changes of the uterus (Adams and Wolfner 2007). Sperm storage begins at this time, since the entrances to the female sperm storage organs lie at the anterior end of the uterus. D. melanogaster females store sperm in two types of organ: the long, coiled seminal receptacle and the paired spermathecae. It is thought that sperm from the seminal receptacle are used first, with the spermathecae acting as long-term storage organs (see Bloch Qazi et al. 2003 and references therein). Interestingly, the spermathecae appear to secrete substances required for sperm survival in both types of storage organ, since sperm stored in the seminal receptacles of lozenge or Hr39 mutant females (which lack spermathecae and additionally lack female accessory glands in the latter case) have reduced viability (Anderson 1945; Allen and Spradling 2008).
While the physiological mechanisms of sperm storage have been well described in several systems (e.g., Suarez 2002; Bloch Qazi et al. 2003; Adams and Wolfner 2007; Rodriguez-Martinez 2007), and its evolutionary implications explored in detail (e.g., Birkhead and Møller 1998; Parker 1998; Simmons 2001; Cameron et al. 2007), the identities of the molecules responsible for sperm storage are still relatively mysterious. Work in mammals and in Drosophila has, however, begun to identify both male and female molecular contributions to sperm storage. For example, expression of the enzyme glucose dehydrogenase (Gld) in the female reproductive tract is required for normal sperm storage, since in its absence females store reduced numbers of sperm and exhibit defects in the release of stored sperm (Iida and Cavener 2004). Recent studies have identified a number of genes expressed in the sperm storage organs (Lawniczak and Begun 2007; Allen and Spradling 2008; Prokupek et al. 2008), which should lead to further progress in identifying female-expressed genes involved in sperm storage.
Males also play roles in sperm storage beyond simply providing sperm. Several male-derived proteins have known roles in sperm storage in D. melanogaster. The carboxylesterase Est-6, which is produced in the male ejaculatory duct and bulb and is transferred to the female during copulation, appears to be involved in the release of sperm from storage (Gilbert and Richmond 1981). Moreover, mutational, RNAi, and directed cell-ablation studies have shown that seminal fluid proteins produced by the male accessory gland (Acps, for Accessory gland proteins) are necessary for the entry of sperm into storage, as well as for their maintenance and release from storage (Neubaum and Wolfner 1999a; Tram and Wolfner 1999; Bloch Qazi and Wolfner 2003; Ravi Ram and Wolfner 2007b). Several specific Acps have been identified that play important roles in sperm storage: Acp36DE, a large glycoprotein, is required for the accumulation of sperm in storage (Bloch Qazi and Wolfner 2003) and—probably as a consequence of its function in sperm storage—plays a role in sperm competition (Clark et al. 1995; Chapman et al. 2000). An additional four Acps—the predicted lectins CG1652 and CG1656, the cysteine-rich secretory protein (CRISP) CG17575, and the serine protease homolog CG9997—were recently shown to be necessary for the release of sperm from storage (Ravi Ram and Wolfner 2007b).
The finding that two predicted lectins (a class of sugar-binding proteins) are involved in sperm storage in D. melanogaster raises interesting parallels to sperm storage in other animals. In a number of mammals, sperm are stored for several hours in an oviductal reservoir, in which sperm are bound tightly to the epithelium of the oviduct (e.g., Suarez and Osman 1987). Carbohydrates mediate the adherence of sperm to the epithelium, with different sugars playing important roles in different species (e.g., Demott et al. 1995; Lefebvre et al. 1995, 1997; Ekhlasi-Hundrieser et al. 2005). In cows, for example, biochemical studies suggest that fucose residues on oviductal annexins act as receptors for sperm, with several sperm-bound seminal proteins recognizing the fucose moiety (Ignotz et al. 2001, 2007; Gwathmey et al. 2006).
In this study, we provide evidence that the seminal fluid protein Acp29AB, another predicted lectin, contributes to sperm storage in D. melanogaster, with a role distinct from those of CG1652 and CG1656. The Acp29AB gene was first identified in a screen for genes differentially expressed in the male accessory gland (Wolfner et al. 1997) and is predicted to encode a 234-amino-acid-secreted Ca2+-dependent (C-type) lectin (Wolfner et al. 1997; Mueller et al. 2004). Three lines of evidence suggest that Acp29AB might play a role in sperm storage. First, Clark et al. (1995) and Fiumera et al. (2005) found associations between naturally occurring alleles at the Acp29AB locus and a male's sperm competitive ability, a pattern that could be generated by differences in sperm storage between males bearing different Acp29AB alleles. Second, consistent with the hypothesis that Acp29AB could affect sperm competition and/or cryptic female choice, Aguadé (1999) found evidence for positive selection on Acp29AB, with an excess of amino acid substitutions between D. melanogaster Acp29AB and its ortholog in a close relative, D. simulans. Finally, Acp29AB's predicted molecular function as a lectin suggests a possible role in sperm storage, given the role of the predicted lectins CG1652 and CG1656 in Drosophila sperm storage (Ravi Ram and Wolfner 2007b), and of protein–sugar interactions in mammalian sperm storage (see above), and in sperm–egg interactions in many animals (see Mengerink and Vacquier 2001 for a review).
We show here that Acp29AB localizes to female sperm storage organs following mating, consistent with a role for this protein in sperm storage. We identified an apparent loss-of-function mutation, Acp29AB1, that affects sperm storage and sperm competition. Sperm from males bearing the Acp29AB1 mutation are not maintained efficiently in storage, and Acp29AB1 mutant males perform poorly in sperm competition, possibly as a consequence of reduced numbers of stored sperm. These results suggest that Acp29AB is an important protein affecting male fertility, and suggest that postcopulatory sexual selection could be a powerful force driving the evolution of this gene. Moreover, our findings highlight the role of sugar–protein interactions in mediating reproduction across diverse animal taxa.
MATERIALS AND METHODS
Fly handling and rearing:
All fly lines were maintained on yeast–glucose media at room temperature on 12-hr light:12-hr dark cycles. Males and virgin females were aged for 3–5 days before mating and/or dissection.
Production and affinity purification of anti-Acp29AB antibodies:
Amino acids 22–128 of Acp29AB, tagged at the N terminus with His-patch thioredoxin, were produced in Escherichia coli using the vector pBAD-DEST49 (Invitrogen) according to standard protocols (Ravi Ram et al. 2005). Following SDS–PAGE, the 29-kDa fusion protein was gel purified and used to inject rabbits; rabbit injection and boosts were carried out by CRAR/Cornell. Antibodies specific to Acp29AB's N terminus were affinity purified against the original fusion protein using a strip purification protocol as described in (Monsma et al. 1990). Anti-Acp29AB antibody was used at a 1:250 concentration for Western blotting, with horseradish peroxidase conjugated anti-rabbit secondary antibodies used at a 1:1000–1:2000 concentration.
Acp29AB transfer and localization:
To confirm transfer of Acp29AB to females during mating, and to localize it in mated female reproductive tracts, whole reproductive tracts (minus ovaries) from 3- to 5-day-old mated females, or portions thereof, were dissected in Ringer's solution (Yamamoto et al. 1988). Whole reproductive tracts with ovaries removed were homogenized in Ringer's solution with protease inhibitors (Roche), and 2× SDS sample buffer was added. Spermathecae were homogenized in 2× SDS sample buffer (Monsma et al. 1990; Ravi Ram et al. 2005); in this study we were not able to also assess seminal receptacles. To investigate the timing of Acp29AB transfer, whole female reproductive tracts without ovaries were collected 5 or 7 min after the start of mating (ASM). Localization of Acp29AB to the sperm mass, the mass of sperm and seminal fluid transferred to the female during mating, was assayed immediately after the end of mating, ∼20 min ASM (Ravi Ram et al. 2005). Spermathecal samples were collected 45 min and 1 hr ASM, since the first sperm typically enter the sperm storage organs within 20 min ASM, and number of sperm stored reaches a maximum at ∼1 hr ASM (Bloch Qazi and Wolfner 2003). Hemolymph sample collection and Western blotting were performed as previously described (Lung and Wolfner 1999; Ravi Ram et al. 2005). Several other Acps with known patterns of localization were used as controls (ovulin and Acp26Ab, Monsma and Wolfner 1988; Acp76A, Coleman et al. 1995; Acp62F, Lung et al. 2002).
Sperm binding assays:
We used in vitro sperm binding assays (following Neubaum and Wolfner 1999a) to investigate associations between Acp29AB and sperm. Briefly, at 20 min ASM, eight sperm masses were dissected out of mated female uteri into an eppendorf tube containing 500 μl of buffer 4 or 5 (Neubaum and Wolfner 1999a) and rotated at 4° for 1 hr. The samples were centrifuged at 5000 rpm for 5 min to separate the sample into pellet and supernatant fractions. The supernatant fraction was collected into a different tube. The pellet fraction, which contained the sperm, was homogenized in 40 μl of 2× SDS sample buffer. The supernatant fraction was concentrated using YM-10 Centriprep columns (Millipore, Billerica, MA) as per the manufacturer's instructions, and samples were prepared by mixing the concentrated supernatant fraction (∼10 μl) with 10 μl of 2× SDS sample buffer. These pellet and supernatant samples were separated on 15% SDS polyacrylamide gels and subjected to Western blotting using anti-Acp29AB antibody.
Identification of an Acp29AB mutant:
To identify an Acp29AB mutant, we screened ∼4500 lines from the Zuker collection (Koundakjian et al. 2004) bearing EMS-induced mutations on a cn bw second chromosome for altered amounts of Acp29AB protein. Total protein was extracted from males homozygous for EMS-mutagenized chromosomes. For each line, two whole 3- to 5-day-old (where possible) mutant male or control female (negative control) adult flies were ground in TE (50 mm Tris-HCl and 10 mm EDTA, pH 7.5), and then SDS sample buffer was added to 2× concentration. Western blotting was performed according to standard protocols (Ravi Ram et al. 2005).
The mutagenesis could have resulted in >1 lesion per chromosome and indeed the Acp29AB mutant line we identified (Acp29AB1) carries a linked but independent sperm transfer mutation (data not shown). To avoid interference of this and any other linked mutation in the phenotypic analyses, all experiments described below were carried out using Acp29AB1 hemizygotes over the deficiency Df(2L)ED611 (Ryder et al. 2007), unless mentioned otherwise. Fertility in these hemizygotes was normal (data not shown), suggesting that the sperm transfer mutation was fully complemented. Acp29AB protein was not detectable in the accessory glands of hemizygote males. Sibling Acp29AB1/CyO males were used as controls.
Nucleic acid and sequencing analysis:
For Northern blot analysis, total RNA was isolated from adult male flies using Trizol reagent (Gibco BRL) and then poly(A)+ purified using the PolyATtract mRNA isolation kit (Promega). Northern blots were prepared using standard procedures as described in Ausubel et al. (2007). Approximately 10 μg of poly(A)+ RNA were run per lane and the blot was probed with Acp29AB or, as a control, β1-tubulin random-primed probes (Bialojan et al. 1984). For reverse transcriptase PCR, cDNA was prepared from RNA extracted from 30 each of homozygous Acp29AB1, background matched Acp29AB+ flies from the Zuker collection, and Canton-S (CS) male flies and 30 CS females following the Superscript (Gibco) instructions. Primers that amplify full-length Acp29AB or Acp76A (as a control) were used in PCR reactions against previously mentioned cDNA samples and a CS male genomic control. For sequence analysis of candidate mutants, the Acp29AB coding region was PCR amplified using primers 5Acp29AB (5′ GGATCTCACACGCTTGAAATCTTCC 3′) and 3Acp29AB (5′ GTGGGTGTTGCAAATAGCTTGAATGA 3′) from genomic DNA prepared as in Hamilton and Zinn (1994). Both strands of the amplified products were sequenced directly by Cornell's BioResource Center with the following primers: 1875Acp29AB (5′ CAAATCTGGCCACAAATATACATAACC 3′), 1083Acp29AB (5′ GCCAACTTTCTCGAATCGTCTCAT 3′), and 1107Acp29AB (5′ GAGACGATTCGAGAAAGTTGGCT 3′).
Phenotypic analysis of the Acp29AB1 mutant:
To assess the effects of the Acp29AB1 mutation on sperm storage, sperm counts were carried out as in Neubaum and Wolfner (1999a) and Mueller et al. (2008). Sperm stored in the storage organs (spermathecae and seminal receptacle) of females mated to control or mutant males were counted at 2 hr ASM and at 4 days ASM. The slides were coded to avoid bias and decoded after counting for data analysis.
For analysis of the effects of the presence or absence of Acp29AB in the male ejaculate on female remating behavior, 3- to 5-day-old virgin CS females were allowed to mate with Acp29AB1/Df(2L)ED611 males or their control siblings for 1 hr, after which males were discarded. Females that mated successfully during this time were then given access to a new CS male 24 hr or 4 days later, again for 1 hr, during which time the pairs were observed and matings counted. Egg laying, fertility, and hatchability were assayed as described in Ravi Ram and Wolfner (2007b) for a period of 10 days postmating.
The role of Acp29AB in sperm competition was assayed by the estimation of two relevant parameters: P1, the proportion of offspring sired by a male when he is the first of two males to mate, and P2, the proportion of offspring sired by a male when he is the second of two males to mate. For the estimation of P1, 3- to 5-day-old cn bw females (which have white eyes) were first mated to Acp29AB1 males or their control siblings; these males were heterozygous for both cn and bw and hence produced half white-eyed (cn bw Acp29AB1/cn bw) and half red-eyed (cn bw/Df(2L)ED611 or cn bw/CyO) progeny. Control experiments confirmed that half of the offspring from these matings had white eyes and half had red eyes (data not shown). Two days later, the same females were allowed to mate with cn bw males, whose progeny from this mating were all white eyed; matings were observed, and only females that successfully mated both times were kept. Progeny from eggs laid 10 days after the second mating were scored for eye color. P1 was estimated as twice the number of red-eyed progeny (since half of the first male's progeny had white eyes) divided by total progeny. Estimation of P2 was conducted in a similar manner, except that females were first mated to a cn bw male and subsequently to Acp29AB1 males or their control siblings.
Statistical analyses:
Statistical analyses were performed using R version 2.5.1 (R Core Development Team 2008) or JMP version 5.1 (SAS Institute, Cary, NC).
RESULTS
Acp29AB is produced in the male accessory gland and localizes to the female spermathecae and hemolymph following mating:
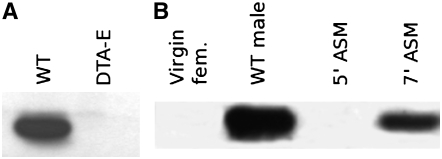
Western blot analysis using affinity-purified antibodies against Acp29AB detects a 29-kDa protein in extracts of CS male accessory glands, but not in extracts of DTA-E males that lack accessory gland main cells (Kalb et al. 1993) (Figure 1A), consistent with the initial identification of Acp29AB as a male accessory gland-specific transcript (Wolfner et al. 1997). The predicted molecular weight of Acp29AB (excluding the putative signal peptide) is 24.8 kDa. The higher apparent molecular weight may be due to post-translational modifications, such as glycosylation; other Acps are known to be glycosylated (Monsma et al. 1990; Bertram et al. 1996), and sequence data predict that Acp29AB can be N-glycosylated at amino acids 61 and 164 (Wolfner et al. 1997). Following mating, Acp29AB is transferred from the male to the female: Acp29AB is absent from the reproductive tracts of virgin females and females 5 min ASM, but is transferred to the female reproductive tract by 7 min ASM (Figure 1B).
Figure 1.—
Acp29AB is produced in the male accessory glands (A) and is transferred to females during mating (B). (A) Western blots using α-Acp29AB antibodies detect Acp29AB in wild-type (CS) male accessory glands but not in reproductive tracts from DTA-E males, which lack the products of the main secretory cells of the accessory gland. (B) Reproductive tracts of 10 virgin females or 10 females 5 min after the start of mating (ASM) contain no detectable Acp29AB, but Acp29AB is detected in reproductive tracts from 10 females 7 min ASM.
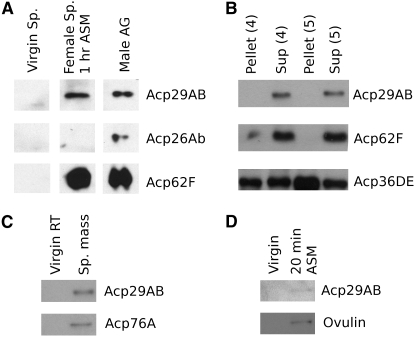
To examine the targets of Acp29AB in the mated female, we performed Western blot analyses of Acp29AB in extracts of mated female spermathecae, the sperm mass, and mated female hemolymph. We find that Acp29AB localizes to the spermathecae 1 hr ASM (Figure 2A); similar results were obtained 45 min ASM (data not shown). Given the localization of Acp29AB to the spermathecae, we performed sperm binding assays at 20 min ASM to determine if Acp29AB is tightly bound to sperm after transfer to the female uterus, but prior to sperm storage (Figure 2B). We found no evidence that Acp29AB binds to, or otherwise associates tightly with sperm, since it was never observed in the pelleted sperm fraction. However, at 20 min ASM, Acp29AB is present in the sperm mass, the mass of sperm and seminal fluid present in the female uterus after copulation (Figure 2C). Finally, we found that Acp29AB is detectable in the hemolymph of mated females (Figure 2D); several Acps have been shown to enter the mated female's hemolymph, which would allow them to elicit their effects via the neuroendocrine system (Monsma et al. 1990; Lung and Wolfner 1999; Ravi Ram et al. 2005). Localization of Acp29AB to the ovary and oocytes could not be examined due to the presence of proteins in the oocyte and ovary that cross-reacted with our anti-Acp29AB antibody (data not shown).
Figure 2.—
Acp29AB localizes to the sperm storage organs and hemolymph in the mated female. (A) Western blots using α-Acp29AB antibodies detect Acp29AB in the spermathecae (Sp.) of mated females 1 hr ASM, but not in the spermathecae of virgin females. The male accessory gland lane (male AG) shows accessory gland extracts from two males; the first two lanes each contain protein extracts from 160 spermathecae. Acp26Ab, a negative control, does not localize to the spermathecae as previously reported (Ravi Ram et al. 2005), while Acp62F does localize to the spermathecae (Lung et al. 2002) and thus acts as a positive control. (B) Acp29AB is not detectable on sperm following centrifugation. Sperm were pelleted in two buffers (4 and 5; Neubaum and Wolfner 1999a) such that sperm-bound proteins remain in the pellet, with soluble proteins in the supernatant (Sup). Acp62F is a negative control (Lung et al. 2002), and Acp36DE is a positive control (Neubaum and Wolfner 1999a). (C) Acp29AB is found in the sperm mass (Sp. mass), the mass of sperm and seminal fluid proteins present in the uterus after mating. Blotting was performed on extracts from two uteri. Acp76A is a positive control (Ravi Ram et al. 2005). (D) Western blots of hemolymph from 60 females. A small quantity of Acp29AB enters the hemolymph ∼20 min ASM. Full-length ovulin (shown) is known to enter the hemolymph, while the absence of low molecular weight ovulin proteolysis products (data not shown) indicates that the hemolymph sample is not contaminated with protein from the reproductive tract (Monsma et al. 1990).
Identification of an Acp29AB mutant:
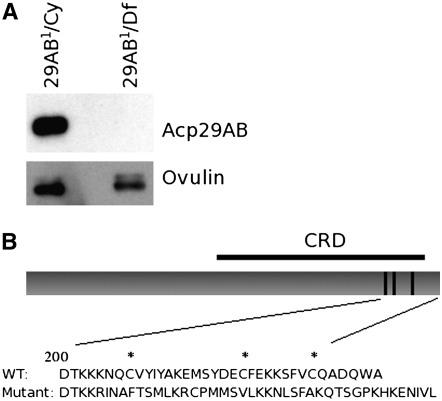
We performed Western blot analysis of protein extracts of whole males from each of ∼4500 second chromosome EMS-mutagenized fly lines (Koundakjian et al. 2004; kindly provided by Charles Zuker) to identify lines whose males either lacked Acp29AB or made an altered version of the protein. From initial Western blots, we identified 13 potential Acp29AB mutant lines (data not shown). Upon retesting, one line, 83-65, consistently showed no detectable Acp29AB protein; we designate this mutant allele Acp29AB1 (Figure 3A).
Figure 3.—
No Acp29AB protein is detectable in Acp29AB1 mutants. (A) Anti-Acp29AB (top) or anti-ovulin (bottom) antibodies were used to probe Western blots of protein extracted from the accessory glands of Acp29AB1 hemizygotes or their control siblings. (B) Schematic of Acp29AB protein and the inferred sequence alterations due to the Acp29AB1 mutation. The top drawing depicts wild-type Acp29AB, showing the carbohydrate recognition domain (CRD; black bar above) and sugar-binding sites (each dark line within the CRD designates two amino acid residues; (Mueller et al. 2004). The predicted amino acid sequences of the C terminus of wild-type Acp29AB, and of the Acp29AB1 mutant allele, are shown below, with putative structurally important cysteines marked by asterisks.
Sequence analysis of the open reading frame of Acp29AB1 shows that there is a single-base-pair deletion (A602) that disrupts the reading frame within Acp29AB1's predicted carbohydrate recognition domain (CRD) (Figure 3B). Due to this frameshift mutation, all amino acids after 200 are misencoded, and the polypeptide chain is predicted to be 242 amino acids long instead of the normal 234 (Figure 3B). Although Acp29AB is not detected in the mutant, Acp29AB mRNA levels are normal, as assessed by reverse transcriptase PCR (RT–PCR) and Northern blot analyses (supplemental material). Since our antibody was raised against the N terminus of Acp29AB, these data suggest that the protein encoded by the Acp29AB1 allele is unstable and/or degraded. Consistent with this hypothesis, the frameshift mutation eliminates three cysteines; in other CRD-containing proteins, homologous cysteines participate in structurally important disulfide bonds (Gronwald et al. 1998).
The Acp29AB1 allele is likely a null mutation, since it disrupts the predicted CRD and makes no detectable Acp29AB protein. We therefore used this allele to examine the role of Acp29AB in mated females by examining postmating responses in mates of Acp29AB1 males.
Acp29AB is necessary for the retention of sperm in storage:
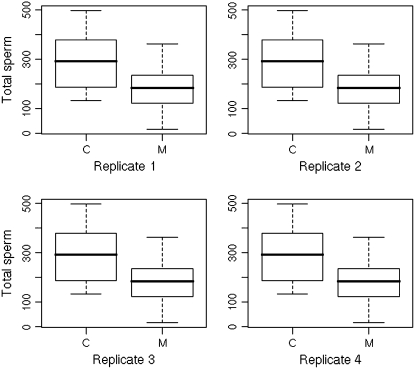
To assess the role of Acp29AB in sperm storage, we counted sperm present in the sperm storage organs of mates of Acp29AB1/Df(2L)ED611 and control males. Two hours ASM, we found no differences between mates of Acp29AB1/Df(2L)ED611 and control males in sperm stored in either the spermathecae (mean sperm stored: 241.1 vs. 235.9, respectively; two-tailed t-test, P = 0.90; n = 32) or the seminal receptacle (mean sperm stored: 363.6 vs. 404.2, respectively; two-tailed t-test, P = 0.22; n = 27), indicating that sperm are able to enter the sperm storage organs normally in the absence of Acp29AB. By contrast, 4 days ASM, we found a significant effect of male genotype on number of sperm stored in the spermathecae and in the seminal receptacle (Figure 4; Table 1). Specifically, fewer sperm were present in the sperm storage organs of mates of Acp29AB1/Df(2L)ED611 males in comparison to controls. Four independent replicate experiments were performed 4 days ASM; we found significant effects of genotype (P < 0.0001) and replicate (P = 0.0004) on sperm storage, but no genotype × replicate interaction effect (i.e., genotypes performed similarly in each replicate). Thus, the sperm of Acp29AB1 males do not appear to be retained efficiently in storage.
Figure 4.—
Sperm storage is reduced 4 days after mating in mates of Acp29AB1 mutants. Total number of sperm stored by mates of control (C) or Acp29AB1/Df(2L)ED611 (M) males in each of four replicates is shown. In each plot, the middle horizontal line represents the median number of sperm stored, the lower and upper margins of the box represent the 25th and 75th quartiles, and the whiskers extend to 1.5 times the interquartile range from the quartiles, such that points lying outside the whiskers are potential outliers.
TABLE 1.
Sperm storage by Acp29AB1 and control males 4 days after mating
| Effect tests
|
|||||
|---|---|---|---|---|---|
| Tissue | Source | d.f. | Sum of squares | F ratio | P |
| Spermathecae | Genotype | 1 | 19154.069 | 5.9280 | 0.0164 |
| Replicate | 3 | 45632.342 | 4.7076 | 0.0039 | |
| Genotype × replicate | 3 | 4066.584 | 0.4195 | 0.7393 | |
| Seminal receptacle | Genotype | 1 | 99078.50 | 13.7314 | 0.0003 |
| Replicate | 3 | 300420.67 | 13.8785 | <0.0001 | |
| Genotype × replicate | 3 | 48748.79 | 2.2520 | 0.0857 | |
| Combined | Genotype | 1 | 252746.97 | 22.6812 | <0.0001 |
| Replicate | 3 | 221491.23 | 6.6254 | 0.0004 | |
| Genotype × replicate | 3 | 87103.30 | 2.6055 | 0.0551 | |
Acp29AB plays a role in sperm competition:
We assessed the effects of the Acp29AB1 mutation on a male's sperm competitive ability, focusing on two aspects of sperm competition: P1, the proportion of offspring sired by a mutant male when he is the first of two males to mate, and P2, the proportion of offspring sired by a mutant male when he is the second of two males to mate. Pooling across three replicate experiments, we found a strong effect of Acp29AB genotype on P1 (P = 0.009; Table 2). In all three replicates, P1 was lower for Acp29AB1 mutant males than for their control siblings. This effect was statistically significant in one replicate (replicate 3; P = 0.01; Mann–Whitney U-test) and tended toward significance in replicate 1 (P = 0.09). We could not test for an interaction between replicate and treatment in the pooled analysis as the combined data violate the assumption of normality typically made in ANOVA, necessitating the use of nonparametric tests. Several different data transformations failed to improve normality. We did not find an effect of Acp29AB genotype on P2 (Table 3).
TABLE 2.
Proportion of offspring sired by Acp29AB1 or control males when mating first to a doubly mated female (P1)
| Replicate 1
|
Replicate 2
|
Replicate 3
|
Combined
|
||||
|---|---|---|---|---|---|---|---|
| Male genotype | n | Median P1 | n | Median P1 | n | Median P1 | Median P1 |
| Acp29AB1/Df(2L)ED611 | 50 | 0.184 | 44 | 0.024 | 41 | 0.026 | 0.050 |
| Control | 57 | 0.281 | 41 | 0.026 | 40 | 0.071 | 0.101 |
| P (Mann–Whitney U-test) | — | 0.09 | — | 0.66 | — | 0.01 | 0.009 |
TABLE 3.
Proportion of offspring sired by Acp29AB1 or control males when mating second to a doubly mated female (P2)
| Replicate 1
|
Replicate 2
|
Combined
|
||||
|---|---|---|---|---|---|---|
| Male genotype | n | Median P2 | n | Median P2 | Median P2 | |
| Acp29AB1/Df(2L)ED611 | 43 | 0.903 | 35 | 0.897 | 0.900 | |
| Control | 35 | 0.910 | 26 | 0.882 | 0.909 | |
| P (Mann–Whitney U-test) | — | 0.91 | — | 0.44 | 0.55 | |
Mates of Acp29AB-deficient males do not show altered postmating behaviors:
Stored sperm are required for several aspects of the female postmating response, including increased egg laying and decreased willingness to remate (Manning 1962, 1967; Chapman and Davies 2004; Ravi Ram and Wolfner 2007a). Given the sperm storage phenotype of Acp29AB1 males, we tested whether mates of Acp29AB1 males showed altered egg-laying or remating behaviors. Mates of Acp29AB1 males showed no difference in remating propensity compared to controls at 1 or 4 days postmating (Table 4). Similarly, we found no differences in total eggs laid by a female, total progeny, or egg-to-adult survivorship (hatchability) over 10 days between mates of Acp29AB1 and control males (Table 5). Subsequent experiments focusing on late (7–10 days after mating) egg laying similarly failed to find a significant effect of male genotype (data not shown).
TABLE 4.
Remating behavior of mates of Acp29AB1 and control males 1 and 4 days after mating
| 1 day ASM
|
4 days ASM
|
|||
|---|---|---|---|---|
| First male genotype | Rep. 1 | Rep. 2 | Rep. 1 | Rep. 2 |
| Acp29AB1/Df(2L)ED611 | 1/30a | 1/20 | 15/20 | 19/38 |
| Control | 1/26 | 4/20 | 9/18 | 22/36 |
| P (Fisher's exact test) | 1 | 0.34 | 0.18 | 0.36 |
ASM, after the start of mating; Rep., replicate.
The fraction indicates the number of females, of the total number assayed, that remated to a CS male 1 or 4 days after the first mating.
TABLE 5.
Fertility parameters of mates of Acp29AB1 and control males: totals over 10 days after mating
| Effect tests (genotype)
|
|||||
|---|---|---|---|---|---|
| Phenotype | Mutant mean (±SD) | Control mean (±SD) | d.f. | F ratio | P |
| Total eggs laid | 420.4 (±94.5) | 452.5 (±101.9) | 1 | 2.3877 | 0.13 |
| Total progeny | 346.8 (±85.8) | 326.9 (±79.6) | 1 | 1.4582 | 0.23 |
| Hatchability | 0.76 (±0.14) | 0.79 (±0.15) | 1 | 0.6021 | 0.44 |
“Mutant” refers to Acp29AB1/Df(2L)ED611 males. All data were Box–Cox transformed for ANOVA to improve fit to normality.
DISCUSSION
Sperm storage is vital for reproduction in many animal species and contributes to the phenomenon of sperm competition. In this study, we investigated the role of the D. melanogaster seminal fluid protein Acp29AB, a predicted lectin, in sperm storage. We showed that Acp29AB localizes to the female sperm storage organs following mating and also enters the mated female's hemolymph. We identified a presumed loss-of-function mutation, Acp29AB1, that disrupts the protein's predicted carbohydrate binding domain and that drastically reduces or eliminates the amount of Acp29AB protein present in the male accessory glands. Using this mutant, we have demonstrated that Acp29AB is necessary for the retention of sperm in storage at wild-type levels, although sperm entry into storage appears to be normal. In addition, Acp29AB1 males perform poorly in the defense component of sperm competition, likely as a result of reduced numbers of stored sperm.
The Acp29AB protein is a predicted C-type lectin, and thus likely interacts with carbohydrates and/or glycoproteins in the male seminal fluid, bound to sperm, or in the female reproductive tract. Protein–carbohydrate interactions play important roles in many aspects of reproduction across a wide range of animal species. Such interactions are vital for sperm–egg fusion in both invertebrates and vertebrates (Rosati et al. 2000; Mengerink and Vacquier 2001; Intra et al. 2006), with egg glycoproteins acting as primary sperm receptors in many species. Moreover, recent studies have shown that protein–carbohydrate interactions are directly involved in the establishment and maintenance of the oviductal sperm reservoir in mammals (Suarez 2002; Ekhlasi-Hundrieser et al. 2005; Ignotz et al. 2007).
Predicted lectins appear to be important for several aspects of sperm storage in Drosophila: whereas Acp29AB is essential for retaining sperm in storage, CG1652 and CG1656 are necessary for their release. Ravi Ram and Wolfner (2007b) used RNA interference (RNAi) to attribute sperm management functions to the latter two Acps, which are also predicted C-type lectins. Sperm from their knockdown males are able to enter into storage normally, but are released from storage more slowly than normal, thereby reducing female fecundity.
Although sugar–protein interactions appear to play important roles in sperm storage in flies and in mammals, it is unlikely that identical mechanisms operate across these divergent taxa. In cows, for example, fucose mediates the tight attachment of sperm to the oviductal epthelium (Lefebvre et al. 1997), inhibiting sperm movement, whereas D. melanogaster sperm maintain some motility while in storage (Lefevre and Jonsson 1962). We instead propose three potential mechanisms that might explain Acp29AB's role in sperm storage:
Acp29AB may promote interactions between sperm and components of the lumen of the sperm storage organs that promote sperm survival and/or retention. While Acp29AB does not bind tightly to sperm in the uterus (Figure 2B), it does localize to the spermathecae and associates with the sperm mass. This loose association with sperm may be sufficient to mediate interactions between sperm and other molecules. Moreover, it is possible that Acp29AB binds sperm in the sperm storage organs, which can be investigated in future studies.
Acp29AB may stimulate the production or release of molecules that affect sperm storage or survival, perhaps through interaction with a glycosylated receptor. Acp29AB could exert such an effect either from within the reproductive tract or through the neuroendocrine system, given that Acp29AB can be detected in the female's hemolymph. Previous experiments have suggested the existence of female-derived substances that contribute to sperm survival: as noted previously, sperm stored in the seminal receptacles of mutant females lacking spermathecae suffer from reduced viability (Anderson 1945; Allen and Spradling 2008), suggesting that the spermathecae produce viability-promoting substances.
Acp29AB may help to protect sperm from pathogens and/or the female's immune system. Lectins play important roles in the recognition of pathogens in insects (e.g., Pace and Baum 2004), raising the possibility that Acp29AB might help to prevent sperm loss due to pathogens in the female reproductive tract.
We note that altered patterns of sperm storage could, in some cases, be linked to differences in the rate of egg production and/or egg laying, if egg passage through the female reproductive tract is tied in any way to release of sperm from storage. Indeed, RNAi knockdown of the predicted lectins CG1652 and CG1656 results in both reduced egg laying and increased sperm storage (Ravi Ram and Wolfner 2007b). Acp29AB's effects on sperm storage, by contrast, appear to be independent of egg-production and egg laying, since mates of Acp29AB1 mutants retain fewer sperm than their control siblings (Figure 4, Table 1), but lay similar numbers of eggs (Table 5). Thus, our data suggest that the sperm storage phenotype of the Acp29AB1 mutant is not caused by differences in the egg-laying process.
Although the Acp29AB1 allele's effect on sperm storage is evidently sufficient to impair a male's sperm competitive ability, we did not see an effect on other female postmating behaviors that depend on the presence of sperm. Specifically, to the level of sensitivity of our assays, neither egg laying nor remating propensity was affected by the presence or absence of Acp29AB in the male ejaculate. By contrast, mates of males mutant for the sperm storage protein Acp36DE do show increased remating propensity and decreased egg laying (Neubaum and Wolfner 1999a). The sperm storage phenotype of mates of Acp29AB1 males is, however, less pronounced than that of mates of Acp36DE mutants—lack of Acp29AB in the ejaculate leads to an ∼40% reduction in sperm storage, whereas absence of Acp36DE leads to a ∼80–90% reduction in sperm storage (Neubaum and Wolfner 1999a). It is possible, then, that mates of Acp29AB1 males have sufficient sperm in storage to manifest a normal posmating behavioral response.
In this context, it is interesting to note that Acp29AB has several paralogs in the D. melanogaster genome, at least one of which (lectin 29Ca) has accessory gland specific or biased expression (Holloway and Begun 2004). The subtlety of the Acp29AB1 mutant phenotype in comparison to that of the Acp36DE1 mutant (Neubaum and Wolfner 1999a) may therefore derive from partial functional redundancy between Acp29AB and at least one of its paralogs.
Several previous studies have suggested a role for Acp29AB in sperm competition, on the basis of genotype–phenotype associations. Two large association studies (Clark et al. 1995; Fiumera et al. 2005) found correlations between alleles of Acp29AB and sperm competitive ability: Clark et al. (1995) found an effect of Acp29AB genotype on P1, while Fiumera et al. (2005) found a strong effect on P2 (P < 0.01) and a weaker effect on P1 (P < 0.05). We note that our failure to find an effect of Acp29AB genotype on P2 is not necessarily in conflict with the second study. The natural polymorphisms used in association studies may not be strict loss-of-function alleles, and their effects likely additionally depend on female genotype (e.g., Clark et al. 1999) or the genetic background via polymorphisms at other Acp loci (Fiumera et al. 2007). We propose that our finding, that Acp29AB1 males suffer from reduced sperm storage, suggests a mechanism for the effects seen by Clark et al. (1995) and Fiumera et al. (2005): differences in sperm competitive ability likely reflect differences in numbers of sperm stored due to differences in Acp29AB genotype and perhaps also due to interactions between a male's Acp29AB genotype and his mate's genotype.
The high variability between blocks in the defense experiment (Table 2) suggests that some form of unaccounted environmental variation impacted the proportion of offspring sired by both the control and the experimental males. Although it seems unlikely that subtle food batch differences or even relatively large variation in larval density are the cause (McGraw et al. 2007), these results do point to both a strong genetic and environmental component to sperm competitive ability and highlight the complex nature of these phenotypes.
At least one other protein involved in sperm storage in Drosophila has also been implicated in sperm competition. Clark et al. (1995) found associations between sperm competitive ability and alleles of Acp36DE, and males null for Acp36DE perform poorly in sperm competition assays (Chapman et al. 2000). Neubaum and Wolfner (1999a) and Chapman et al. (2000) argued that differences in sperm storage likely underlie differences in P1 and P2 associated with Acp36DE genotype. Thus, proteins involved in different stages of sperm storage (accumulation in storage for Acp36DE and maintenance in storage for Acp29AB) can have similar effects during sperm competition. These data suggest that differential storage of sperm from different males due to seminal protein polymorphism may play an important role in determining the outcome of sperm competition.
Patterns of molecular evolution at Acp29AB are consistent with positive selection on this locus: Aguadé (1999) and Zurovcova et al. (2006) have found evidence for an excess of nonsynonymous (amino acid altering) substitutions between D. melanogaster and D. simulans at Acp29AB, suggesting multiple adaptive fixations of favorable amino acid variants between these two species. The inferred history of positive selection on Acp29AB may well result from its role in sperm competition, as noted by Aguadé (1999): any variant that confers an advantage in the context of sperm competition or that increases a sperm's probability of being used by a female, will be favored (barring antagonistic pleiotropy; e.g., see Fiumera et al. 2005). Consequently, genes whose products are involved in sperm storage, retention, or use, and that hence may contribute to variation in postcopulatory sexual selection, are predicted to experience elevated rates of positive selection (e.g., Civetta and Singh 1995; Aguadé 1999; Clark et al. 2006; Panhuis et al. 2006). Our findings, that the predicted lectin Acp29AB is necessary for the maintenance of sperm in storage, combined with previous studies demonstrating that natural variation at Acp29AB associates with sperm competitive ability, support the hypothesis that postcopulatory sexual selection is driving the evolution of this male reproductive gene.
Acknowledgments
We thank Tracey Chapman and an anonymous reviewer, as well as Laura Sirot, Frank Avila, and Michael Goldberg for helpful comments on this manuscript, and Charles Zuker and members of the Zuker lab (particularly Ed Koundakjian) for generously making available their second-chromosome mutant fly lines. Members of the Wolfner lab, particularly Ella Lynch and Ramya Rajagopalan, helped in screening the Zuker collection for Acp29AB mutants. Kevin Kraus (Luther College) generously provided us with α-Acp76A antibodies. This work was funded by National Institutes of Health grant HD38921 to M.F.W. (including a diversity supplement for S.N.A.). For part of this work, S.N.A. was funded by a State University of New York minority fellowship. A.W. was a Howard Hughes Medical Institute (HHMI) predoctoral fellow. J.G. received funding from the Cornell/HHMI summer undergraduate research program and from Cornell's College of Agriculture and Life Sciences.
References
- Adams, E. M., and M. F. Wolfner, 2007. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J. Insect Physiol. 53 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguadé, M., 1999. Positive selection drives the evolution of the Acp29AB accessory gland protein in Drosophila. Genetics 152 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A. K., and A. C. Spradling, 2008. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 135 311–321. [DOI] [PubMed] [Google Scholar]
- Anderson, R. C., 1945. A study of the factors affecting fertility of lozenge females of Drosophila melanogaster. Genetics 30 280–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman et al. (Editors), 2007. Current Protocols in Molecular Biology. John Wiley & Sons, Hoboken, NJ.
- Bertram, M. J., D. M. Neubaum and M. F. Wolfner, 1996. Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochem. Mol. Biol. 26 971–980. [DOI] [PubMed] [Google Scholar]
- Bialojan, S., D. Falkenburg and R. Renkawitz-Pohl, 1984. Characterization and developmental expression of beta tubulin genes in Drosophila melanogaster. EMBO J. 3 2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead, T. R., and A. P. Møller (Editors), 1998. Sperm Competition and Sexual Selection. Academic Press, San Diego, CA.
- Bloch Qazi, M. C., Y. Heifetz and M. F. Wolfner, 2003. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 256 195–211. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi, M. C., and M. F. Wolfner, 2003. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J. Exp. Biol. 206 3521–3528. [DOI] [PubMed] [Google Scholar]
- Cameron, E., T. Day and L. Rowe, 2007. Sperm competition and the evolution of ejaculate composition. Am. Nat. 169 e158–e172. [DOI] [PubMed] [Google Scholar]
- Chapman, T., and S. J. Davies, 2004. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides 25 1477–1490. [DOI] [PubMed] [Google Scholar]
- Chapman, T., D. M. Neubaum, M. F. Wolfner and L. Partridge, 2000. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc. Biol. Sci. 267 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta, A., and R. S. Singh, 1995. High divergence of reproductive tract proteins and their association with postzygotic reproductive isolation in Drosophila melanogaster and Drosophila virilis group species. J. Mol. Evol. 41 1085–1095. [DOI] [PubMed] [Google Scholar]
- Clark, A. G., M. Aguadé, T. Prout, L. G. Harshman and C. H. Langley, 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A. G., D. J. Begun and T. Prout, 1999. Female x male interactions in Drosophila sperm competition. Science 283 217–220. [DOI] [PubMed] [Google Scholar]
- Clark, N. L., J. E. Aagaard and W. J. Swanson, 2006. Evolution of reproductive proteins from animals and plants. Reproduction 131 11–22. [DOI] [PubMed] [Google Scholar]
- Coleman, S., B. Drahn, G. Petersen, J. Stolorov and K. Kraus, 1995. A Drosophila male accessory gland protein that is a member of the serpin superfamily of proteinase inhibitors is transferred to females during mating. Insect. Biochem. Mol. Biol. 25 203–207. [DOI] [PubMed] [Google Scholar]
- DeMott, R. P., R. Lefebvre and S. S. Suarez, 1995. Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol. Reprod. 52 1395–1403. [DOI] [PubMed] [Google Scholar]
- Eberhard, W. G., 1996. Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press, Princeton, NJ.
- Ekhlasi-Hundrieser, M., K. Gohr, A. Wagner, M. Tsolova, A. Petrunkina et al., 2005. Spermadhesin AQN1 is a candidate receptor molecule involved in the formation of the oviductal sperm reservoir in the pig. Biol. Reprod. 73 536–545. [DOI] [PubMed] [Google Scholar]
- Fiumera, A. C., B. L. Dumont and A. G. Clark, 2005. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics 169 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera, A. C., B. L. Dumont and A. G. Clark, 2007. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics 176 1245–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, D. G., and R. C. Richmond, 1981. Studies of esterase 6 in Drosophila melanogaster. VI. Ejaculate competitive abilities of males having null or active alleles. Genetics 97 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwald, W., M. C. Loewen, B. Lix, A. J. Daugulis, F. D. Sonnichsen et al., 1998. The solution structure of type II antifreeze protein reveals a new member of the lectin family. Biochemistry 37 4712–4721. [DOI] [PubMed] [Google Scholar]
- Gwathmey, T. M., G. G. Ignotz, J. L. Mueller, P. Manjunath and S. S. Suarez, 2006. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol. Reprod. 75 501–507. [DOI] [PubMed] [Google Scholar]
- Hamilton, B. A., and K. Zinn, 1994. From clone to mutant gene. Methods Cell. Biol. 44 81–94. [DOI] [PubMed] [Google Scholar]
- Hölldobler, B., and E. O. Wilson, 1990. The Ants. Harvard University Press, Cambridge, MA.
- Holloway, A. K., and D. J. Begun, 2004. Molecular evolution and population genetics of duplicated accessory gland protein genes in Drosophila. Mol. Biol. Evol. 21 1625–1628. [DOI] [PubMed] [Google Scholar]
- Holman, L., R. P. Freckleton and R. R. Snook, 2008. What use is an infertile sperm? A comparative study of sperm-heteromorphic Drosophila. Evol. Int. J. Org. Evol. 62 374–385. [DOI] [PubMed] [Google Scholar]
- Holman, L., and R. R. Snook, 2006. Spermicide, cryptic female choice and the evolution of sperm form and function. J. Evol. Biol. 19 1660–1670. [DOI] [PubMed] [Google Scholar]
- Ignotz, G. G., M. C. Lo, C. L. Perez, T. M. Gwathmey and S. S. Suarez, 2001. Characterization of a fucose-binding protein from bull sperm and seminal plasma that may be responsible for formation of the oviductal sperm reservoir. Biol. Reprod. 64 1806–1811. [DOI] [PubMed] [Google Scholar]
- Ignotz, G. G., M. Y. Cho and S. S. Suarez, 2007. Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol. Reprod. 77 906–913. [DOI] [PubMed] [Google Scholar]
- Iida, K., and D. R. Cavener, 2004. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J. Exp. Biol. 207 675–681. [DOI] [PubMed] [Google Scholar]
- Intra, J., F. Cenni and M. E. Perotti, 2006. An alpha-L-fucosidase potentially involved in fertilization is present on Drosophila spermatozoa surface. Mol. Reprod. Dev. 73 1149–1158. [DOI] [PubMed] [Google Scholar]
- Kalb, J. M., A. J. DiBenedetto and M. F. Wolfner, 1993. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc. Natl. Acad. Sci. USA 90 8093–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian, E. J., D. M. Cowan, R. W. Hardy and A. H. Becker, 2004. The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics 167 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak, M. K., and D. J. Begun, 2007. Molecular population genetics of female-expressed mating-induced serine proteases in Drosophila melanogaster. Mol. Biol. Evol. 24 1944–1951. [DOI] [PubMed] [Google Scholar]
- Lefebvre, R., P. J. Chenoweth, M. Drost, C. T. LeClear, M. MacCubbin et al., 1995. Characterization of the oviductal sperm reservoir in cattle. Biol. Reprod. 53 1066–1074. [DOI] [PubMed] [Google Scholar]
- Lefebvre, R., M. C. Lo and S. S. Suarez, 1997. Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol. Reprod. 56 1198–1204. [DOI] [PubMed] [Google Scholar]
- Lefevre, Jr., G., and U. B. Jonsson, 1962. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics 47 1719–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung, O., and M. F. Wolfner, 1999. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem. Mol. Biol. 29 1043–1052. [DOI] [PubMed] [Google Scholar]
- Lung, O., U. Tram, C. M. Finnerty, M. A. Eipper-Mains, J. M. Kalb et al., 2002. The Drosophila melanogaster seminal fluid protein Acp62F is a protease inhibitor that is toxic upon ectopic expression. Genetics 160 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, A., 1962. A sperm factor affecting the receptivity of Drosophila melanogaster. Nature 194 252–253. [Google Scholar]
- Manning, A., 1967. The control of sexual receptivity in female Drosophila. Anim. Behav. 15 239–250. [DOI] [PubMed] [Google Scholar]
- McGraw, L. A., A. C. Fiumera, M. Ramakrishnan, S. Madhavarapu, A. G. Clark et al., 2007. Larval rearing environment affects several post-copulatory traits in Drosophila melanogaster. Biol. Lett. 3 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengerink, K. J., and V. D. Vacquier, 2001. Glycobiology of sperm-egg interactions in deuterostomes. Glycobiology 11 37R–43R. [DOI] [PubMed] [Google Scholar]
- Miller, G. T., and S. Pitnick, 2002. Sperm-female coevolution in Drosophila. Science 298 1230–1233. [DOI] [PubMed] [Google Scholar]
- Monsma, S. A., and M. F. Wolfner, 1988. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 2 1063–1073. [DOI] [PubMed] [Google Scholar]
- Monsma, S. A., H. A. Harada and M. F. Wolfner, 1990. Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Dev. Biol. 142 465–475. [DOI] [PubMed] [Google Scholar]
- Mueller, J. L., D. R. Ripoll, C. F. Aquadro and M. F. Wolfner, 2004. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc. Natl. Acad. Sci. USA 101 13542–13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, J. L., J. Linklater, K. Ravi Ram, T. Chapman and M. F. Wolfner, 2008. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics 178 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum, D. M., and M. F. Wolfner, 1999. a Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 153 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum, D. M., and M. F. Wolfner, 1999. b Wise, winsome, or weird? Mechanisms of sperm storage in female animals. Curr. Top. Dev. Biol. 41 67–97. [DOI] [PubMed] [Google Scholar]
- Pace, K. E., and L. G. Baum, 2004. Insect galectins: roles in immunity and development. Glycoconj. J. 19 607–614. [DOI] [PubMed] [Google Scholar]
- Panhuis, T. M., N. L. Clark and W. J. Swanson, 2006. Rapid evolution of reproductive proteins in abalone and Drosophila. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, G. A., 1998. Sperm competition and the evolution of ejaculates: towards a theory base, pp. 3–54 in Sperm Competition and Sexual Selection, edited by T. R. Birkhead and A. P. Møller. Academic Press, San Diego.
- Prokupek, A., F. Hoffmann, S. I. Eyun, E. Moriyama, E. Zhou et al., 2008. An evolutionary expressed sequence tag analysis of Drosophila spermatheca genes. Evolution (in press). [DOI] [PubMed]
- R Core Development Team, 2008. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
- Ravi Ram, K., S. Ji and M. F. Wolfner, 2005. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem. Mol. Biol. 35 1059–1071. [DOI] [PubMed] [Google Scholar]
- Ravi Ram, K., and M. F. Wolfner, 2007. a Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 47 427–445. [DOI] [PubMed] [Google Scholar]
- Ravi Ram, K., and M. F. Wolfner, 2007. b Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 3 e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martinez, H., 2007. Role of the oviduct in sperm capacitation. Theriogenology 68(Suppl 1): S138–S146. [DOI] [PubMed] [Google Scholar]
- Rosati, F., A. Capone, C. D. Giovampaola, C. Brettoni and R. Focarelli, 2000. Sperm-egg interaction at fertilization: glycans as recognition signals. Int. J. Dev. Biol. 44 609–618. [PubMed] [Google Scholar]
- Ryder, E., M. Ashburner, R. Bautista-Llacer, J. Drummond, J. Webster et al., 2007. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics 177 615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, L., 2001. Sperm Competition and Its Evolutionary Consequences. Princeton University Press, Princeton, NJ.
- Suarez, S. S., 2002. Formation of a reservoir of sperm in the oviduct. Reprod. Domest. Anim. 37 140–143. [DOI] [PubMed] [Google Scholar]
- Suarez, S. S., and R. A. Osman, 1987. Initiation of hyperactivated flagellar bending in mouse sperm within the female reproductive tract. Biol. Reprod. 36 1191–1198. [DOI] [PubMed] [Google Scholar]
- Tram, U., and M. F. Wolfner, 1999. Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics 153 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner, M. F., H. A. Harada, M. J. Bertram, T. J. Stelick, K. W. Kraus et al., 1997. New genes for male accessory gland proteins in Drosophila melanogaster. Insect Biochem. Mol. Biol. 27 825–834. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K., A. Chadarevian and M. Pellegrini, 1988. Juvenile-hormone action mediated in male accessory- glands of Drosophila by calcium and kinase-C. Science 239 916–919. [DOI] [PubMed] [Google Scholar]
- Zurovcova, M., A. Tatarenkov and L. Berec, 2006. Differences in the pattern of evolution in six physically linked genes of Drosophila melanogaster. Gene 381 24–33. [DOI] [PubMed] [Google Scholar]