Abstract
We previously reported that mice lacking JSAP1 (jsap1-/-) were lethal and the brain of jsap1-/- at E18.5 exhibited multiple types of developmental defects, which included impaired axon projection of the corpus callosum and anterior commissures. In the current study, we examined whether the early telencephalic commissures were formed abnormally from the beginning of initial development or whether they arose normally, but have been progressively lost their maintenance in the absence of JSAP1. The early corpus callosum in the brain of jsap1+/+ at E15.5-E16.5 was found to cross the midline with forming a distinct U-shaped tract, whereas the early axonal tract in jsap1-/- appeared to cross the midline in a diffuse manner, but the lately arriving axons did not cross the midline. In the brain of jsap1-/- at E17.5, the axon terminals of lately arriving collaterals remained within each hemisphere, forming an early Probst's bundle-like shape. The early anterior commissure in the brain of jsap1+/+ at E14.5-E15.5 crossed the midline, whereas the anterior commissure in jsap1-/- developed, but was deviated from their normal path before approaching the midline. The axon tracts of the corpus callosum and anterior commissure in the brain of jsap1-/- at E16.5-E17.5 expressed phosphorylated forms of FAK and JNK, however, their expression levels in the axonal tracts were reduced compared to the respective controls in jsap1+/+. Considering the known scaffolding function of JSAP1 for the FAK and JNK pathways, these results suggest that JSAP1 is required for the pathfinding of the developing telencephalic commissures in the early brains.
Keywords: axons; focal adhesion kinase 1; JNK mitogen-activated protein kinases; MAPK8IP3 protein, human
Introduction
JNK/stress-activated protein kinase-associated protein 1 (JSAP1), also called JIP3, functions as a scaffold protein for components of the c-Jun N-terminal kinase (JNK) pathway (Whitmarsh et al., 1998; Yasuda et al., 1999; Kelkar et al., 2000). JSAP1 also interact with focal adhesion kinase (FAK), which is activated during cell adhesive interactions with the extracellular matrix (Takino et al., 2002, 2005). Moreover, JSAP1 is distributed in growth cones and plasma membrane as well as in the soma of neurons during development (Kelkar et al., 2000; Sato et al., 2004, 2008, 2011; Abe et al., 2009). Thus, these findings indicate that JSAP1 has a function to regulate axonal development. Indeed, mice lacking JSAP1 show pathfinding defects of the thalamocortical tracts in developing brain (Ha et al., 2005).
FAK and JNKs are highly expressed in the developing brain, and are important in neurite outgrowth and cell migration (Burgaya et al., 1995; Huang et al., 2003; Zhan et al., 2003; Rico et al., 2004; Robles and Gomez, 2006). FAK-deficient cells form focal adhesion/complexes, but show a decreased rate of cell migration due to the delayed disassembly of focal adhesions (Ilić et al., 1995; Reiske et al., 1999; Sieg et al., 1999; Jacamo et al., 2007). Moreover, phosphorylated JNK (phospho-JNK) is colocalized with FAK in focal adhesions of fibroblasts cultured on fibronectin (Almeida et al., 2000; Takino et al., 2005), suggesting that JNK is also involved in cell adhesion. And, jnk1-/- mice exhibit disrupted tract formation of the anterior commissure (AC) in the brain resulting from the progressive loss of microtubules within axons and dendrites. It has been shown that MAP2 and MAP1B have a reduced ability to bind microtubules and to promote their assembly in the absence of JNK1 (Chang et al., 2003). Consistent with the known roles of JNK in cell adhesion, the ectopic expression of the mixed lineage kinase, MUK/DLK/ZPK, in neural precursor cells impairs radial migration, whereas it allows these cells to leave the ventricular zone and differentiate into neural cells, suggesting that MUK/DLK/ZPK and JNK regulate radial cell migration via microtubule-based events (Hirai et al., 2002; Eto et al., 2010).
Recent molecular genetic studies have reported that E18.5 brains with the JSAP1 null mutation show multiple developmental defects, such as impaired axon guidance of the corpus callosum (CC) and AC, pathfinding defects of thalamocortical tracts and migration defects of anti-calretinin positive cells in the developing cerebral cortex (Kelkar et al., 2003; Ha et al., 2005). The developmental defects identified in the E18.5 brain of jsap1-/- should occur during developmental stages earlier than E18.5, probably as a consequence of the altered function of JSAP1-assisted signaling pathways (Ha et al., 2005; Chae et al., 2006). Moreover, because JSAP1 functions as a scaffold protein for JNK and FAK, the potential organization of JNK and FAK by JSAP1 might be important for the distribution or activation of these cellular factors in developing neurons. Thus, it would be intriguing to determine whether the JSAP1 dependent distribution and activations of JNK and FAK are involved in the impaired axon guidance of telencephalic commissures.
In the present study, we examined the appearance and progression of developmental defects in the early jsap1-/- brain. In addition, we investigated whether the null mutation of JSAP1 affects the temporal distributions of phospho-JNK and phospho-FAK in the axon tracts of the telencephalic commissures.
Results
Temporal patterns of developmental defects of the telencephalic commissures in the brains of jsap1-/-
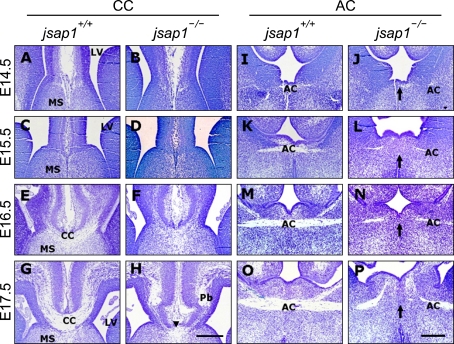
In a previous study, we reported that the E18.5 jsap1-/- brain showed impaired axon projections of the CC and AC (Ha et al., 2005). In the current study, we investigated the temporal patterns of the developmental defects during the early development of jsap1-/- brains. Previous studies reported that the early axonal extension of the CC, which stems from the cingulate cortex, pioneers across the midline of the telencephalon during E15.5-E16.5, and subsequently the late arriving collaterals originating from other regions of the neocortex join the existing tract (Koester and O'Leary, 1994; Ozaki and Wahlsten, 1998; Rash and Richards, 2001). Cresyl violet-stained brain sections showed that the overall morphology of the telencephalon of jsap1-/- at E14.5-E15.5 did not obviously differ from that of jsap1+/+ (Figures 1A-1D; see also Figures 2-5). Our cresyl violet-stained brain sections revealed that jsap1+/+ brains formed the CC during E15.5-E16.5 as reported previously (Koester and O'Leary, 1994; Ozaki and Wahlsten, 1998), while the early axons of the CC in the jsap1-/- brain at E15.5-E16.5, at least in part, crossed the midline (Figures 1C-1F). In jsap1+/+ brains at E17.5, the axon bundle of the CC became thicker and formed a typical U-shaped tract, whereas in jsap1-/- brains, late arriving neocortical-originated callosal axons failed to cross the midline. Instead, these axons projected only within each hemisphere, and formed Probst's bundle-like structures bilaterally in the cingulate cortex (Figures 1G and 1H). The Probst's bundles are known to be comprised of axonal tips that have failed to develop properly to cross the midline after their initial extension to the medial hemispheric walls (des Neves et al., 1999; Shen et al., 2002).
Figure 1.
Developmental defects of the corpus callosum and anterior commissures in developing brains of jsap1-/-. (A-N) Cresyl violet staining of coronal sections of paraffin-embedded brains at developmental stages; E14.5 (A, B, I, J), E15.5 (C, D, K, L), E16.5 (E, F, M, N) and E17.5 (G, H, O, P). In E14.5-E15.5 brains, the histologic configuration of the corpus callosum in jsap1-/- was indistinguishable from that of jsap1+/+ (A-D). Note that the early axon tracts of the corpus callosum in E16.5 brains (E, F) were identifiable in both jsap1+/+ and jsap1-/-. Probst's bundle-like structures (Pb) formed in the cingulate cortex at E17.5 in jsap1-/- brain were indicated (H). Note that the axons of the anterior commissures were distracted from their normal path long before approaching the midline (I-P), and thus were disconnected during development (arrows). AC, anterior commissures; CC, corpus callosum; LV, lateral ventricle; MS, medial septum; Th, thalamus; arrows and arrow heads, defected area. Scale bars, 500 µm.
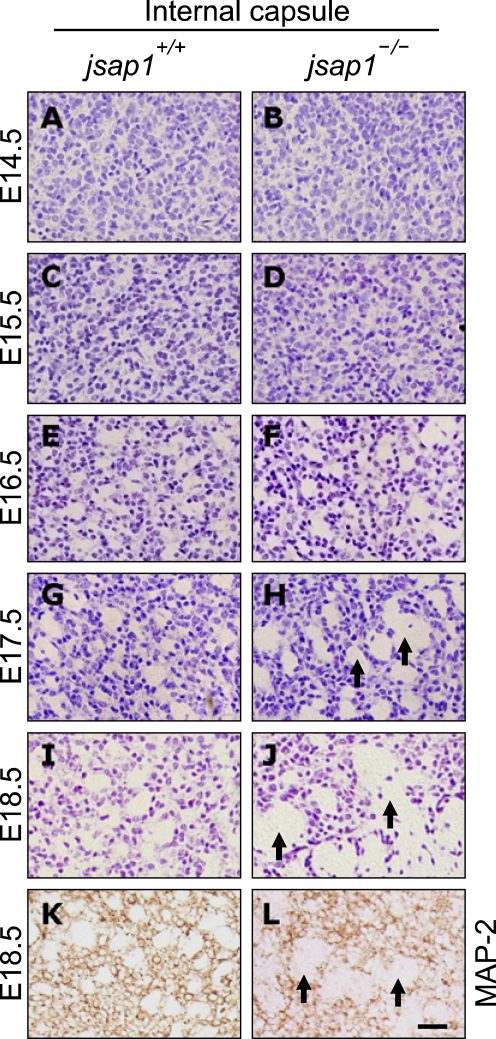
Figure 2.
Developmental defects of the internal capsule in developing brains of jsap1-/-. (A-L) Cresyl violet (A-J) or anti-MAP-2 (K-L) stained coronal sections of E14.5-E18.5 brains. The fiber tracts of the internal capsule were not clearly detected until E15.5 in both genotypes. The thickness of the fiber tracts in jsap1-/- at E17.5-E18.5 was larger than that of jsap1+/+. Arrows indicate the fiber tracts of the internal capsule. Scale bar, 100 µm.
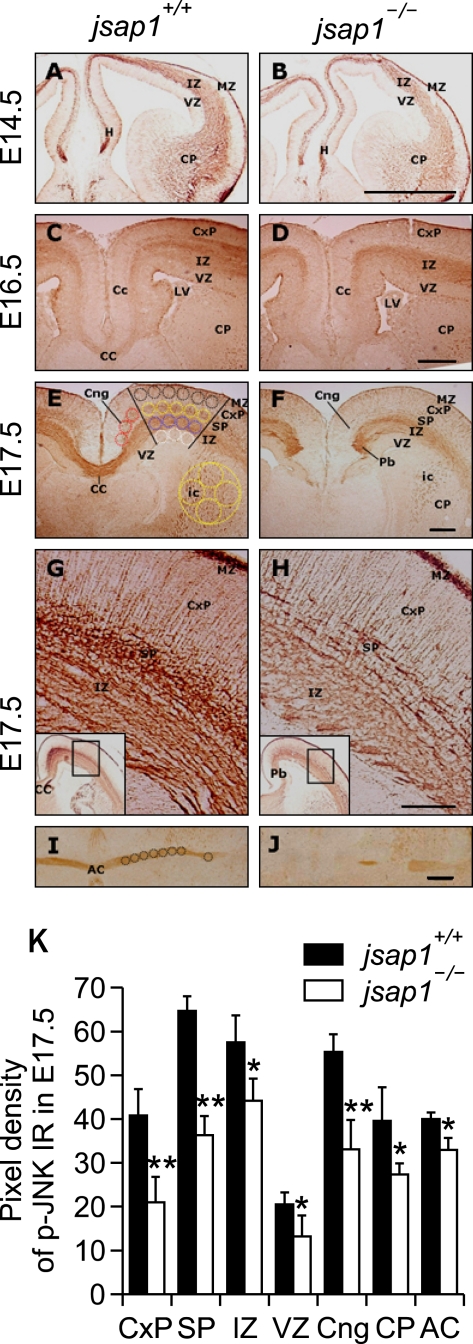
Figure 5.
Reduced expression of phospho-JNK levels in developing brains of jsap1-/-. (A-J) Anti-phospho-JNK stained coronal sections of cryo-sectioned brains at E14.5 (A, B), E16.5 (C, D), and E17.5 (E-J). Phospho-JNK was distributed in the soma and neuritic processes of neuronal cells in broad regions of developing jsap1+/+ and jsap1-/- brains. Anti-phospho-JNK labeled neuronal cells in the marginal zone, subplate, intermediate zone, subventricular zone, caudoputamen, and cingulum bundles, and also labeled radial glial fibers in the cortical plate (G, H). G and H are magnified images of the rectangles in the insets. The expression level of phospho-JNK in the U-shaped axon tract of the corpus collasum (C-F), and anterior commissure (I, J) in jsap1-/- brains was lower than that in jsap1+/+ brains. (K) Quantification of the expression levels of phospho-JNK in indicated brain regions in both genotypes. *, P < 0.05 and **, P < 0.01, differences between jsap1+/+ and jsap1-/- brains (Student's t-test). AC, anterior commissure; CC, corpus callosum; cng, cingulum bundle; CP, caudo-putamen; CxP, cortical plate; ic, internal capsule; IZ, intermediate zone; LV, lateral ventricle; MZ, molecular zone; Pb, Probst's bundle; SP, subplate; tca, thalamocortical axons; VZ, ventricular zone. A, C, E, and G; jsap1+/+. B, D, F, and H; jsap1-/-. Scale bars: A-F, 250 µm; G and H, 100 µm.
The second telencephalic commissure, AC, comprises of two major axon pathways; the anterior limb that connects the anterior olfactory nucleus and anterior piriform cortex, and the posterior limb that connects the piriform and temporal cortices (Abbie, 1940). The early extension of the AC in jsap1+/+ was clearly detected and appeared to cross the midline at E14.5. The axonal projection of the AC in jsap1-/- appeared at E14.5, but did not cross the midline (Figures 1I and 1J). The developing axons of the AC during E15.5-E17.5 appeared to be thinner than jsap1+/+. Moreover, developing axons of the AC were deviated from their normal path before approaching the midline and the AC axons in jsap1-/- headed to the thalamus (Figures 1K-1P). The average diameter of axonal tracts of the AC in jsap1-/- was relatively thinner than jsap1+/+ (Figures 1K-1P).
In contrast to the telencephalic commissures described above, the average diameter of axonal tracts (internal capsule) that pass through the striatum in jsap1-/- was thicker than that in jsap1+/+ at E16.5-E18.5 (Figures 2A-2L). In E16.5, the fiber tracts of the internal capsule were clearly present and their thickness was similar in both genotypes (Figures 2E and 2F). After E17.5, however, the thickness of the fiber tracts in jsap1-/- was larger than that of jsap1+/+ (Figures 2G-2L). It remained unknown whether these axonal tracts all reach to their target neurons in the telencephalic cortex properly.
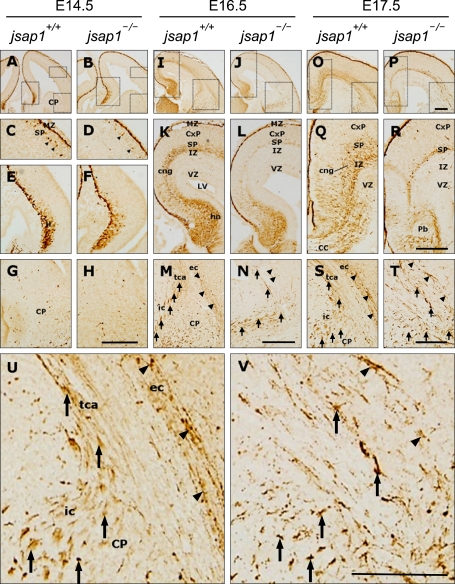
In a previous study, we demonstrated that calretinin (calcium-binding protein) labels a subset of neurons and axonal tracts in the brain of E18.5 embryo, permitting to detect distinct developmental deficits in jsap1-/- brain (Ha et al., 2005). In the present study, we continued to explore the distribution of calretinin-positive cells and axonal tracts in earlier embryonic brains. In the E14.5 brain, anti-calretinin labeled the marginal zone, subplate, hippocampus and caudoputamen, and their expressions were similar between the two genotypes (Figures 3A-3H). In E16.5-E17.5 jsap1+/+ brains, anti-calretinin labeled cell bodies and axonal processes of neurons in the marginal zone, cortical plate, subplate, intermediate zone, hippocampal neuroepithelium, and caudoputamen. While in jsap1-/-, anti-calretinin similarly labeled the cell bodies and axonal processes of neurons in these regions, but staining levels were lower than those of jsap1+/+ (Figures 3I-3T).
Figure 3.
Anti-calretinin staining showed altered expression levels and developmental defects in thalamocortical fibers in the brains of jsap1-/-. (A-V) Anti-calretinin stained coronal sections of the cortices of developing brains at E14.5 (A-H), E16.5 (I-N) and E17.5 (O-V). Anti-calretinin immunoreactivity in jsap1-/- brains at E16.5-17.5 was reduced in many brain regions compared to jsap1+/+ brains (I-V). Anti-calretinin labeled thalamocortical fibers (arrows) in jsap1-/- brains at E16.5-17.5 appeared to have a pathfinding defect (M, N, S-V). High magnification of the panels S and T (U, V). cng, cingulum bundle; CP, caudo-putamen; CxP, cortical plate; hn, hippocampal neuroepithelium; ic, internal capsule; IZ, intermediate zone; ec, external capsule; LV, lateral ventricle; MZ, molecular zone; SP, subplate; tca, thalamocortical axons; VZ, ventricular zone. Scale bars, 500 µm.
Developing thalamocortical axons (TCAs) were also stained by anti-calretinin. Anti-calretinin barely labeled TCAs at E14.5 (Figures 3G and 3H). In E16.5-E17.5 jsap1+/+ brains, anti-calretinin labeled TCAs running along the intermediate zone of the cortical cortex. Whereas in E16.5-E17.5 jsap1-/-, anti-calretinin staining showed the pathfinding defects of TCAs at the junction between the caudoputamen and the cortical wall, and their axon collaterals ran diffusely in the intermediate zone (Figures 3N and 3T). Stained axons at high magnification revealed that in jsap1+/+, calretinin was distributed in a patched manner along the thalamocortical and/or corticofugal projections, whereas in jsap1-/-, it was detected in the axonal tracts, but with an infrequent abnormal heavy accumulation in certain axons (Figures 3S and 3T).
Distribution of the phosphorylated forms of FAK and JNK in developing neurons in the early brains of jsap1-/-
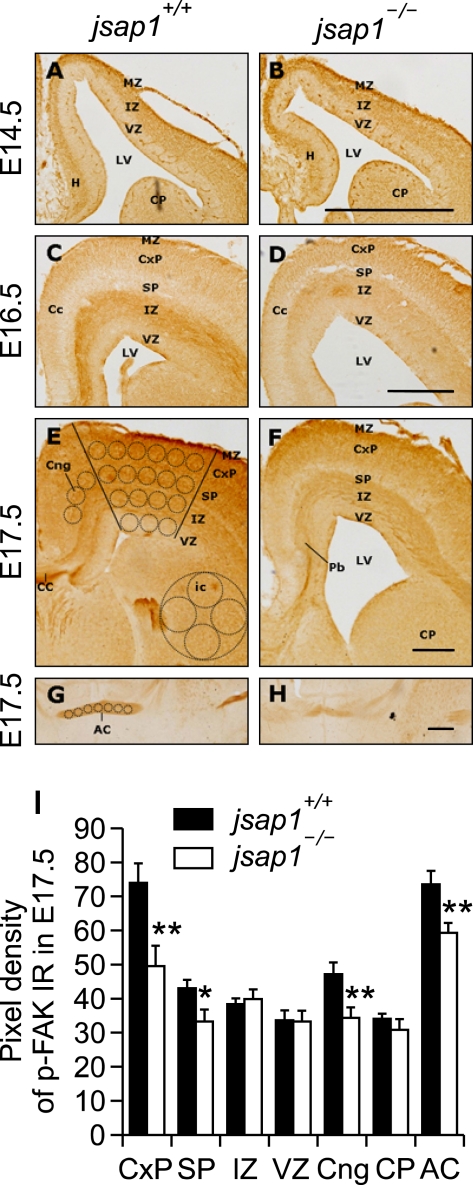
In E14.5 jsap1+/+ brain, phospho-FAK was distributed in broad brain areas with enhanced expression in the intermediate and molecular zones of the cortical plate. This distribution pattern did not obviously differ from that in the respective brain of jsap1-/- (Figures 4A and 4B). In E16.5 brain, anti-phospho-FAK clearly labeled U-shaped early callosal axons in both genotypes, though staining level in the axon tract in jsap1-/- was lower than that in jsap1+/+ (Figures 4C and 4D). In E17.5 jsap1+/+ brain, phospho-FAK distributed in most brain regions, including the corpus callosum, anterior commissure intermediate zone, and cortical plate, wherein phospho-FAK was distributed mainly in neuritic processes and soma of neurons, and also labeled radial glial fibers in the cortical plate (Figure 4E). While in the E17.5 brain of jsap1-/-, the immunoreactivity of p-FAK was decreased and particularly in the axon tract of the corpus callosum (Figures 4E, 4F and 4I) and the anterior commissure (Figures 4G-4I). In E14.5-E17.5 brains of both genotypes, FAK was expressed in most brain regions, and this expression pattern was similar to that of phospho- FAK (data not shown).
Figure 4.
Reduced expression of phospho-FAK levels in developing brains of jsap1-/-. (A-H) Anti-phospho-FAK stained coronal sections of cryo-cut brains at E14.5 (A, B), E16.5 (C, D), and E17.5 (E-H). Phospho-FAK was distributed in the soma and neuritic processes of neuronal cells in broad regions in the developing jsap1+/+ and jsap1-/- brains. The expression level of phospho-FAK in the U-shaped axon tract of the corpus collasum (C-F) and anterior commissure (G, H) in jsap1-/- brains was lower than that in jsap1+/+ brains. (I) Quantification of the expression levels of phospho-FAK in indicated brain regions in both genotypes. The pixel density of immunoreactivity was measured, as described in Materials and Methods. *, P < 0.05 and **, P < 0.01, differences between jsap1+/+ and jsap1-/- brains (Student's t-test). AC, anterior commissure; CC, corpus callosum; CxP, cortical plate; IZ, intermediate zone; LV, lateral ventricle; MZ, molecular zone; Pb, Probst's bundle; VZ, ventricular zone. A, C, and E; jsap1+/+. B, D, and F; jsap1-/-. Scale bars, 500 µm.
Similarly, anti-phospho-JNK also broadly labeled developing brains. In E14.5 brains, enhanced phospho-JNK immunoreactivities were detected in the marginal zone and intermediate zone of the cortical plate, caudoputamen, ventricular zone, and hippocampus in jsap1+/+. The anti-phospho-JNK levels in the corresponding regions of jsap1-/- were slightly, though not dramatically, lower than jsap1+/+ (Figures 5A and 5B). In E16.5-E17.5 brains, anti-phospho-JNK labeled developing neurons in the marginal zone, subplate, intermediate zone, subventricular zone, caudoputamen, and cingulum bundles in the cortical plate in both genotypes (Figures 5C-5J). Radial glial fibers, which function in neuronal migration and laminar patterning of developing cortex (Parnavelas, 2000; Ever and Gaiano, 2005), were also labeled by anti-phospho-JNK in similar levels in both genotypes (Figures 5E-5H). Whereas phospho-JNK levels in the subplate and the cingulum bundle in E16.5- E17.5 jsap1-/- brains were lower than those in jsap1+/+. Anti-phospho-JNK also labeled the callosal axons crossing the midline in the E16.5-E17.5 brains in both genotypes, though the staining levels in jsap1-/- were lower than those in jsap1+/+ (Figures 5C-5F and 5K). The expression level of anti-phospho-JNK in the anterior commissure was also decreased in E17.5 jsap1-/- (Figures 5I-5K). At E17.5, anti-phospho-JNK stained callosal axon tracts were disrupted and formed Probst's bundle-like structures bilaterally in the cingulate cortex (Figure 5F). Quantification of immunoreactive images revealed that the immunoreactivity of phospho-JNK in jsap1-/- brains was reduced in all brain regions examined compared with those in jsap1+/+ (Figure 5K). In E14.5-E17.5 brains of both genotypes, JNK1 was expressed in most brain regions, and the distribution of JNK1 was overlapped with that of phospho-JNK (data not shown).
Because JSAP1 knockout mice showed reduction in the cortical thickness of the brain (Ha et al., 2005) and overexpression of JSAP1 increased JNK activity and apoptosis following ischemiareperfusion (Xu et al., 2010), we investigated whether the JSAP1 deficiency affect cell death during development. TUNEL staining showed that TUNEL-positive cells in each brain section were distributed in many brain regions, including the frontal cortex, dorsal striatum, and the areas around the lateral ventricles (data not shown). TUNEL-positive cells distributed around the corpus callosum at the midline area of the brain of jsap1-/- were tended to be reduced in numbers compared to jsap1+/+ (Supplemental Data Figure S1), although this study did not examine the possibility that such reduction was due to the delayed progression of development in jsap1-/- compared to jsap1+/+.
Discussion
We recently reported that the JSAP1 deficient E18.5 brain exhibits various types of developmental defects in the brain, including impaired axon guidance of the CC and AC (Ha et al., 2005). The CC is the largest axon bundle that connects many areas between the two hemispheres, including the inferior frontal lobes, deeper limbic structures, supplementary motor cortices and cingulate gyri (Abbie, 1940). Early callosal axons originating from the cingulate cortex (cingulate cortex-originated axons) pioneer and cross the midline of the telencephalon during E15.5-E16.5, and late arriving collaterals of the CC that originate from other regions of the neocortex (neocortex-originated axons) follow the pre-existing axon tract, and join to form the typical U-shaped axonal tract (Koester and O'Leary, 1994; Ozaki and Wahlsten, 1998; Rash and Richards, 2001). As described, the early axonal extension of the CC in jsap1-/- was detected during E15.5-E16.5 (Figure 1). A careful examination of the callosal axons in the E16.5 brain revealed that some collaterals of these early axons lost U-shaped axonal integrity, though their tract trace was identified in all cases, and that the remaining axon tract, as examined at E16.5, was lightly stained by anti-phospho-JNK or anti-phospho-FAK (Figures 4 and 5). In contrast, the axon tract of the CC in E17.5 jsap1-/- brain was interrupted at the midline, and instead formed Probst's bundle-like structures in the cingulate cortex bilaterally (Figures 1G and 1H; Figures 5E and 5F). The disrupted tract formation of the CC is likely to be due to the degeneration of early developed axons, and the pathfinding defect of late developing axons. Thus our results suggest that the JSAP1-regulated signal pathway may play a role in the development of the telencephalic commissure, particularly for axon pathfinding and probably the maintenance of the early tract.
The finding that the callosal axons in jsap1+/+ were strongly labeled by antibodies for the active forms of FAK and JNK (that is, phospho-JNK and phospho-FAK, respectively) during E14.5-E17.5 (Figures 4 and 5). Moreover, the expression levels of phospho-JNK and phospho-FAK in developing callosal axons in jsap1-/- were lower than those in jsap1+/+, and this reduction was correlated with the disruption of axon tract formation. In consistent with these findings, previous studies have shown that FAK and JNK pathways are involved in axonal or dendritic development. For examples, targeted neuron-specific FAK deletion alters dendritic morphology in the developing brain (Beggs et al., 2003; Shi et al., 2009), and JNK deletion induces disrupted tract formation of the AC and the progressive loss of microtubules within axons and dendrites in neonate brains, although the CC was unaffected (Chang et al., 2003). Regarding the JSAP1-dependent mechanism of axon development, it is of interest that the JSAP1 deficiency resulted in reduced levels of phospho-FAK and phospho-JNK in developing callosal axons, but it did not completely abolish their expression. These results suggest that FAK and JNK signaling pathways have a role in the development and/or maintenance of this axon tract, though it remains to be determined whether the JSAP1-dependent down-regulation of the FAK or JNK pathways is the primary cause of defective telencephalic commissure development. We recently demonstrated that mouse embryonic fibroblasts (MEF cells) prepared from jsap1-/- embryos showed a whole sequence of cell spreading and cell adhesion on culture plates like jsap1+/+ MEF cells, but they look significantly longer to go through the sequence of cell spreading and adhesion-induced FAK activation was slightly reduced. These jsap1-/- MEFs showed the reduced cell spreading and cell adhesion properties in in vitro cell adhesion assay (Chae et al., 2006). Moreover, in a previous study, we reported that axon guidance defects of the CC and AC, and the reduced levels of phospho-FAK and phospho-JNK in the JSAP1-deficient brain were partially rescued by the transgenic expression of JIP1/SKIP (Ha et al., 2005), although JSAP1 and JIP1/SKIP are distinct proteins. Thus, it is likely that overlapping components of the JSAP1- and JIP1-dependent functional networks play a role in axon guidance, which suggest that JSAP1-like proteins play a compensatory role in unaffected neurons of jsap1-/-, and that they might participate to an extent even in affected neurons, and mitigate JSAP1-deficient phenotypes. The developmental phenotypes displayed by the jsap1-/- brains are highly complex, yet available information gathered from molecular biologic and knockout mouse studies is too limited to explain the observed phenotypes. Although JSAP1-dependent regulation of FAK and JNK occurs and their proper regulation might be important for the tract formation of the CC, further work is needed to reveal the mechanisms by which the FAK and JNK pathways regulate axon tract maintenance and axon pathfinding of the CC.
Because JSAP1 is known to function as a scaffold protein for FAK and JNK (Whitmarsh et al., 1998; Yasuda et al., 1999; Takino et al., 2002), we were interested in determining whether JSAP1 regulates the subcellular localization of FAK and JNK in neuronal cells and their subcellular distributions are correlated with developmental defects. We found no evidence that the JSAP1 null mutation restricts the distribution of FAK and JNK in axons. Consequently, though the jsap1-/- brain showed reduced phospho-JNK and phospho-FAK expression in the callosal axons, it is not yet known whether JSAP1 is required for the activation of FAK and JNK, or for the sustained maintenance of activated FAK or JNK.
The impaired axon guidance of the late arriving CC collaterals in the jsap1-/- brain is particularly interesting because these axons showed pathfinding defects. Previous studies have reported that mice lacking growth cone-related genes, such as GAP-43 and MAP1B, display impaired axon guidance of the CC and the AC (Richard, 2002). JSAP1 forms a complex with FAK (Takino et al., 2002), which is also a component in growth cones (Burgaya et al., 1995), and JSAP1 is strongly expressed in growth cones and affects neurite outgrowth (Xu et al., 2003). Therefore, it will be interesting to determine whether JSAP1 interacts any of these growth cone components or whether the intracellular signals essential for growth cone function are commonly regulated by JSAP1 and these growth cone components. Phospho-JNK positive TCAs running through the internal capsule also showed pathfinding defects, and projected with an aberrant lateroventral, rather than dorsolateral, trajectory (Figure 5F). Subplate neurons and their fibers are known to play a role in the guidance of cortical afferents, such as thalamocortical afferents (Zhou et al., 1999; Soria and Fairén, 2000; Deng and Elberger, 2001; Shinozaki et al., 2002). Though it is not known whether the pathfinding defects of callosal axons and TCAs are due to cell autonomous or not, organized regulation of cell adhesion-related factors by JSAP1 might play a role.
The present study and our previous study (Ha et al., 2005) together show that almost all neuroanatomical abnormalities, such as impaired axon guidance of the CC and AC, the pathfinding defects of thalamocortical tracts and migration defects of anti-calretinin positive cells in the developing cerebral cortex, with the exception of calretinin transport along axons (Ha et al., 2005), are primarily related to various forms of defective cell adhesion. Thus, our results strongly support the notion that JSAP1 regulates signal factors essential for cell adhesion in vivo. Given the known functions of JNK and FAK pathway in neurite outgrowth and cell migration, proper distribution and formation of functional modules of JNK and FAK pathway components might be essential to afford specificity and efficiency of these signaling pathways, and JSAP1 may participate in these processes.
Methods
JSAP1 deficient embryos
JSAP1 knockout mice were described in a previous study (Ha et al., 2005). JSAP1 deficient embryos were obtained by intercrossing JSAP1 knockout heterozygotes, and the genotypes of individual embryos were determined by PCR using tail tip DNA, as previously described (Ha et al., 2005).
Preparation of brain sections
Fetuses were removed from dams by caesarian section at embryonic ages E12.5 to E18.5. To prepare paraffin-embedded sections, brains were quickly removed, fixed in fresh 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4℃ overnight, dehydrated through a graded alcohol series, cleared with xylene, and embedded in Paraplast (Oxford, UK). Serial 4 µm coronal sections were then prepared, and subjected to cresyl violet staining to examine cellularity. For immunohistochemistry, brains were fixed in 4% paraformaldehyde at 4℃ overnight and equilibrated in 10%, 20%, and 30% sucrose solutions and then frozen in OCT compound (Tissue Tek, Elkhart). Brains were sectioned coronally into 20 µm sections using a cryostat (CM1850, Leica Ins., Germany). Sections were immunologically stained after mounting onto gelatin-coated subbed slides or following a floating method. The numbers of jsap1+/+ or jsap1-/- embryos examined in detail at each age were as follows: E14.5, n = 4 and 5; E15.5, n = 2 and 2; E16.5, n = 10 and 10; E17.5, n = 10 and 10; E18.5, n = 13 and 15.
Immunohistochemistry and quantification
For immunohistochemistry, sections were incubated for 30 min with 3% H2O2 in 0.1 M PBS (pH 7.4) to remove endogenous peroxidase activity, and washed in PBS. Sections were then blocked with a solution containing 5% normal goat/or horse serum, 2% BSA, 2% FBS and 0.1% triton X-100 for 2 h at room temperature (RT). Primary antibody in blocking buffer was then added to sections, and sections were incubated overnight at 4℃ and washed in PBS. They were then incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) at a dilution of 1:200 for 1 h at RT, avidin and biotinylated HRP complex (Vector Laboratories) at 1:200 for 1 h at RT, and visualized with 0.05% 3.3'-diaminobenzidine and 0.001% H2O2 in 0.1 M Tris, pH 7.4. After counterstaining, the brain sections were dehydrated and cover-slipped using a standard procedure.
Primary antibodies used were as follows: rabbit anti-phospho-JNK (Biosource, CA), rabbit anti-phospho-FAK (Y397) (Biosource, CA), mouse anti-FAK (Santa Cruz, CA), mouse anti-JNK1 (Pharmingen, San Diego, CA) and rabbit anti-calretinin (Chemicon International Inc. Temecula, CA), mouse anti-neuronal nuclei (NeuN) (Chemicon), and anti-MAP-2 (Upstate, Lake placid, NY).
For quantification, immunoreactive images were uploaded on the Adobe Photoshop software (Adobe Systems, San Jose, CA). The pixel density of immunoreactivity was measured using Image J software (http://rsbweb.nih.gov/ij/index.html) in a blinder manner, with a modification of the quantification method described previously (Kopniczky et al., 2005). Briefly, open circular cursors with a diameter of 50, 100, or 200 µm were placed on the corpus callosum (6 circles), cingulum bundle (3 circles), ventricular zone (5 circles), intermediate zone (5 circles), subplate (5 circles), cortical plate (5 circles), caudo-putamen (4 circles) and anterior commissure (5-10 circles), as illustrated in Figures 4 and 5. The average of 10 background determinations performed near the brain areas being counted was subtracted from the average pixel densities measured for the brain regions to study.
In situ detection of fragmented DNA (terminal deoxynucleotidyl transferase-mediated UTP nick end labelling, TUNEL)
The fragmentation of DNA was examined using an ApopTag® Peroxidase In situ Apoptosis Dection Kit (S7100) (Millipore, MA) according to the manufacturer's instructions. Briefly, after deparaffinization and hydration using xylene and graded alcohol, brain sections were placed to enzymatic digestion with 20 µg/ml of proteinase K for 5 min, treated with 5% hydrogen peroxide for 20 min to exhaust endogenous peroxidase activity, and washed with phosphate-buffered saline (PBS, 0.1 M, pH 7.4). They were then immersed in an ApopTag® Equilibration Buffer to label the 3'-OH ends of fragmented DNA for 10 min and incubated with terminal deoxynucleotidyl transferase enzyme at 37℃ for 1 h. After washing with PBS, sections were incubated with anti-digoxygenin conjugated peroxidase and the peroxidase substrate (diaminobenzidine) to detect signs of apoptotic cell death.
Statistical analysis
Differences between two groups were assessed using a Student's t-test, at a minimum confidence level of P < 0.05.
Supplemental data
Supplemental data include a figure and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-8-04.pdf.
Acknowledgements
This research was supported by a grant (2010K000814) from Brain Research Center, The 21st Century Frontier Research Program of the Ministry of Education, Science and Technology, Republic of Korea.
Abbreviations
- AC
anterior commissure
- CC
corpus callosum
- FAK
focal adhesion kinase
- JSAP1
JNK/stress-activated protein kinase-associated protein 1
- MEF
mouse embryonic fibroblasts
- TCAs
thalamocortical axons
Supplementary Material
Supplemental Data
References
- 1.Abbie AA. The origin of the corpus callosum and the fate of structures related to it. J Comp Neurol. 1940;70:9–44. [Google Scholar]
- 2.Abe N, Almenar-Queralt A, Lillo C, Shen Z, Lozach J, Briggs SP, Williams DS, Goldstein LS, Cavalli V. Sunday driver interacts with two distinct classes of axonal organelles. J Biol Chem. 2009;284:34628–34639. doi: 10.1074/jbc.M109.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida EA, Ilić D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH2-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgaya F, Menegon A, Menegoz M, Valtorta F, Girault JA. Focal adhesion kinase in rat central nervous system. Eur J Neurosci. 1995;7:1810–1821. doi: 10.1111/j.1460-9568.1995.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 6.Chae HJ, Ha HY, Im JY, Song JY, Park S, Han PL. JSAP1 is required for the cell adhesion and spreading of mouse embryonic fibroblasts. Biochem Biophys Res Commun. 2006;345:809–816. doi: 10.1016/j.bbrc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 8.Deng J, Elberger AJ. The role of pioneer neurons in the development of mouse visual cortex and corpus callosum. Anat Embryol (Berl) 2001;204:437–453. doi: 10.1007/s429-001-8001-3. [DOI] [PubMed] [Google Scholar]
- 9.das Neves L, Duchala CS, Tolentino-Silva F, Haxhiu MA, Colmenares C, Macklin WB, Campbell CE, Butz KG, Gronostajski RM. Disruption of the murine nuclear factor I-A gene (Nfia) results in perinatal lethality, hydrocephalus, and agensis of the corpus callosum. Proc Natl Acad Sci U S A. 1999;96:11946–11951. doi: 10.1073/pnas.96.21.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eto K, Kawauchi T, Osawa M, Tabata H, Nakajima K. Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth. Neurosci Res. 2010;66:37–45. doi: 10.1016/j.neures.2009.09.1708. [DOI] [PubMed] [Google Scholar]
- 11.Ever L, Gaiano N. Radial glial progenitors: neurogenesis and signaling. Curr Opin Neurobiol. 2005;15:29–33. doi: 10.1016/j.conb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Ha HY, Cho IH, Lee KW, Lee KW, Song JY, Kim KS, Yu YM, Lee JK, Song JS, Yang SD, Shin HS, Han PL. The axon guidance defect of the telencephalic commissures of the JSAP1-deficient brain was partially rescued by the transgenic expression of JIP1. Dev Biol. 2005;277:184–199. doi: 10.1016/j.ydbio.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Hirai S, Kawaguchi A, Hirasawa R, Baba M, Ohnishi T, Ohno S. MAPK-upstream protein kinase (MUK) regulates the radial migration of immature neurons in telencephalon of mouse embryo. Development. 2002;129:4483–4495. doi: 10.1242/dev.129.19.4483. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 15.Ilić D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 16.Jacamo R, Jiang X, Lunn JA, Rozengurt E. FAK phosphorylation at Ser-843 inhibits Tyr-397 phosphorylation, cell spreading and migration. J Cell Physiol. 2007;210:436–444. doi: 10.1002/jcp.20870. [DOI] [PubMed] [Google Scholar]
- 17.Jacobowitz DM, Abbott LC. Chemoarchitectonic Atlas of the Developing Mouse Brain. Boca Raton, FL, USA: CRC Press; 1997. [Google Scholar]
- 18.Kelkar N, Gupta S, Dickens M, Davis RJ. Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol Cell Biol. 2000;20:1030–1043. doi: 10.1128/mcb.20.3.1030-1043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelkar N, Delmotte MH, Weston CR, Barrett T, Sheppard BJ, Flavell RA, Davis RJ. Morphogenesis of the telencephalic commissure requires scaffold protein JNK-interacting protein 3 (JIP3) Proc Natl Acad Sci USA. 2003;100:9843–9848. doi: 10.1073/pnas.1733944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koester SE, O'Leary DD. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. J Neurosci. 1994;14:6608–6620. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopniczky Z, Dobó E, Borbély S, Világi I, Détári L, Krisztin-Péva B, Bagosi A, Molnár E, Mihály A. Lateral entorhinal cortex lesions rearrange afferents, glutamate receptors, increase seizure latency and suppress seizure-induced c-fos expression in the hipp ocampus of adult rat. J Neurochem. 2005;95:111–124. doi: 10.1111/j.1471-4159.2005.03347.x. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki HS, Wahlsten D. Timing and origin of the first cortical axons to project through the corpus callosum and the subsequent emergence of callosal projection cells in mouse. J Comp Neurol. 1998;400:197–206. doi: 10.1002/(sici)1096-9861(19981019)400:2<197::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Parnavelas JG. The origin and migration of cortical neurons: new vistas. Trends Neurosci. 2000;23:126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- 24.Rash BG, Richards LJ. A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol. 2001;434:147–157. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- 25.Reiske HR, Kao SC, Cary LA, Guan JL, Lai JF, Chen HC. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 26.Résibois A, Rogers JH. Calretinin in the rat brain: an immunohistochemical study. Neuroscience. 1992;46:101–134. doi: 10.1016/0306-4522(92)90012-q. [DOI] [PubMed] [Google Scholar]
- 27.Richards LJ. Axonal pathfinding mechanisms at the cortical midline and in the development of the corpus callosum. Braz J Med Biol Res. 2002;35:1431–1439. doi: 10.1590/s0100-879x2002001200004. [DOI] [PubMed] [Google Scholar]
- 28.Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Ito M, Ito T, Yoshioka K. Scaffold protein JSAP1 is transported to growth cones of neurites independent of JNK signaling pathways in PC12h cells. Gene. 2004;329:51–60. doi: 10.1016/j.gene.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Sato T, Torashima T, Sugihara K, Hirai H, Asano M, Yoshioka K. The scaffold protein JSAP1 regulates proliferation and differentiation of cerebellar granule cell precursors by modulating JNK signaling. Mol Cell Neurosci. 2008;39:569–578. doi: 10.1016/j.mcn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, Enkhbat A, Yoshioka K. Role of plasma membrane localization of the scaffold protein JSAP1 during differentiation of cerebellar granule cell precursors. Genes Cells. 2011;16:58–68. doi: 10.1111/j.1365-2443.2010.01465.x. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002;22:239–247. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci. 2009;29:8129–8142. doi: 10.1523/JNEUROSCI.4681-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinozaki K, Miyagi T, Yoshida M, Miyata T, Ogawa M, Aizawa S, Suda Y. Absence of Cajal-Retzius cells and subplate neurons associated with defects of tangential cell migration from ganglionic eminence in Emx1/2 double mutant cerebral cortex. Development. 2002;129:3479–3492. doi: 10.1242/dev.129.14.3479. [DOI] [PubMed] [Google Scholar]
- 36.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for intergrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 37.Soria JM, Fairén A. Cellular mosaics in the rat marginal zone define an early neocortical territorialization. Cereb Cortex. 2000;10:400–412. doi: 10.1093/cercor/10.4.400. [DOI] [PubMed] [Google Scholar]
- 38.Takino T, Yoshioka K, Miyamori H, Yamada KM, Sato H. A scaffold protein in the c-Jun N-terminal kinase signaling pathway is associated with focal adhesion kinase and tyrosine-phosphorylated. Oncogene. 2002;21:6488–6497. doi: 10.1038/sj.onc.1205840. [DOI] [PubMed] [Google Scholar]
- 39.Takino T, Nakada M, Miyamori H, Watanabe Y, Sato T, Gantulga D, Yoshioka K, Yamada KM, Sato H. JSAP1/JIP3 cooperates with focal adhesion kinase to regulate c-Jun N-terminal kinase and cell migration. J Biol Chem. 2005;280:37772–37781. doi: 10.1074/jbc.M505241200. [DOI] [PubMed] [Google Scholar]
- 40.Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis R. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 41.Xu B, Zhou Y, O K, Choy PC, Pierce GN, Siow YL. Regulation of stress-associated scaffold proteins JIP1 and JIP3 on the c-Jun NH2-terminal kinase in ischemia-reperfusion. Can J Physiol Pharmacol. 2010;88:1084–1092. doi: 10.1139/y10-088. [DOI] [PubMed] [Google Scholar]
- 42.Xu P, Yoshioka K, Yoshimura D, Tominaga Y, Nishioka T, Ito M, Nakabeppu Y. In vitro development of mouse embryonic stem cells lacking JSAP1 scaffold protein revealed its requirement during early embryonic neurogenesis. J Biol Chem. 2003;278:48422–48433. doi: 10.1074/jbc.M307888200. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis R. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, Iwao H. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- 45.Zhou C, Qiu Y, Pereira FA, Crair MC, Tsai SY, Tsai MJ. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24:847–859. doi: 10.1016/s0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data