Abstract
The cytokine interleukin-3 (IL-3) is a critical regulator of inflammation and immune responses in mammals. IL-3 exerts its effects on target cells via receptors comprising an IL-3-specific α-subunit and common β-subunit (βc; shared with IL-5 and granulocyte-macrophage colony-stimulating factor) or a β-subunit that specifically binds IL-3 (βIL-3; present in mice but not humans). We recently identified two splice variants of the α-subunit of the IL-3 receptor (IL-3Rα) that are relevant to hematopoietic progenitor cell differentiation or proliferation: the full length (“SP1” isoform) and a novel isoform (denoted “SP2”) lacking the N-terminal Ig-like domain. Although our studies demonstrated that each mouse IL-3 (mIL-3) Rα isoform can direct mIL-3 binding to two distinct sites on the βIL-3 subunit, it has remained unclear which residues in mIL-3 itself are critical to the two modes of βIL-3 recognition and whether the human IL-3Rα SP1 and SP2 orthologs similarly instruct human IL-3 binding to two distinct sites on the human βc subunit. Herein, we describe the identification of residues clustering around the highly conserved A-helix residue, Glu23, in the mIL-3 A- and C-helices as critical for receptor binding and growth stimulation via the βIL-3 and mIL-3Rα SP2 subunits, whereas an overlapping cluster was required for binding and activation of βIL-3 in the presence of mIL-3Rα SP1. Similarly, our studies of human IL-3 indicate that two different modes of βc binding are utilized in the presence of the hIL-3Rα SP1 or SP2 isoforms, suggesting a possible conserved mechanism by which the relative orientations of receptor subunits are modulated to achieve distinct signaling outcomes.
Keywords: Cell Surface Receptor, Cytokine, Inflammation, Interleukin, Signal Transduction, GM-CSF, IL-3, IL-5
Introduction
Interleukin-3 (IL-3)3 is a cytokine produced principally by activated T-cells during immune responses that is known to be a key regulator of inducible hematopoiesis and inflammation. Importantly, IL-3 serves a vital role in stimulating basophil and mast cell responses to parasite infections (1). Because basophils are now known to play a critical role in Th2 immune responses (2–4), IL-3 is a critical regulator of allergic inflammation. In addition, IL-3 is expressed in the major embryonic vessels and serves an essential function in regulating the survival and proliferation of hematopoietic stem cells in the early stages of embryonic development (5). Despite these critical biological functions, our understanding of the mechanism by which IL-3 can stimulate its cell surface receptor remains limited.
IL-3 acts upon target cells via cell surface receptors composed of an IL-3-specific α-subunit and a common β-subunit (hβc) that is shared with the related cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-5 (IL-5). Although α-subunits bind their cognate ligands with low affinity (Kd, 1–100 nm) (6–8), formation of a high affinity signaling complex with the β-subunit (Kd, 50–500 pm) (9, 10) is essential for the activation of Janus kinase 2 (Jak2), which is constitutively associated with the intracellular portions of the receptor subunits, and induction of several signaling pathways (11, 12). In mice, but not humans, an additional β-subunit, known as βIL-3, is also utilized that specifically binds mouse IL-3 (mIL-3), but not GM-CSF or IL-5 (13). This receptor provides a useful model for studying the molecular details of cytokine recognition of the β-subunits, because mIL-3 binds βIL-3 directly with an affinity of ∼10–20 nm in the absence of the mIL-3Rα subunit, even though the latter is absolutely required for the formation of a signaling complex (14, 15).
Recently, we reported that both the human IL-3 (hIL-3) and mIL-3Rα subunits exist as two naturally occurring alternative splice variants: the archetypal full-length receptor with an ectodomain comprising an N-terminal Ig-like domain and a cytokine receptor homology module, designated the “SP1” variant, and a novel form lacking the N-terminal Ig-like domain designated “SP2” (16). These isoforms add additional complexity to the receptor system, because the two IL-3Rα isoforms have the capacity to elicit different signaling outcomes (16). In the case of mIL-3, we established that mIL-3Rα SP2 directs mIL-3 binding to the βIL-3 ligand-binding interface formed at the “elbow” between domains 1 and 4 (16), analogous to the ligand-binding interface of human βc (hβc) (15, 17–19), whereas the mIL-3Rα SP1 isoform directs mIL-3 binding to a distinct epitope on βIL-3 (15), which remains to be identified.
We sought to characterize the molecular mechanisms of receptor recognition and activation by studying the ligand, mIL-3. In the absence of structures of mIL-3 and its receptors, we prepared a structural model of the hIL-3·hβc interaction based on the recently solved hGM-CSF receptor complex crystal structure (20) and identified putative hβc-interacting residues within hIL-3. From alignment of the hIL-3 and mIL-3 sequences, we identified the homologous, candidate receptor-binding residues in mIL-3 and prepared a panel of mIL-3 mutants in which the highly conserved residue, Glu23, and neighboring residues in the A- and C-helices were individually mutated to alanine. The A-helix glutamic acid residue is very highly conserved throughout IL-3 orthologs (21), and the homologous A-helix glutamic acid residues in the related cytokines, hGM-CSF, hIL-5, and hIL-3, are known to be to central to hβc recognition (22–25). Importantly, we found that the mIL-3 Glu23 and the surrounding cluster comprising Lys27 (A-helix), Glu65 (adjacent to C-helix), Val69, Asn73, and Lys76 (all C-helix) are critical for βIL-3 binding and receptor activation in the presence of the mIL-3Rα SP2 isoform, whereas an overlapping epitope involving Lys27 and Glu65 is required for binding and activation of the mIL-3Rα SP1/βIL-3 receptor. Our studies implicate these distinct epitopes on mIL-3 as important contributors to the two different modes of βIL-3 binding utilized in the presence of the SP1 or SP2 mIL-3Rα isoforms. Our subsequent studies implicated the homologous A-helix glutamate, Glu22, within the human ortholog, hIL-3, as playing a critical role in hβc binding in the presence of the hIL-3Rα SP2 isoform, but with a less critical role in the presence of the SP1 isoform. Consistent with a role for the hIL-3Rα SP2 isoform directing hIL-3 interaction with the established domain 1-domain 4 interface in hβc, mutation of domain 4 B′-C′ loop residues similarly disrupted high affinity ligand binding in the presence of hIL-3Rα SP2, but not SP1. Overall, these studies indicate that two distinct IL-3-binding sites exist on both βIL-3 and hβc, and their utilization within the respective signaling complexes is dictated by the two IL-3Rα isoforms, SP1 and SP2, and involves distinct β-receptor-binding epitopes within IL-3. Our findings suggest a molecular mechanism by which isoform-specific ectodomain engagements could influence the relative receptor orientations, leading to initiation of distinct intracellular signaling programs.
EXPERIMENTAL PROCEDURES
Cell Lines
The generation of CTLL2 cell lines stably expressing wild-type or mutant mIL-3Rα SP1, mIL-3Rα SP2, hIL-3Rα SP1, or hIL-3Rα SP2 and wild-type or mutant hβc or βIL-3 subunits was described previously (16). These cell lines were maintained in RPMI 1640 containing 10% (v/v) fetal bovine serum (FBS), 50 μm 2-mercaptoethanol, and murine IL-2 at 10 units/ml in the presence of 2 μg/ml puromycin and 0.4 mg/ml G418 to select for cDNAs introduced via the pEFIRES-P and pEFIRES-N vectors, respectively, as before (16). COS7 cells were maintained in Dulbecco's modified Eagle's medium containing 10% FBS. Receptor expression was achieved by electroporation of cells with 10 μg of wild-type hIL-3Rα SP1 or SP2 and 25 μg of wild-type or mutant hβc DNA constructs at 200 V and 960 microfarads, after the cells were harvested by trypsinization, as described previously (15, 18). COS7 binding studies were performed on cells 64–68 h after transfection. The growth medium for the CTLL2 and COS7 cell lines was supplemented with 60 μg/ml benzyl penicillin, 100 μg/ml streptomycin, and 10 μg/ml gentamicin.
The murine pro-B cell line Ba/F3 was kindly provided by Dr. H. Warren (John Curtin School of Medical Research, The Australian National University) and cultured in RPMI 1640 supplemented with 10% (v/v) FBS and 0.2% (v/v) mIL-3 conditioned insect cell medium. Stem cell factor (SCF) ER-Hoxb8 neutrophil progenitor cells (26) were kindly provided by Prof. M. P. Kamps (Department of Pathology & Molecular Pathology Graduate Program, School of Medicine, University of California at San Diego) and cultured in OptiMEM medium (Invitrogen) containing 10% (v/v) FBS, 1% (v/v) penicillin-streptomycin-glutamine solution (Invitrogen), 1% (v/v) mouse SCF conditioned medium, 30 μm 2-mercaptoethanol, and 1 μm β-estradiol (Sigma). SCF-conditioned medium was produced by culturing the Chinese hamster ovary (SCF) cell line (kindly provided by Prof. M. P. Kamps) in OptiMEM medium supplemented with 10% (v/v) FBS, penicillin-streptomycin-glutamine solution, and 2-mercaptoethanol.
Site-directed Mutagenesis of mIL-3
Site-directed mutagenesis was performed using the QuikChange method with Pfu Turbo polymerase (Stratagene) according to the manufacturer's instructions to prepare the following mIL-3 mutants: I20A, E23A, K27A, and E30A (A-helix), E65A (neighboring the C-helix), and Y68A, V69A, S72A, N73A, and K76A (C-helix). The sequences were verified by Big Dye Terminator sequencing (Applied Biosystems).
Expression and Purification of mIL-3 and hIL-3
Wild-type and mutant mIL-3 and hIL-3 were expressed using the baculovirus/insect cell system according to established protocols (27). Briefly, cDNAs encoding C-terminally His6-tagged mIL-3 or hIL-3 within the pBacPak8 vector (Novagen) were co-transfected with FlashBAC DNA (Oxford Biosystems) into Sf9 cells. Subsequently, viruses were amplified and used to infect High5 insect cells to produce cytokines. Wild-type and mutant mIL-3 were secreted into the culture medium and used without further purification as growth stimuli for BaF/3 and SCF ER-Hoxb8 cell lines as indicated. For ligand binding and proliferation studies, the secreted mIL-3 and hIL-3 proteins were dialyzed extensively before purification by nickel chromatography and gel filtration.
Hot and Cold Saturation Assays
Murine IL-3 hot and cold saturation binding assays were performed on CTLL2 cells stably expressing the relevant receptors as described before (15). Human IL-3 hot saturation assays were performed on CTLL2 cells stably expressing hIL-3RαSP1 or SP2 via the pEFIRES-N vector and hβc via the pEF-IRES-P vector as described before (15, 16). COS7 cells transiently transfected with hIL-3RαSP1 or SP2 in pEFIRES-N vector and hβc in pCEX-V3-Xba vector were also used in hIL-3 hot saturation binding assays. 106 cells were resuspended in binding medium (RPMI1640 supplemented with 10 mm HEPES, pH 7.5, and 0.5% (w/v) bovine serum albumin) and incubated with increasing concentrations of radioligand in a total volume of 200 μl for 2–3 h at 4 °C. Nonspecific binding was determined by the addition of a 100-fold excess of unlabeled ligand to samples in which high radioligand concentrations were added. The nonspecific binding was interpolated for lower radioligand concentrations using linear regression. The assays were terminated by centrifugation of each binding solution through 0.2 ml of 2:1 (v/v) dibutylphthalate (Aldrich):dinonylphthalate (Fluka) at 12,000 × g for 4 min. The tips of the tubes, containing the visible pellet and associated radio-iodinated ligand, were counted using a Packard 5780 Auto-gamma counter. The dissociation constant (Kd) and Scatchard transformations were calculated from specific binding data using the EBDA (28) and LIGAND (29) programs that are contained in the KELL software (Biosoft, Cambridge, UK). Scatchard plots were used to provide a graphical representation of the binding data, and iterative curve fitting by LIGAND was used to estimate Kd values. Multiple data files were coanalyzed to obtain more accurate estimates of Kd values. One- and two-site models were evaluated in LIGAND, and only when statistically significant (p < 0.05), a two-site model was used to determine Kd values.
Cold Competition Binding Assays
To assess the ability of wild-type and mutant mIL-3 or hIL-3 variants to replace 125I-labeled wild-type mIL-3 or hIL-3, respectively, to saturate relevant receptors stably expressed in CTLL2 cells, cold competition binding assays were performed on CTLL2 cells stably transfected with relevant receptors. Stable transfectants expressing βIL-3 and either mIL-3α SP1 or SP2 used in this assay showed a single binding site in hot saturation assays as evidenced by a linear Scatchard plot (not shown), as observed before (16). Likewise, CTLL2 cells stably expressing hβc and either hIL-3α SP1 or SP2 also exhibited a one-site binding model in hot saturation hIL-3 binding assays (see Fig. 6, C and D). The cells were prepared as described above. 100 pm, 1 nm, and 1 nm 125I-labeled wild-type mIL-3 and a serial dilution of unlabeled wild-type or mutant mIL-3 (ranging from 20 pm to 200 nm) were added to 106 cells expressing βIL-3 and mIL-3Rα SP1, βIL-3 and mIL-3Rα SP2, and βIL-3 only, respectively, resuspended in binding medium to give a total volume of 200 μl. 100 pm 125I-labeled wild-type hIL-3 and a serial dilution of unlabeled wild-type or hIL-3 E22A were added to 106 cells expressing hIL-3Rα SP1 or SP2 and hβc resuspended in binding medium to give a total volume of 200 μl. After incubation for 4 h at 4 °C with intermittent agitation, the cells were washed and processed as described above. All of the assays were run in triplicate, in three independent experiments.
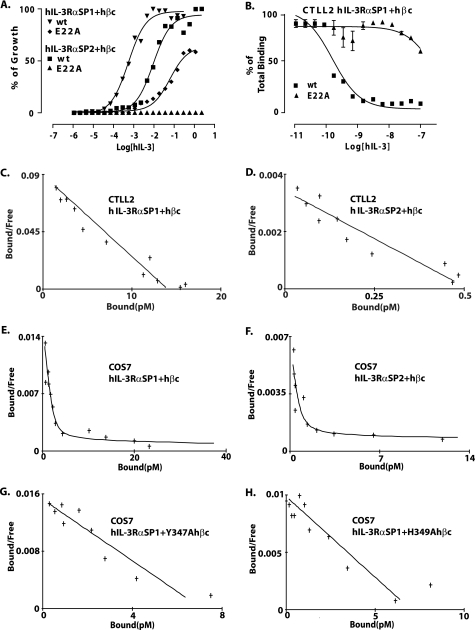
FIGURE 6.
Mutational analysis of the hIL-3·hβc interface. A, growth stimulation of CTLL2 cells expressing the hIL-3Rα SP1/hβc or hIL-3Rα SP2/hβc receptors by hIL-3 E22A and wild-type hIL-3. B, competition binding assays measuring the ability of hIL-3 E22A and wild-type hIL-3 to compete with 125I-radiolabeled wild-type hIL-3 for binding to the hIL-3Rα SP1/hβc receptor. C–H, hot saturation binding assays using 125I-radiolabeled wild-type hIL-3 on CTLL2 cells co-expressing hIL-3Rα SP1/hβc (C) and hIL-3Rα SP2/hβc (D) and COS7 cells co-expressing hIL-3Rα SP1/hβc (E), hIL-3Rα SP2/hβc (F), hIL-3Rα SP1/hβc Y347A (G), and hIL-3Rα SP1/hβc H349A (H).
The data were analyzed using the nonlinear regression analysis program GraphPad Prism (version 5.0; GraphPad Software Inc., San Diego, CA). Inhibition constants (mean Ki values with 95% confidence interval) of competitors for radiolabeled ligand-binding sites were calculated according to the formula Ki = IC50/(1 + L/Kd), where L is the concentration of radiolabeled ligand, Kd is the dissociation constant of the radioligand, and IC50 denotes the concentration of unlabeled ligand producing 50% inhibition of specific binding by the radiolabeled ligand. The Kd of radiolabeled ligand for relevant receptors was determined in parallel hot or cold saturation studies carried out as described elsewhere (15, 18, 30).
Proliferation Assays
Growth responses of CTLL-2 cells expressing IL-3Rα and relevant β-subunit receptors to wild-type or mutant hIL-3 or mIL-3 were measured in triplicate by [3H]thymidine incorporation as described previously (18). For CTLL-2 cells, proliferation was measured after 2 days of incubation. The proliferation data were analyzed by GraphPad Prism (version 5.0; GraphPad Software Inc.). The EC50 values were determined from sigmoidal dose response curve fitting and expressed relative to the wild-type response as ED50 values.
Western Blot Analysis of Jak2 and Erk1/2 Signaling in Ba/F3 and SCF ER-Hoxb8 Cell Lines
The following antibodies were purchased from Cell Signaling: rabbit anti-phospho-Jak2 (Tyr1007/1008), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti-Jak2 (D2E12), anti-p44/42 MAPK (137F5). For the preparation of lysates of Ba/F3 and SCF ER-Hoxb8 neutrophil progenitors, Ba/F3 or SCF ER-Hoxb8 cells were washed with plain RPMI 1640 or OptiMEM, respectively, three times and then starved in plain RPMI 1640 or OptiMEM, respectively, for 4 h before incubation with or without 1% (v/v) conditioned insect cell medium containing either wild-type or E23A mutant mIL-3 for 10 min. Wild-type and E23A mutant mIL-3 express at equivalent levels from High5 insect cells, as deduced by SDS-PAGE gel electrophoresis and Coomassie Blue staining. The lysates were either prepared in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 1 mm EGTA, 1 mm EDTA, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 10 mm Na3VO4, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, 10 mm NaF, 250 μm cathepsin G inhibitor I (only for SCF ER-Hoxb8 cells), 1 mm phenylmethylsulfonyl fluoride) or boiling SDS buffer (1% (w/v) SDS in 50 mm Tris-HCl, pH 6.8). The proteins were resolved by NuPAGE Novex 4–12% Bis-Tris gel electrophoresis (Invitrogen) and transferred to polyvinylidene difluoride for Western blot analyses with antibodies diluted according to the manufacturer's instructions. Total protein loading was controlled by gel staining with GelCode Blue (Pierce) and visualizing protein bands using a Fuji LAS-1000 Plus Imager (Fujifilm).
RESULTS
Identification and Mutation of Putative Receptor-binding Residues within mIL-3
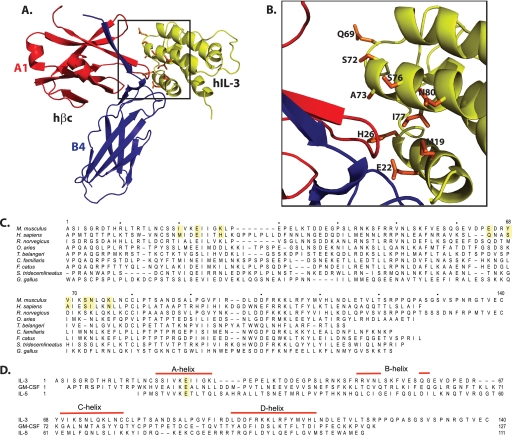
In the absence of a structure of mIL-3 or a model of mIL-3 interaction with the βIL-3 receptor, we generated a structural model of the hIL-3·hβc complex (Fig. 1, A and B) by superimposing the solution structure of a hIL-3 analog onto that of hGM-CSF within the experimentally determined hGM-CSF receptor complex crystal structure (20). The orthologous residues in mIL-3 were then identified by alignment of the human and mouse IL-3 sequences (Fig. 1C). The suitability of using the hIL-3 analog structure in such a model in the absence of an mIL-3 structure is validated by our recent NMR spectroscopy studies of an mIL-3 analog (21), where chemical shift indices confirm that the positions of the key α-helices, A–D, align with those in hIL-3 (32). The identified candidate βIL-3-interacting residues comprise the mIL-3 residues, Ile20, Lys27 (A-helix), Glu65 (preceding the C-helix), Tyr68, Val69, Ser72, Asn73, and Lys76 (C-helix), all of which cluster around the key A-helix residue, Glu23 (Fig. 1, A and B), which is conserved in all known IL-3 orthologs except the tree shrew, Tupaia belangeri (Fig. 1C), and in the related cytokines, IL-5 and GM-CSF (Fig. 1D). Although the functional role of mIL-3 Glu23 has not been examined experimentally to date, the homologous residue in hIL-3, Glu22, was shown to be important for receptor binding and activation (33, 34). We mutated each of these putative receptor-binding residues individually to alanine within full-length mIL-3 and expressed each mutein and wild-type mIL-3 from High5 insect cells using the baculovirus system. Unfortunately, mIL-3 I20A and Y68A mutants did not express (data not shown), and thus the functional consequences of these mutations could not be examined. The side chains of the Ile20 and Tyr68 human IL-3 homologs are buried in the hydrophobic core of the hIL-3 four-helix bundle, which indicates that I20A and Y68A mIL-3 were likely to be not secreted from insect cells because of the loss of structural integrity and consequent degradation. The remaining mutants, E23A, K27A, E65A, V69A, S72A, N73A, and K76A, were successfully expressed from High5 cells and purified for functional studies (Fig. 2A).
FIGURE 1.
Structural model of the hIL-3·hβc interface and IL-3 alignments. A, a schematic depicting a model of hIL-3 in complex with the domain 1-domain 4 interface of the hβc ectodomain. hIL-3 is drawn in green, domain 1 from chain A of the hβc homodimer is drawn in red, and domain 4 from chain B of the hβc homodimer is drawn in blue. The model was prepared by superimposition of the solution structure of a hIL-3 analog (Protein Data Bank accession code 1jli) on to hGM-CSF within the hGM-CSF receptor complex crystal structure (Protein Data Bank accession code 3cxe) using Coot (31). This figure was drawn using PyMOL. B, close-up view of the boxed region in A. The side chains of candidate hβc-interacting residues within hIL-3 are drawn as orange sticks and labeled according to the hIL-3 sequence. C, multi-species alignments of IL-3 amino acid sequences. hIL-3 residues identified as interactors of hβc by inspection of the model in A and B and their homologs in mIL-3 selected for alanine substitution mutagenesis are highlighted. The residue numbers correspond to the mIL-3 sequence. A more comprehensive alignment of IL-3 ortholog sequences is published in Ref. 21. D, structural alignment of mIL-3, mGM-CSF, and mIL-5 amino acid sequences, with the highly conserved A-helix glutamic acid residue highlighted. The positions of helices, marked with red bars above the sequences, were inferred from chemical shift indices determined by NMR spectroscopy for an mIL-3 analog (32) and mGM-CSF alignment with the hGM-CSF structure (Protein Data Bank accession code 2gmf). The mIL-5 secondary structure was deduced from the crystal structure (Protein Data Bank accession code 3b5k).
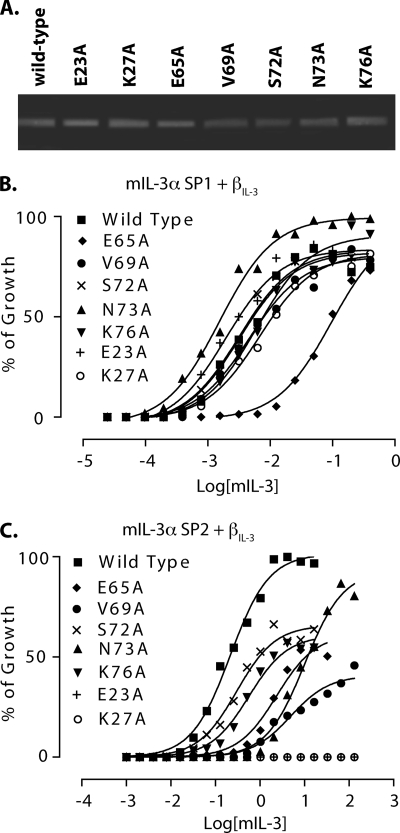
FIGURE 2.
Biological activity of purified mIL-3 mutants. A, reducing SDS-PAGE analysis of wild-type and mutant mIL-3 variants purified by Ni2+ affinity chromatography and gel filtration. The gel was stained with Coomassie Blue. B and C, proliferation of CTLL2 cells co-expressing mIL-3Rα SP1/βIL-3 (B) or mIL-3Rα SP2/βIL-3 (C) receptors in response to increasing concentrations of each mIL-3 mutant was measured. % of Growth represents the proportion of maximal [3H]thymidine incorporation.
Mutation of the Cluster Surrounding Glu23 Affects mIL-3 Growth Signaling via the SP2 but Not SP1 mIL-3Rα Isoform
Initially, we examined whether each of the mIL-3 mutants could stimulate the proliferation of the factor-dependent cell line, CTLL2, stably expressing βIL-3 and either the full-length (SP1 isoform) mIL-3Rα (Fig. 2B) or a naturally occurring splice variant of mIL-3Rα lacking the N-terminal Ig-like domain (D1; the SP2 isoform; Fig. 2C). Remarkably, the CTLL2 cells co-expressing the mIL-3Rα SP1 and βIL-3 subunits were relatively insensitive to mutation of Glu23 and residues in the surrounding cluster (Fig. 2B and Table 1). Only mIL-3 E65A exhibited any marked deficit in receptor activation relative to wild-type mIL-3, with 29-fold higher concentrations of mIL-3 E65A than wild-type mIL-3 required to give comparable growth stimulation.
TABLE 1.
Growth stimulation and receptor binding activities of wild-type and mutant mIL-3 to CTLL2 cells co-expressing βIL-3 and mIL-3Rα SP1 or SP2
| mIL-3 | ED50a |
Ki ± S.E.b |
||
|---|---|---|---|---|
| mIL-3RαSP1/βIL-3 | mIL-3RαSP2/βIL-3 | mIL-3RαSP1/βIL-3 | mIL-3RαSP2/βIL-3 | |
| nm | ||||
| Wild-type | 1 ± 0.03 | 1 ± 0.06 | 0.168 ± 0.001 | 0.626 ± 0.002 |
| E23A | 0.61 ± 0.02 | c | 0.298 ± 0.002 | e |
| K27A | 2.09 ± 0.05 | c | 1.56 ± 0.011 | e |
| E65A | 29.60 ± 1.71 | 11.01 ± 2.34d | 1.75 ± 0.010 | 1.94 ± 0.009 |
| V69A | 1.43 ± 0.04 | 22.56 ± 2.80d | 0.223 ± 0.001 | 5.73 ± 0.040 |
| S72A | 0.98 ± 0.03 | 1.40 ± 0.17d | 0.191 ± 0.001 | 0.282 ± 0.003 |
| N73A | 0.48 ± 0.01 | 46.96 ± 2.54 | 0.181 ± 0.001 | e |
| K76A | 1.88 ± 0.05 | 2.63 ± 0.61d | 0.304 ± 0.002 | 0.273 ± 0.003 |
a ED50 values are calculated relative to growth stimulation by wild-type mIL-3 for each of the two receptor systems. The ED50 values for wild-type mIL-3 growth stimulation of CTLL2 cells expressing mIL-3RαSP1/βIL-3 or mIL-3RαSP2/βIL-3 were each set as 1.
b The Ki values are the means and standard errors derived from three independent experiments. The numbers in parentheses represent the 95% confidence intervals. The Ki values were determined using GraphPad Prism software.
c No detectable growth stimulation.
d The growth responses of this mIL-3 mutant were approximately half the maximum percentage of growth observed for wild-type mIL-3.
e No detectable competition of mutant with wild-type mIL-3 for receptor binding.
In contrast, each of these mIL-3 mutants, except S72A, less effectively stimulated proliferation of CTLL2 cells co-expressing βIL-3 and the mIL-3Rα isoform lacking D1 (SP2) relative to wild-type mIL-3 (Fig. 2C and Table 1). The most severely affected mIL-3 mutants were E23A and K27A: two mutations that led to complete abolition of growth signaling, even at mIL-3 concentrations >500-fold higher than those required for half-maximal stimulation with wild-type mIL-3. For both of these mutants, no proliferative response was observed for βIL-3/mIL-3RαSP2-expressing CTLL2 cells in growth titrations extending to mIL-3 concentrations in excess of 100 times the wild-type mIL-3 effective concentration required for half-maximal growth stimulation (EC50). Mutation of neighboring residues located in the C-helix of mIL-3 led to profound, but less severe, defects in the sensitivity of mIL-3RαSP2/βIL-3-expressing CTLL2 cells to mIL-3 stimulation. Of these mutants, mIL-3 E65A, V69A, and N73A were the least effective stimuli for CTLL2 mIL-3RαSP2/βIL-3 growth, with respective 9.6-, 19-, and 41-fold higher mIL-3 concentrations required to achieve growth stimulation comparable with wild-type mIL-3 (Fig. 2C and Table 1). mIL-3 S72A, in comparison, retained stimulatory activity toward CTLL2 mIL-3RαSP2/βIL-3 cells comparable with wild-type mIL-3, albeit with reductions in the maximal levels of cell proliferation, whereas mIL-3 K76A exhibited a modest reduction in proliferative activity (2-fold relative to wild-type mIL-3) (Fig. 2C).
Receptor Binding Activities of mIL-3 Mutants
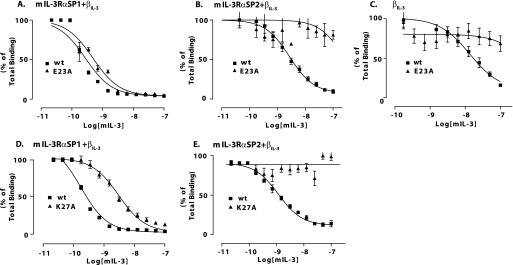
To further probe the molecular basis for mIL-3 receptor recognition, we performed binding assays to measure the capacity of our panel of mIL-3 mutants to bind receptors composed of βIL-3 and either the mIL-3Rα SP1 or SP2 isoform. These studies utilized a cold competition assay in which a titration of unlabeled mIL-3 (either wild type or mutant) ranging from 20 pm to 200 nm competed with a fixed amount of 125I-radiolabeled wild-type mIL-3 for binding to the appropriate mIL-3 receptors stably expressed on CTLL2 cells (100 pm, 1 nm, and 1 nm radiolabeled mIL-3 for mIL-3RαSP1/βIL-3, mIL-3RαSP2/βIL-3, and βIL-3, respectively). From these data, we calculated the concentration of wild-type or mutant mIL-3 required for 50% inhibition of 125I-labeled wild-type mIL-3 binding to the relevant receptor (IC50 values), which were converted to inhibition constants (Ki values). Initially, we performed a detailed examination of the mIL-3 E23A receptor binding properties (Fig. 3, A-C, and Table 1). These studies revealed that the mIL-3 E23A shows only a modest decrease in affinity for CTLL2 mIL-3RαSP1/βIL-3 cells (Ki = 0.30 nm versus 0.17 nm for wild-type mIL-3), whereas mIL-3 E23A did not detectably bind CTLL2 mIL-3RαSP2/βIL-3 cells (Fig. 3, A and B, and Table 1). We used the cold competition assay to measure the affinity of mIL-3 E23A for CTLL2 cells expressing solely the βIL-3 receptor. In this assay, mIL-3 E23A did not detectably bind directly to the βIL-3, whereas the Ki for wild-type mIL-3 binding was measured as 11.8 nm (Fig. 3C). These data suggest that Glu23 is an essential determinant of mIL-3 direct binding to βIL-3 and, similarly, mIL-3 binding to βIL-3 in the presence of the mIL-3Rα SP2 isoform. These findings mirror our prior work showing that mutagenesis of ligand-binding residues in βIL-3 domain 1-domain 4 functional epitope abolishes direct binding to mIL-3 as well as mIL-3 binding in the presence of mIL-3Rα SP2 (16). As a result, because βIL-3 utilizes the same epitope to bind mIL-3 both directly and in the presence of mIL-3Rα SP2, we measured binding for the remaining mIL-3 mutants to CTLL2 cells co-expressing βIL-3 and either the mIL-3Rα SP1 or SP2 isoform, but not βIL-3 alone. As shown in Fig. 3 (D and E), mIL-3 K27A exhibited dramatically reduced binding to CTLL2 mIL-3RαSP1/βIL-3 cells (Ki, 1.56 nm relative to 0.17 nm for wild-type mIL-3; Table 1) and no detectable binding to CTLL2 cells co-expressing βIL-3 and mIL-3RαSP2 subunits. Thus, Lys27 is a critical residue for high affinity binding to the mIL-3RαSP2/βIL-3 receptor, but in contrast to Glu23, it also plays a key role in the formation of the mIL-3·mIL-3Rα SP1·βIL-3 complex.
FIGURE 3.
Receptor binding properties of A-helix mutants relative to wild-type mIL-3. Competition binding assays were used to examine the capacity of mIL-3 E23A to compete with 125I-radiolabeled mIL-3 for binding to the mIL-3Rα SP1/βIL-3 (A), mIL-3Rα SP2/βIL-3 (B), and βIL-3 (C) receptors. The binding of mIL-3 K27A to mIL-3Rα SP1/βIL-3 (D) and mIL-3Rα SP2/βIL-3 (E) was also measured. In each plot, the control experiment examining competition by unlabeled wild-type (wt) mIL-3 for receptor binding is shown.
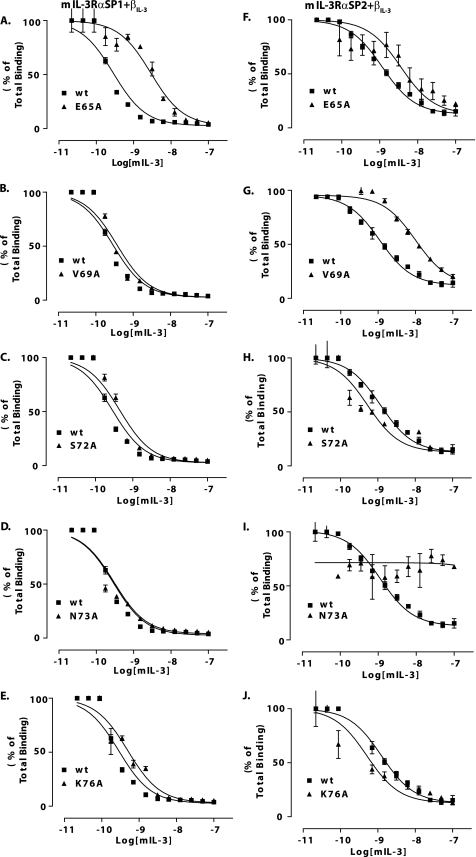
We then examined the capacity of mIL-3 C-helix mutants to bind CTLL2 cells co-expressing βIL-3 and either mIL-3Rα SP1 or SP2. Of these mutants, only mIL-3 E65A showed any substantial deficit in binding to CTLL-2 cells co-expressing the βIL-3 and mIL-3Rα SP1 subunits, with a 10-fold increase in Ki relative to wild-type mIL-3 (Fig. 4A and Table 1). In contrast, binding affinities of the mIL-3 mutants, V69A, S72A, N73A, and K76A, for CTLL2 mIL-3Rα SP1/βIL-3 cells were comparable with wild-type mIL-3 (Fig. 4, B–E, and Table 1). We also evaluated the capacity of the mIL-3 C-helix mutants to bind CTLL2 cells co-expressing mIL-3Rα SP2 and βIL-3 subunits. These experiments revealed an essential role for Asn73 of mIL-3 in receptor binding, because mIL-3 N73A does not detectably bind the mIL-3Rα SP2/βIL-3 receptor system (Fig. 4I), and mutation of the neighboring residues, Glu65 and Val69, led to >3- and >9-fold elevations in Ki values relative to wild-type mIL-3. However, by comparison, mutation of either Ser72 or Lys76 does not substantially compromise the affinity of mIL-3 for the mIL-3Rα SP2/βIL-3 receptor (Fig. 4, H and J, and Table 1).
FIGURE 4.
Receptor binding properties of C-helix mutants relative to wild-type mIL-3. Competition binding assays were used to examine the capacity of mIL-3 E65A (A or F), V69A (B or G), S72A (C or H), N73A (D or I), and K76A (E or J) to compete with 125I-radiolabeled mIL-3 for binding to the mIL-3Rα SP1/βIL-3 or mIL-3Rα SP2/βIL-3 receptors, respectively. In each plot, the control experiment examining competition by unlabeled wild-type (wt) mIL-3 for receptor binding is shown.
It should be noted that internalization of receptor·ligand complexes will have minimal bearing on the correlation between ligand binding and receptor activation in the present study. First, all binding experiments were conducted at 4 °C to minimize the possibility of internalization over the 2–3-h time course of binding assays. Second, studies of the related IL-5 receptor have shown that internalization of human βc occurs only after IL-5 binding and the activation of downstream signaling pathways (35), and as a result, we can assume that receptor·ligand complex internalization during the course of our proliferation assays is a key step in “turning off” a signal and will correlate with the capacity of wild-type or mutant IL-3 to activate the IL-3 receptor and initiate intracellular signaling.
Activation of Jak2 and Erk1/2 Signaling by Wild-type and E23A mIL-3
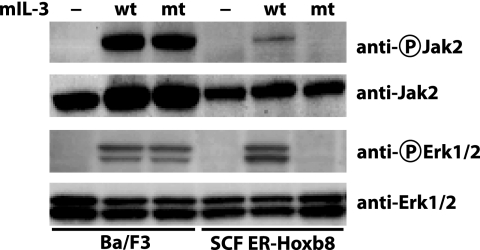
To examine the roles of the two distinct mIL-3 binding modes in governing the initiation of intracellular signaling, we investigated the capacity of wild-type and E23A mIL-3 to activate Jak2 and Erk1/2 in two mIL-3-dependent self-renewal cell line models: Ba/F3, a pro-B cell line; and SCF ER-Hoxb8, a neutrophil progenitor cell line prevented from undergoing differentiation when cultured in the presence of β-estradiol, which is required for the activity of a retrovirally introduced Hoxb8 gene (26). We compared the signals arising from wild-type mIL-3 with mIL-3 E23A, because unlike most other mIL-3 mutants described herein, mIL-3 E23A can elicit a wild-type growth response in CTLL2 cells co-expressing βIL-3 and mIL-3α SP1 but no detectable growth response in an analogous cell line expressing mIL-3α SP2. Ba/F3 cells exhibit comparable proliferation responses when stimulated with wild-type and E23A mIL-3,4 and because of its insensitivity to the E23A mutation, we classified Ba/F3 as a cell line that signals primarily via mIL-3Rα SP1. In contrast, SCF ER-Hoxb8 is a neutrophil progenitor cell line that showed a >100-fold reduction in proliferative response to the E23A mIL-3 mutant relative to wild-type mIL-3,4 thereby indicating that this cell line signals primarily via the mIL-3Rα SP2 isoform. We conducted stimulations of these cell lines with 1% (v/v) conditioned insect cell media containing of wild-type or E23A mIL-3, lysed the cells, and examined the activation status of the key signaling effector kinases, Jak2 and Erk1/2, by Western blot, using antibodies that recognize phosphorylation of Tyr1007/Tyr1008 of Jak2 and Thr202/Tyr204 of Erk1/2 (Fig. 5). Consistent with our findings in the proliferation assay (Fig. 2), Ba/F3 cells showed no measurable differences in Jak2 and Erk1/2 signaling when stimulated with wild-type or E23A mIL-3. In contrast, SCF ER-Hoxb8 cells showed a dramatic reduction in Jak2 and Erk activation when stimulated with E23A mIL-3 (Fig. 5).
FIGURE 5.
Activation of Jak2 and Erk1/2 signaling in Ba/F3 and SCF ER-Hoxb8 cells by wild-type and E23A mIL-3. BaF/3 cells cultured in mIL-3 (left three lanes) and SCF ER-Hoxb8 cells cultured in SCF (right three lanes) were starved for 4 h before stimulation with wild-type (wt), E23A (mt), or no (—) mIL-3 for 10 min. Western blots were performed to detect phospho-Jak2 (Tyr1007/1008) and total Jak2 (upper two panels) and phospho-Erk1/2 (Thr202/Tyr204) and total Erk1/2 (lower two panels). The data shown are representative of the outcomes of a minimum of two independent experiments.
hIL-3·hβc Binding Interfaces in Complexes with hIL-3Rα SP1 and SP2
Although the involvement of hIL-3 Glu22 in receptor activation and binding to hβc within the hIL-3Rα SP1·hβc high affinity complex has been demonstrated (33, 34), its role in receptor binding and activation with hβc in the presence of the novel hIL-3Rα SP2 isoform has not been examined. In the present work, we compared the capacity of hIL-3 E22A to bind and activate hβc in the presence of either the hIL-3Rα SP1 or SP2 isoform (Fig. 6A). Growth signaling was measured using CTLL2 cells stably expressing hIL-3Rα SP1/hβc or hIL-3Rα SP2/hβc for both wild-type and hIL-3 E22A. In the case of wild-type hIL-3, 16-fold higher concentrations were required for full growth stimulation via the hIL-3Rα SP2/hβc receptor relative to the hIL-3Rα SP1/hβc receptor, in agreement with our previous findings (16). In contrast, hIL-3 E22A gave a much reduced growth stimulation of cells expressing hIL-3Rα SP1/hβc (115-fold increased ED50 for hIL-3 E22A relative to wild-type hIL-3) and no growth stimulation of cells expressing hIL-3Rα SP2/hβc (Fig. 6A). These data illustrate that Glu22 is essential for growth signaling via the hIL-3Rα SP2/hβc receptor and plays an important, albeit nonessential, role in signaling via the hIL-3Rα SP1/hβc receptor. Consistent with the growth signaling data described above, binding of hIL-3 E22A to the hIL-3Rα SP1/hβc receptor was measured using cold competition assays and was found to be dramatically reduced relative to wild-type hIL-3 (Fig. 6B) (Ki = 123 nm versus wild type 0.125 nm). However, because of the low levels of 125I-radiolabeled hIL-3 binding to CTLL2 cells co-expressing the hIL-3Rα SP2 and hβc subunits, it was not possible to determine the Ki for hIL-3 E22A binding using cold competition assays.
We measured the Kd for high affinity binding to the hIL-3Rα SP2/hβc receptor stably expressed in CTLL2 cell lines and transiently expressed in COS7 cells using hot saturation binding assays with 125I-radiolabeled hIL-3. In CTLL2 cells, the Kd values for hIL-3Rα SP1/hβc and hIL-3Rα SP2/hβc binding to wild-type hIL-3 were quite similar (157 and 150 pm, respectively), with similar values obtained from binding studies in COS7 cells (172 and 126 pm, respectively) (Table 2). This is the first time that the Kd has been determined for high affinity binding with the naturally occurring hIL-3Rα SP2 isoform. Intriguingly, these data reveal for the first time that hIL-3 binds to the hIL-3Rα SP2/hβc receptor with an affinity comparable with hIL-3Rα SP1/hβc binding when expressed in either CTLL2 or COS7 cells (Fig. 6C and Table 2). Notably, far fewer high affinity binding sites/cell were detected in CTLL2 cells (78 sites with SP2 and 1864 sites with SP1), albeit with a more comparable number detected in COS7 cells (88 sites with SP2 and 275 sites with SP1). This is an intriguing observation, because comparable SP1 and SP2 isoform expression can be detected by flow cytometry on the surface of these cells (16),4 and comparable numbers of low affinity hIL-3-binding sites were measured in COS7 cells (10,928 sites for SP2 and 14,965 sites for SP1). Overall, we conclude that despite being properly transported to the cell surface, the hIL-3Rα SP2 subunit exhibits a lower propensity to engage in high affinity hIL-3 binding in CTLL2 cells. This situation clearly differs from the Ig-like domain deletion mutants of the mouse and human IL-6α receptor, which fail to localize to the cell surface (37), and the human GM-CSF receptor α-subunit, which requires its Ig-like domain for high affinity GM-CSF binding and normal receptor activation (38).
TABLE 2.
Receptor binding of wild-type hIL-3 to CTLL2 or COS7 cells co-expressing wild-type or mutant hβc with hIL-3Rα SP1 or SP2 determined from hot saturation binding assays
| Cell line | hIL-3Rα | hβc | Position of mutation | High affinity Kd ± S.E.a | Low affinity Kd ± S.E. | No. of experiments |
|---|---|---|---|---|---|---|
| pm | nm | |||||
| CTLL2 | SP1 | Wild-type | 157 ± 15 | c | 3 | |
| CTLL2 | SP2 | Wild-type | 150 ± 14 | c | 2 | |
| COS7 | SP1 | Wild-type | 162 ± 40 | 100d | 3 | |
| COS7 | SP2 | Wild-type | 126 ± 49.6 | 100d | 2 | |
| COS7 | SP1 | Y347A | B′-C′ loop | 466 ± 44 | c | 3 |
| COS7 | SP2 | Y347A | B′-C′ loop | b | 3 | |
| COS7 | SP1 | H349A | B′-C′ loop | 710 ± 76 | c | 3 |
| COS7 | SP2 | H349A | B′-C′ loop | b | 3 | |
| COS7 | SP1 | Y15A | A-B loop | b | 2 | |
| COS7 | SP2 | Y15A | A-B loop | b | 2 | |
| COS7 | SP1 | F79A | E-F loop | b | 2 | |
| COS7 | SP2 | F79A | E-F loop | b | 2 |
a Binding was determined using the hot saturation binding assay, and Kd ± S.E. was determined by co-analysis of data from multiple experiments using LIGAND (29).
b Binding was not detectable above nonspecific binding.
c The one-site binding model was statistically significant.
d To improve the accuracy of determination of the high affinity Kd, the low affinity site Kd (for hIL-3RαSP1 binding) was fixed at 100 nm when a two-site binding model was found to be statistically significant, according to established protocols (36).
In earlier work, the key ligand-binding interface on hβc was shown to comprise residues at the elbow formed between domains 1 and 4, contributed by the two chains of the hβc homodimer (15, 17, 18). Previously, we showed that the domain 1 hβc residues Tyr15 (A-B loop) and Phe79 (E-F loop) play important roles in hIL-3 binding and growth signaling with hIL-3Rα SP1/hβc (15, 18), and other studies have demonstrated that the domain 4 hβc residue, Tyr403 (F′-G′ loop), is also critical for hIL-3 binding (36). Interestingly, the domain 4 residues Tyr347, His349, and Ile350 in the B′-C′ loop, which are critical for IL-5 and GM-CSF high affinity binding, do not appear to play a role in IL-3 binding with hIL-3Rα SP1/hβc (39). We investigated the role of the hβc residues Tyr15, Phe79, Tyr347, and His349 in high affinity binding with the hIL-3Rα SP2 isoform relative to the hIL-3Rα SP1 isoform. These experiments were carried out in COS7 cells using transient expression. Neither hβc Y15A nor hβc F79A gave detectable hIL-3 binding when co-expressed with either hIL-3Rα SP1 or hIL-3Rα SP2, indicating that the domain 1 residues Tyr15 and Phe79 are both critical for high affinity binding with each of the IL-3Rα isoforms. The lack of binding is not due to poor expression of hβc Y15A or hβc F79A, because the expression of these two mutants was comparable with wild-type hβc expression in COS7 cells, as shown previously (Ref. 18 and data not shown). Interestingly, with hIL-3Rα SP1, the hβc Y347A and H349A mutants resulted in only modest reductions in wild-type high affinity binding, as described in earlier studies (39). The hβc Y347A and H349A mutants were previously shown to be expressed at levels comparable with wild-type hβc (39), a finding supported by the high affinity hIL-3 binding observed in the present work. In contrast, when co-expressed with hIL-3Rα SP2, the hβc Y347A and H349A mutants gave no detectable high affinity hIL-3 binding. These data clearly illustrate differences in the relative contributions of functional epitope residues located at the hβc domain 1-domain 4 elbow region to hIL-3 high affinity in the presence of the two hIL-3Rα isoforms. With both hIL-3Rα SP1 and SP2, mutation of the key domain 1 residues, Tyr15 and Phe79, completely abrogates high affinity hIL-3 binding. However, upon mutation of Tyr347 or His349 in the domain 4 B′-C′ loop, high affinity hIL-3 binding is ablated in the presence of the hIL-3Rα SP2 isoform but only modestly reduced with hIL-3Rα SP1. These data mirror the differential contribution of hIL-3 Glu22 to recognition and activation of hβc co-expressed with the hIL-3Rα SP1 and SP2 isoforms, as described above, where hIL-3Rα SP1/hβc receptors could be activated by hIL-3 E22A, and hIL-3Rα SP2/hβc receptors could not. Collectively, these data support the role of the hβc domain 1-domain 4 elbow region in hIL-3 binding but suggest that there are differences in the epitopes bound by hIL-3 depending on whether the complex involves hIL-3Rα SP1 or SP2.
DISCUSSION
The molecular mechanisms underlying the capacity of the IL-3 receptor to achieve differential signaling outcomes in response to IL-3 stimulation, such as the proliferation or differentiation of hematopoietic progenitors, have remained an enigma ever since the discovery of IL-3. By identifying a naturally occurring splice variant of the IL-3 α-subunit termed SP2 that lacks the N-terminal Ig-like domain that is otherwise present in the full-length receptor (termed SP1) in addition to the membrane-proximal cytokine receptor homology module (16), we have uncovered additional complexity within the IL-3 receptor system that would enable IL-3 to activate distinct signaling outcomes. Interestingly, whereas the IL-3Rα subunits in both mouse and humans exist as either SP1 or SP2 splice variants, no such naturally occurring isoforms have been identified in the related GM-CSF or IL-5 α receptors (38), consistent with the essential roles of the N-terminal Ig-like domains within these α-subunits for receptor activation (38, 40).
In the present work, we sought to establish which mIL-3 residues are crucial to recognition and activation of the βIL-3 receptor in the presence of either the mIL-3Rα SP1 or SP2 isoforms. In the absence of the detailed analyses of mIL-3, it has remained unclear: (a) which residues in the ligand itself are critical for βIL-3 binding and (b) whether the same key mIL-3 residues contribute to the two distinct modes of βIL-3 recognition, as dictated by the mIL-3Rα SP1 or SP2 splice variants. Initially, we identified candidate βIL-3-interacting residues based on a model of the hIL-3 interaction with the related hβc receptor (Fig. 1A), which was derived from the recent hGM-CSF·receptor complex crystal structure (20). Residues located in the A- and C-helices of hIL-3, which cluster around Glu22, a key residue for receptor binding and activation (33, 34), were selected from the model the hIL-3/hβc interaction, and the homologous residues in mIL-3 were identified by sequence alignment (Fig. 1C). We prepared a panel of mIL-3 mutants, comprising E23A and the neighboring residues K27A (A-helix), E65A (preceding C-helix), V69A, S72A, N73A, and K76A (C-helix), and examined their capacities to activate and bind βIL-3 in the presence of either mIL-3Rα SP1 or SP2 relative to wild-type mIL-3 (Figs. 3 and 4). Of these mutants, only mIL-3 S72A behaved comparably with wild-type mIL-3 in all of our experiments, which is surprising because Ser72 is one of the few residues conserved in this region between mIL-3 and hIL-3 with the exception of hydrophobic core contributors. Among the other mIL-3 mutants, we observed an excellent agreement between their receptor binding properties and their capacity to activate the receptor. The only possible exception is mIL-3 K27A, which exhibits a modest defect in its capacity to stimulate proliferation of CTLL2 mIL-3Rα SP1/βIL-3 cells relative to wild-type mIL-3 (a 2-fold increase in EC50) but with a profound reduction in binding affinity for these cells relative to wild-type mIL-3 (a 9-fold increase in Ki).
It is notable that only the mIL-3 K27A and E65A mutants showed reduced affinities for CTLL2 cells co-expressing mIL-3Rα SP1 and βIL-3, and they were the only two mutants among our panel that showed reduced stimulation of this cell line. These findings suggest that Glu65 is a critical residue for mIL-3Rα SP1/βIL-3 receptor binding and activation, and Lys27 plays a secondary role. Moreover, these data suggest the existence of an epitope on mIL-3 that mediates βIL-3 recognition in the presence of the mIL-3Rα SP1 subunit that is likely to be distinct from, but still overlap, the canonical receptor-binding epitope clustering around Glu23. A complete understanding of the βIL-3 epitope that mediates mIL-3 binding with mIL-3Rα SP1 awaits determination of a complex crystal structure.
The receptor composed of the mIL-3Rα SP2 and βIL-3 subunits was remarkably sensitive to mutation of Glu23 and the surrounding residues in mIL-3, consistent with an important role of the Glu23 cluster in mediating recognition of the domain 1-domain 4 ligand-binding interface that we have previously described for direct mIL-3 binding to βIL-3 (15), the same interface that is utilized for mIL-3 binding by βIL-3 in the presence of the mIL-3Rα SP2 isoform (Fig. 3C and Ref. 16). Proliferation of CTLL2 cells expressing the mIL-3Rα SP2/βIL-3 receptor system was undetectable when the mIL-3 E23A or K27A mutants were used as stimuli, with severe defects observed for mIL-3 E65A, V69A, and N73A and a modest loss of activity for mIL-3 K76A (Fig. 2). The mIL-3 E23A, K27A, and N73A mutants did not detectably bind mIL-3Rα SP2/βIL-3 receptors, whereas more modest reductions (3- and 9-fold, respectively) were observed for mIL-3 E65A and V69A receptor binding (Figs. 3 and 4). Of note, as one would predict from our proliferation studies with stable CTLL2 cell lines, mIL-3 E23A exhibited a reduction relative to wild-type mIL-3 in activation of the key signaling effectors, Jak2 and Erk1/2, in SCF ER-Hoxb8 cells, an mIL-3-dependent cell line that predominantly signals via the mIL-3Rα SP2 isoform (Fig. 5). These data contrast the capacity of wild-type and E23A mIL-3 to activate Jak2 and Erk1/2 equipotently in Ba/F3 cells, an mIL-3-dependent cell line that primarily signals via mIL-3Rα SP1 (Fig. 5).
Our data are reminiscent of prior studies of the hIL-3, where substitution of residues located adjacent to the conserved A-helix glutamate, in particular His26, the ortholog of mIL-3 Lys27, was poorly tolerated and diminished hIL-3 receptor activation and bioactivity (25, 34). In the present study, we unveiled a key role for mIL-3 Lys27 in the recognition and activation of βIL-3 in the presence of either mIL-3Rα SP1 or SP2. The importance of this residue is especially interesting considering that Lys27 is poorly conserved between IL-3 orthologs (Fig. 1C) and, unlike the highly conserved Glu23, is not conserved in the related cytokines, GM-CSF and IL-5 (Fig. 1D). This raises the possibility that Lys27 in mIL-3 is at least partly responsible for conveying the capacity of mIL-3 to directly bind the βIL-3 receptor and may govern the specificity of mIL-3, in preference to GM-CSF and IL-5, for binding to this mIL-3-specific receptor. Our data also indicate that the C-helix residue, Asn73, is an important contributor to mIL-3 recognition of the βIL-3 domain 1-domain 4 ligand-binding epitope (Fig. 4). The role of the mIL-3 C-helix in receptor binding and activation was largely unexpected, because prior mutagenesis studies of the orthologous hIL-3 clearly showed that the C-helix was very tolerant of amino acid substitution (34). One notable exception, however, was alanine substitution of hIL-3 Ile77, the homolog of mIL-3 Asn73, which resulted in a 15-fold reduction in potency (25), consistent with the mIL-3 homolog Asn73 serving a similar, conserved function in receptor binding and activation.
Our recent discovery of the hIL-3Rα SP2 splice variant, which complements the existing full-length or SP1 isoform, led us to investigate whether a similar mechanism to that deduced for βIL-3 binding and activation by mIL-3 operates within the human IL-3 receptor system. The hIL-3-binding epitope on hβc has remained a curiosity, because although hIL-3 binds hβc via the domain 1-domain 4 elbow interface, mutations within the hβc domain 4 B′-C′ loop do not abrogate hIL-3 binding in the presence of the hIL-3Rα SP1 isoform (36) (Fig. 6, G and H). In the present work, we confirmed that the hβc domain 4 B′-C′ loop mutants, Y347A and H349A, do not abrogate high affinity hIL-3 binding when co-expressed with hIL-3Rα SP1 but, conversely, when co-expressed with hIL-3Rα SP2, no high affinity hIL-3 binding could be detected. In comparison, mutation of other established ligand-binding residues in hβc domain 1, Tyr15 and Phe79 (15), abrogated hIL-3 high affinity binding when co-expressed with either of the hIL-3Rα isoforms. These data indicate that hIL-3 recognition of hβc within the hIL-3Rα SP1·hβc and hIL-3Rα SP2·hβc signaling complexes involves overlapping, but subtly different, interaction interfaces on the hβc receptor. The same appears to be true of hIL-3 itself, because alanine substitution of the highly conserved Glu22 within hIL-3 completely abolishes activation of the hIL-3Rα SP2/hβc receptor, whereas activation of the hIL-3Rα SP1/hβc receptor is severely reduced but still detectable.
The studies described in the present work have greatly enhanced our knowledge of the molecular details underlying mIL-3 recognition of βIL-3 within the signaling complexes formed with the mIL-3Rα SP1 or SP2 isoforms. These studies provide further support for the notion that the mIL-3Rα SP1 or SP2 subunits can direct mIL-3 binding to distinct epitopes on the βIL-3 receptor and suggest an elegant mechanism by which the relative orientations of receptor subunits could potentially be tuned to initiate different intracellular signaling programs to elicit distinct signaling outcomes. In addition, the subtle differences in human IL-3 interaction epitopes on the hβc receptor, as governed by the hIL-3Rα SP1 and SP2 isoforms, suggest that a mechanism similar to that deduced for the mIL-3 receptor operates in the human IL-3 receptor system. This concept of tunable receptor activation and signaling outputs has been elegantly explored for the erythropoietin and growth hormone receptors, where constraining the relative orientations of the transmembrane/intracellular domains led to preferential activation of distinct signaling effectors, such as Jak2 or MAPK (41, 42). Although the studies described herein have greatly advanced our knowledge of the mechanisms underlying the mouse and human IL-3 receptor activation, a comprehensive understanding of ligand binding and signal transduction by this fascinating receptor system awaits the crystal structures of IL-3 in complex with the ectodomains of the IL-3Rα SP1 or SP2 and β-subunits.
Acknowledgments
We thank J. Olsen for excellent technical advice; Dr. H Warren (John Curtin School of Medical Research, The Australian National University) for BaF/3 cells; Prof. M. P. Kamps (Department of Pathology & Molecular Pathology Graduate Program, School of Medicine, University of California at San Diego) for SCF ER-Hoxb8 neutrophil progenitor and Chinese hamster ovary (SCF) cells; the John Curtin School of Medical Research Microscopy & Cytometry Facility for assistance with fluorescence-activated cell sorter analyses; and Australian Cancer Research Foundation Biomolecular Resource Facility (The Australian National University) for automated sequencing of plasmids.
This work was supported by grants from the National Health and Medical Research Council of Australia.
S. Mirza and I. G. Young, unpublished data.
- IL
- interleukin
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- SCF
- stem cell factor
- IL-3Rα
- α-subunit of the IL-3 receptor
- βc
- common β-subunit of the GM-CSF, IL-3, and IL-5 receptors
- βIL-3
- mouse IL-3 specific β receptor subunit
- m
- mouse
- h
- human
- Jak
- Janus kinase
- FBS
- fetal bovine serum
- MAPK
- mitogen-activated protein kinase
- Erk
- extracellular signal-regulated kinase.
REFERENCES
- 1.Lantz C. S., Boesiger J., Song C. H., Mach N., Kobayashi T., Mulligan R. C., Nawa Y., Dranoff G., Galli S. J. (1998) Nature 392, 90–93 [DOI] [PubMed] [Google Scholar]
- 2.Perrigoue J. G., Saenz S. A., Siracusa M. C., Allenspach E. J., Taylor B. C., Giacomin P. R., Nair M. G., Du Y., Zaph C., van Rooijen N., Comeau M. R., Pearce E. J., Laufer T. M., Artis D. (2009) Nat. Immunol. 10, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimoto T., Yasuda K., Tanaka H., Nakahira M., Imai Y., Fujimori Y., Nakanishi K. (2009) Nat. Immunol. 10, 706–712 [DOI] [PubMed] [Google Scholar]
- 4.Sokol C. L., Chu N. Q., Yu S., Nish S. A., Laufer T. M., Medzhitov R. (2009) Nat. Immunol. 10, 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robin C., Ottersbach K., Durand C., Peeters M., Vanes L., Tybulewicz V., Dzierzak E. (2006) Dev. Cell 11, 171–180 [DOI] [PubMed] [Google Scholar]
- 6.Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 9655–9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaki S., Murata Y., Kitamura T., Miyajima A., Tominaga A., Takatsu K. (1993) J. Exp. Med. 177, 1523–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gearing D. P., King J. A., Gough N. M., Nicola N. A. (1989) EMBO J. 8, 3667–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura T., Sato N., Arai K., Miyajima A. (1991) Cell 66, 1165–1174 [DOI] [PubMed] [Google Scholar]
- 10.Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. (1991) Cell 66, 1175–1184 [DOI] [PubMed] [Google Scholar]
- 11.Quelle F. W., Sato N., Witthuhn B. A., Inhorn R. C., Eder M., Miyajima A., Griffin J. D., Ihle J. N. (1994) Mol. Cell Biol. 14, 4335–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamaki K., Miyajima I., Kitamura T., Miyajima A. (1992) EMBO J. 11, 3541–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. (1990) Science 247, 324–327 [DOI] [PubMed] [Google Scholar]
- 14.Hara T., Miyajima A. (1992) EMBO J. 11, 1875–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy J. M., Ford S. C., Olsen J. E., Gustin S. E., Jeffrey P. D., Ollis D. L., Young I. G. (2004) J. Biol. Chem. 279, 26500–26508 [DOI] [PubMed] [Google Scholar]
- 16.Chen J., Olsen J., Ford S., Mirza S., Walker A., Murphy J. M., Young I. G. (2009) J. Biol. Chem. 284, 5763–5773 [DOI] [PubMed] [Google Scholar]
- 17.Carr P. D., Gustin S. E., Church A. P., Murphy J. M., Ford S. C., Mann D. A., Woltring D. M., Walker I., Ollis D. L., Young I. G. (2001) Cell 104, 291–300 [DOI] [PubMed] [Google Scholar]
- 18.Murphy J. M., Ford S. C., Wiedemann U. M., Carr P. D., Ollis D. L., Young I. G. (2003) J. Biol. Chem. 278, 10572–10577 [DOI] [PubMed] [Google Scholar]
- 19.Murphy J. M., Young I. G. (2006) Vitam. Horm. 74, 1–30 [DOI] [PubMed] [Google Scholar]
- 20.Hansen G., Hercus T. R., McClure B. J., Stomski F. C., Dottore M., Powell J., Ramshaw H., Woodcock J. M., Xu Y., Guthridge M., McKinstry W. J., Lopez A. F., Parker M. W. (2008) Cell 134, 496–507 [DOI] [PubMed] [Google Scholar]
- 21.Murphy J. M., Metcalf D., Young I. G., Hilton D. J. (2010) Growth Factors. 28, 104–110 [DOI] [PubMed] [Google Scholar]
- 22.Graber P., Proudfoot A. E., Talabot F., Bernard A., McKinnon M., Banks M., Fattah D., Solari R., Peitsch M. C., Wells T. N. (1995) J. Biol. Chem. 270, 15762–15769 [DOI] [PubMed] [Google Scholar]
- 23.Tavernier J., Tuypens T., Verhee A., Plaetinck G., Devos R., Van der Heyden J., Guisez Y., Oefner C. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 5194–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez A. F., Shannon M. F., Hercus T., Nicola N. A., Cambareri B., Dottore M., Layton M. J., Eglinton L., Vadas M. A. (1992) EMBO J. 11, 909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagley C. J., Phillips J., Cambareri B., Vadas M. A., Lopez A. F. (1996) J. Biol. Chem. 271, 31922–31928 [DOI] [PubMed] [Google Scholar]
- 26.Wang G. G., Calvo K. R., Pasillas M. P., Sykes D. B., Häcker H., Kamps M. P. (2006) Nat. Methods 3, 287–293 [DOI] [PubMed] [Google Scholar]
- 27.Gustin S. E., Church A. P., Ford S. C., Mann D. A., Carr P. D., Ollis D. L., Young I. G. (2001) Eur. J. Biochem. 268, 2905–2911 [DOI] [PubMed] [Google Scholar]
- 28.McPherson G. A. (1985) J. Pharmacol. Methods 14, 213–228 [DOI] [PubMed] [Google Scholar]
- 29.Munson P. J., Rodbard D. (1980) Anal. Biochem. 107, 220–239 [DOI] [PubMed] [Google Scholar]
- 30.Murphy J. M., Soboleva T. A., Mirza S., Ford S. C., Olsen J. E., Chen J., Young I. G. (2008) Cytokine 42, 234–242 [DOI] [PubMed] [Google Scholar]
- 31.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 32.Yao S., Murphy J. M., Low A., Norton R. S. (2010) Biomol. NMR Assign., 4, 73–77 [DOI] [PubMed] [Google Scholar]
- 33.Barry S. C., Bagley C. J., Phillips J., Dottore M., Cambareri B., Moretti P., D'Andrea R., Goodall G. J., Shannon M. F., Vadas M. A., Lopez A. F. (1994) J. Biol. Chem. 269, 8488–8492 [PubMed] [Google Scholar]
- 34.Olins P. O., Bauer S. C., Braford-Goldberg S., Sterbenz K., Polazzi J. O., Caparon M. H., Klein B. K., Easton A. M., Paik K., Klover J. A., Thiele B. R., McKearn J. P. (1995) J. Biol. Chem. 270, 23754–23760 [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Moczygemba M., Huston D. P., Lei J. T. (2007) J. Leukocyte Biol. 81, 1137–1148 [DOI] [PubMed] [Google Scholar]
- 36.Woodcock J. M., Bagley C. J., Zacharakis B., Lopez A. F. (1996) J. Biol. Chem. 271, 25999–26006 [DOI] [PubMed] [Google Scholar]
- 37.Vollmer P., Oppmann B., Voltz N., Fischer M., Rose-John S. (1999) Eur. J. Biochem. 263, 438–446 [DOI] [PubMed] [Google Scholar]
- 38.Mirza S., Walker A., Chen J., Murphy J. M., Young I. G. (2010) Biochem. J. 426, 307–317 [DOI] [PubMed] [Google Scholar]
- 39.Woodcock J. M., Zacharakis B., Plaetinck G., Bagley C. J., Qiyu S., Hercus T. R., Tavernier J., Lopez A. F. (1994) EMBO J. 13, 5176–5185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornelis S., Plaetinck G., Devos R., Van der Heyden J., Tavernier J., Sanderson C. J., Guisez Y., Fiers W. (1995) EMBO J. 14, 3395–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seubert N., Royer Y., Staerk J., Kubatzky K. F., Moucadel V., Krishnakumar S., Smith S. O., Constantinescu S. N. (2003) Mol. Cell 12, 1239–1250 [DOI] [PubMed] [Google Scholar]
- 42.Brown R. J., Adams J. J., Pelekanos R. A., Wan Y., McKinstry W. J., Palethorpe K., Seeber R. M., Monks T. A., Eidne K. A., Parker M. W., Waters M. J. (2005) Nat. Struct. Mol. Biol. 12, 814–821 [DOI] [PubMed] [Google Scholar]