Abstract
CD4+ T helper (Th)1 and Th17 cells both can cause autoimmune diseases either alone or collaboratively, if left unchecked. However, what determines the dominant Th effector phenotype in a specific autoimmune disease remains ill understood. Our present investigation shows that null mutation of immediate early response gene X-1 (IEX-1) promotes differentiation of Th17 cells, but compromising the survival of Th1 cells. The differential effect gave rise to a greater number of Th17 cells, a higher level of IL-17 production, and severer arthritis in IEX-1 knockout (KO) mice than in wild type (WT) mice after immunizations with collagen. IEX-1 deficiency-facilitated Th17 cell differentiation was mediated by increased formation of reactive oxygen species (ROS) at mitochondria following T cell activation, as suggested by marked inhibition of Th17 induction with ROS scavenger N-acetylcysteine (NAC) or mitoquinone (MitoQ), a specific inhibitor for mitochondrial ROS production. Mitochondrial ROS augmented the expression of B-cell activating transcription factor (Batf) that may contribute to increased IL-17 production in the absence of IEX-1 in light of its importance in IL-17 transcription. The results demonstrate that mitochondrial ROS contribute significantly to the dominant Th effector phenotype in autoimmunity in addition to the milieu of cytokines.
Keywords: Th1/17 cell balance, mitochondria, ROS, IEX-1, arthritis
Introduction
Our view of autoimmune diseases has changed dramatically in the past decade due to the discovery of an importance of Th17 cells (1). In the traditional model, we believe that Th1 cells are the primary Th effector in tissue-specific autoimmune diseases such as collagen-induced arthritis (CIA), experimental autoimmune encephalomyelitis (EAE), experimental autoimmune uveitis (EAU), and experimental autoimmune myocarditis (EAM) (2). However, mice genetically deficient in IL-17 are resistant to EAE and CIA (3), transfer of IL-17-producing T cells induces severe EAE (4), and treatment of mice with neutralizing antibodies against IL-17 reduces severity of CIA or EAE (4–6), but protects mice from EAU (7). In fact, Th17 and Th1 cells both can cause autoimmune diseases independently or collaboratively (8). These two types of Th cells can co-localize within the region of inflammation or coordinately enter the region in a specific order (9). Strikingly, while IFN-γ-deficient mice are defective in Th1 cell development, the mice develop an elevated Th17 response that exacerbates the disease (7). On the contrary, IL-17 deficiency results in increased differentiation of Th1 cells that cause the pathologic lesion in EAE and EAU (7). That lack of one subset may promote a response driven by the other makes the treatment of these disorders extremely difficult as targeting one may exaggerate the other, leaving disease progression unchecked or even worsened. Thus, a thorough understanding of what determines the dominant Th effector in a given autoimmune disease may help to develop specific strategies for treatment of the disorder.
IEX-1 (Immediate early response gene X-1) can be rapidly induced by various physiological and environmental factors such as irradiation, viral infection, inflammatory cytokines, chemical carcinogens, growth factors, and hormones (10). One of the primary functions of IEX-1 is to target mitochondrial FoF1-ATPase Inhibitor (IF1) for degradation, leading to acceleration of ATP hydrolysis in a cell under stress (11). ATP hydrolysis would stimulate ATP synthesis and continuous proton flux through the electron transport chain, thus preventing accumulation of electrons and ROS production at mitochondria. Transgenic mice that targeted expression of IEX-1 to lymphocytes developed a lupus-like autoimmune disease and T cell lymphoma due to insufficient apoptosis of Th1 cells (12,13). Paradoxically, lack of IEX-1 also exaggerated, rather than suppressed, inflammation following Leishmanial infection in part due to an elevated level of IL-17 production (14). The reciprocal effects of IEX-1 on Th17 and Th1 differentiation and survival were also observed in dextran sodium sulfate (DSS)-induced colitis where IEX-1 deficiency protected mice from DSS-induced colitis by promoting the survival and differentiation of IL-17-producing T cells while enhancing apoptosis in Th1 cells (15).
Overexpression of IEX-1 has been shown to suppress mitochondrial ROS (mtROS) production and protect cells from apoptosis, whereas null mutation of IEX-1 increases ROS production at mitochondria, rendering cells susceptible to apoptosis (11). While the mechanism underlying IEX-1-mediated protection against apoptosis is well studied, how IEX-1 deficiency promotes Th17 differentiation is not known. The present study shows that lack of IEX-1 augments the expression of B-cell activating transcription factor (Batf) by increasing mtROS formation. Increased Batf expression may be one of the mechanisms by which IL-17 expression is augmented in the absence of IEX-1 in light of its importance in regulation of IL-17 transcription previously demonstrated in Batf-deficient T cells (16). The observation, in line with our previous investigations, argues strongly for a crucial role of IEX-1 in the regulation of a balance between Th1 and Th17-driven autoimmune responses by the control of mtROS generation (14,15).
Materials and Methods
Animals
IEX-1 knockout (KO) and wild-type (WT) control mice on a mixed 129Sv/C57BL/6 background (F1) were generated by gene-targeted deletion as described (17). The animals were housed in conventional cages in the animal facilities of Massachusetts General Hospital in compliance with institutional guidelines, and both female and male mice were used. All of the studies were reviewed and approved by the Massachusetts General Hospital Subcommittee of Research Animal Studies.
Induction and assessment of CIA
Chicken type II collagen (CII) (Sigma) was dissolved in 10 mM acetic acid at a concentration of 2 mg/ml and emulsified in an equal volume of complete Freund’s adjuvant (CFA) containing 10mg/ml heat-killed Mycobacterium (M.) tuberculosis (H37Ra; Difco Laboratories, Detroit, MI, USA). To induce CIA, IEX-1 KO and WT control mice at 5~6 weeks of age with equal numbers of females and males in each group were intradermally administered at the tail base with 100 µl freshly prepared emulsion. The injection was repeated 21 days later with the amount of M. tuberculosis reducing from 500 µg to 50 µg per mouse so as to avoid too severe ulceration at the site of immunization in IEX-1 KO mice. The control mice received CFA alone and did not developed arthritis. To determine a role for IL-17 in CIA development in the absence of IEX-1, separate groups of IEX-1 KO mice were immunized similarly as above, along with intraperitoneal (i.p.) injection of anti-IL-17 antibody (Ab) or control Ab at a dose of 100 µg/mouse once a week starting from day 0 for 4 consecutive weeks. The neutralizing anti-mouse IL-17A Ab (TC11-18H10.1) and control Ab were obtained from BioLegend, San Diego, CA. The animals were clinically assessed for arthritis at indicated periods of time by scoring normal 0; slight swelling 1; extensive swelling 2; and joint destruction and/or rigidity, 3. Each paw was graded, giving a maximal score of 12 per mouse as described (18).
Measurement of IL-17 production in the inflamed paw
On week after the booster immunization, mice were killed and the hind paws were dissected, snap-frozen in liquid nitrogen, and stored at −80°C until use. The frozen paws were broken into little pieces on dry ice, and then homogenized on ice in PBS containing 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Amersham, Piscataway, NJ) using a polytron homogenizer (Kinematica AG, Littau/Lucerne, Switzerland). The resultant tissue homogenates were centrifuged at 15,000 g for 15 min at 4°C. The supernatants were collected as paw homogenates, and protein and IL-17A levels were measured by a protein assay kit (Bio-Rad) or an enzyme-linked immunosorbent assay (ELISA) Ready-Set-Go! Kit per the manufacturer’s protocol (eBioscience, San Diego, CA). The amount of IL-17A was normalized to the protein level for comparison.
Th 17 polarization and detection
Single-cell suspensions were prepared from spleens of indicated mice and treated with a mixture of rat anti-mouse Abs against CD19, CD32 and CD8 followed by depletion of Ab-bound cells with BioMag goat anti-rat IgG (Polyscience) as per manufacturer’s instructions. The freshly isolated CD4+ T cells were plated in 6-well plate at 0.6 X 106/well in 2 ml RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% FBS, 2 mM L-glutamine, 50 µM β-mercaptoethanol (β-ME), 1X nonessential amino acids, 1 µM sodium pyruvate, and 1% penicillin-streptomycin and stimulated with 1 µg/ml anti-CD3, 2 µg/ml anti-CD28 (BioLegend), 1 ng/ml TGF-β, and 100 ng/ml IL-6 (PeproTech) for Th17 polarization unless otherwise indicated. After 4 days of incubation, the cells were restimulated with 50 ng/ml PMA (Sigma) and 750 ng/ml ionomycin (Sigma) for 5 h in the presence of 1 µl/ml Golgi-Plug (BD Biosciences) followed by intracellular staining and FACS analysis as described (15).
Intracellular staining of T cells
For intracellular staining of IL-17 and IFN-γ, the cells were fixed with 2% formaldehyde, washed with a permeabilization/blocking buffer (BioLegend), and incubated with Alexa Fluor® 700-conjugated anti-IL-17 and FITC-conjugated anti-IFN-γ Abs (BioLegend). Similarly, intracellular staining of Foxp3 and p-STAT3 were performed by permeabilization of the cells with a Foxp3 Fix/Perm Buffer (BioLegend) and staining with Alexa Fluor® 488-conjugated anti-Foxp3 (BioLegend) or Alexa Fluor® 488-conjugated anti-phospho-STAT3 (Tyr705) (Cell Signaling). The stained cells were evaluated on a FACSAria (BD) and the data were analyzed in flowJo software (Tree Star Inc, Ashland, OR).
Histology analysis
Mice were sacrificed by cervical dislocation 30 days after the first immunization and the paws were removed and fixed in 10% formalin for 4 days. After decalcification in 5% formic acid, the specimens were processed for paraffin embedding, sections and stained with hematoxylin and eosin (H&E).
Quantitative real-time RT-PCR
To analyze retinoic acid receptor-related orphan nuclear receptor (ROR)γt, RORα, and IL-17 gene expression, WT and IEX-1 KO CD4+ T cells were polarized under Th17-polarizing conditions for 24 or 48 hrs. Total RNA was extracted, reverse-transcribed, and amplified by real-time RT-PCR using a SYBR Green PCR kit (Applied Biosystems, Foster City, CA) on an Mx4000TM Multiplex Quantitative PCR System (Stratagene). Threshold cycle (Ct) was used to calculate the relative template quantity as the manufacturer’s recommendation using β-actin as an internal control. The primers used were: forward, CCGCTGAGAGGGCTTCAC and reverse, TGCAGGAGTAGGCCACATTACA for RORγt; forward, TCTCCCTGCGCTCTCCGCAC and reverse, TCCACAGATCTTGCATGGA for RORα; forward CTGGAGGATAACACTGTGAGAGT, and reverse, TGCTGAATGGCGACGGAGTTC for IL-17; forward, CCAGAAGAGCCGACAGAGAC and reverse, GAGCTGCGTTCTGTTTCTCC for Batf; forward, CGCTCAACCTGGCTTACTTC and reverse, GGGCTCAACTTGAGGGCG for IκBζ; and forward, GGCTGTATTCCCCTCCATCG and reverse, CCAGTTGGTAACAATGCCATGT for β-actin.
Transfection and luciferase activity measurement
Expression vectors encoding RORγt were co-transfected into HEK293 cells along with a luciferase reporter vector containing Il17 minimal promoter (19) (all the vectors were kindly provided by Dr. Chen Dong from Department of Immunology, M.D. Anderson Cancer Center). The minimal promoter consisted of DNA sequence −1131 to +1 relative to translation start site in which the conserved noncoding sequence 2 (CNS2) site contained two ROREs (Retinoic acid receptor-related Orphan Receptor binding Element) that could be bound by RORγt or RORα (19). The firefly luciferase activities were assayed and normalized relative to the renilla luciferase activity in each sample per the manufacturer’s instruction (Promega).
Statistical analysis
The statistical analysis was based on the calculation of arithmetic mean and standard deviation (SD). The difference between two means was compared by two-tailed unpaired Student’s t test assuming equal variances. One-way ANOVA was used for multiple group comparisons. A p-value of less than 0.05 was considered statistically significant.
Results
Enhanced Th17 differentiation in IEX-1 KO CD4+ T cells
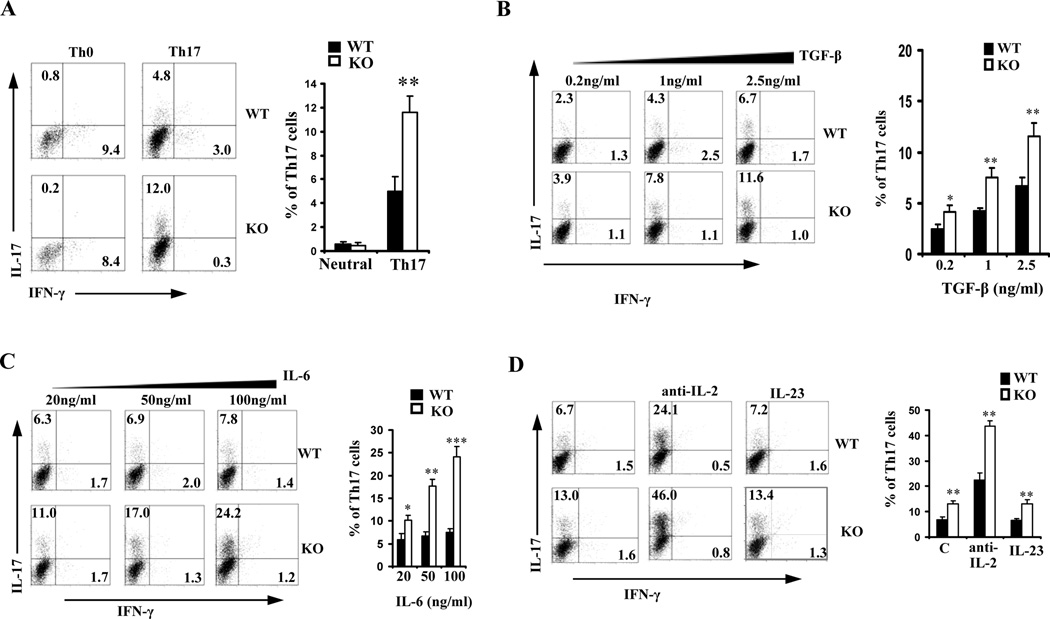
We showed that lack of IEX-1 resulted in expansion of Th17 population and a high level of IL-17 production in DSS-induced colitis and Leishmanial infection (14,15). To address which signaling was affected by IEX-1 null mutation leading to a Th17-biased immune response in the mice, CD4+ T cells from WT and IEX-1 KO mice were stimulated under Th17-polarization conditions with varying concentrations of either TGF-β or IL-6. As can be seen from figure 1A, percentages of Th17 cells were increased from about 5% in the presence of IEX-1 to 12% in the absence of IEX-1 at 1ng/ml TGF-β and 20 ng/ml IL-6 (p< 0.01), concomitant with an inverse reduction in the proportion of Th1 cells from 3% to 0.3%. There was no significant difference in Th17 and Th1 differentiation when the cells were cultured under non-polarization conditions (Th0). The data confirm that enhanced Th17 cell differentiation is an intrinsic characteristic of IEX-1 deficient T cells. Increasing concentrations of TGF-β from 0.2ng/ml to 2.5 ng/ml promoted expansion of Th17 cells similarly in the presence vs. absence of IEX-1 (Figure 1B). Under similar conditions, however, increasing amounts of IL-6 widened the difference in Th17 cell differentiation in the presence compared to the absence of IEX-1 in a dose-dependent fashion (Figure 1C). The maximum effect was observed at a concentration of 100 ng/ml of IL-6, which brought about a 3-fold increase in the percentage of Th17 cells in IEX-1 KO T cells when compared to WT counterparts (8% in WT vs. 24% in KO, Figure 1C). IEX-1 deficiency appeared to affect IL-6-mediated signaling more than TGF-β signaling.
Figure 1.
Null mutation of IEX-1 enhances Th17 differentiation. WT and IEX-1-knockout (KO) CD4+ T cells were incubated with 1 µg/ml anti-CD3 and 2 µg/ml anti-CD28 for 4 days (Th0) or along with 1 ng/ml TGF-β and 20 ng/ml IL-6 (Th17) followed by intracellular staining and FACS analysis for IFN-γ versus IL-17 expression (A). The CD4+ T cells were also differentiated under Th17-polarizing conditions as A except that an increasing concentration of TGF-β (B) or IL-6 (C) was included in the culture. D. The CD4+ T cells were differentiated under Th17 conditions in the presence or absence of 50 ng/ml IL-23 or 10 µg/ml anti-IL-2 antibody as A. Representative flow cytometric profiles are shown on the left and mean percentages ± SD of Th17 cells are summarized on the right in each panel. *, ** and ***, p<0.05, 0.01 or 0.001, respectively, in presence or absence of IEX-1 (n = 6 for all).
It has been shown that IL-2 acts as a negative regulator for Th17 differentiation whereas IL-23 has a supporting effect (4,20). The concentrations of these two cytokines were thus measured in the media collected from Th17 cell culture on day 1 or 2 to corroborate whether lack of IEX-1 affected Th17 cell differentiation via these two cytokines. We found no significant difference in the production of these two cytokines in the presence or absence of IEX-1 (data not shown). In accordance to this, when anti-IL-2 Ab or IL-23 was added to the culture along with 100ng/ml IL-6 and 1 ng/ml TGF-β, IL-23 did not display any impact on Th17 cell differentiation irrespective of IEX-1 expression (Figure 1D), supporting a dispensable role of IL-23 in the initial induction of Th17 cells (21,22). Addition of IL-2 neutralizing Ab to the culture robustly but similarly stimulated Th17 polarization in WT and IEX-1 KO cells (Figure 1D). We also tested whether IFNγ and IL-4 had any impact on the enhanced Th17 cell differentiation lacking IEX-1 and failed to detect any significance in the presence or absence of anti-IFNγ and/or anti-IL4 Ab in this Th17-polarizing condition (data not shown). The observation is similar to previous finding showing no effects of anti-IFNγ and/or anti-IL-4 Ab on Th17 differentiation when purified CD4+ T cells were polarized in the presence of TGF-β and IL-6 (23).
IEX-1 Deficiency-mediated Th17 differentiation depends on ROS
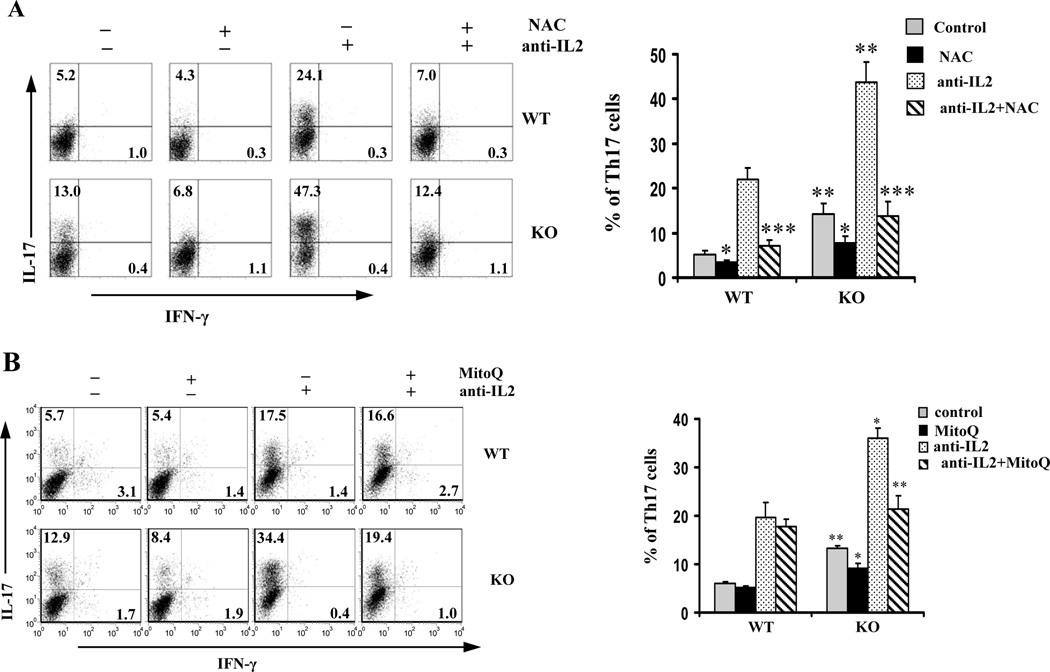
Our previous investigation showed that lack of IEX-1 significantly increased ROS formation in T cells after stimulation with anti-CD3/CD28 (14). We thus sought to test whether Th17 cell differentiation was sensitive to ROS by addition of a cell permeable ROS-scavenger N-acetylcysteine (NAC) to Th17 cell differentiation cultures. As shown in Figure 2A, NAC suppressed Th17 cell development in both WT and IEX-1 KO Th cells and with or without anti-IL2 Ab. Unlike NAC, another ROS scavenger, named mitoquinone (MitoQ), abrogated Th17 cell differentiation in IEX-1 deficient T cells only, with little inhibitory effect on WT Th17 cell differentiation, regardless of whether or not anti-IL2 Ab was added to the culture (Figure 2B).
Figure 2.
Effects of anti-oxidants on Th17 differentiation. WT and IEX-1 KO CD4+ T cells were stimulated with 1 µg/ml anti-CD3, 2 µg/ml anti-CD28, 1 ng/ml TGF-β and 100 ng/ml IL-6 for 4 days with or without anti-IL-2 antibody in the presence or absence of 10 mM of NAC (A) or 200 nM MitoQ (B) followed by intracellular staining and FACS analysis for IFN-γ versus IL-17 expression as Figure 1. Representative flow cytometric profiles are shown on the left and mean percentages ± SD of Th17 cells are summarized on the right in each panel. *, ** and ***, p<0.05, 0.01 or 0.001, respectively, compared to WT cells or untreated IEX-1 KO cells (n = 6).
MitoQ, a coenzyme Q10 analogue, is a specific inhibitor for mtROS formation as it contains a lipophilic triphenylphosphonium cation that causes the antioxidant to accumulate several hundredfold higher within mitochondria than outside the mitochondria because of the high mitochondrial membrane potential (24,25). MitoQ has shown to diminish ROS formation at mitochondria in IEX-1 deficient aortic rings to a WT level (17). We also tested apocynin for its effect on Th17 differentiation in the absence of IEX-1. Apocynin blocks an association of p47phox and p67phox with the gp91phox subunit within the membrane NAD(P)H oxidase complex and acts as a specific inhibitor for ROS formation by the membrane NAD(P)H oxidase. Inclusion of the compound in the culture did not alter Th17 polarization in either WT or IEX-1 KO T cells at the concentration range of 10~250 µM (data not shown). The data is in agreement with our previous investigation suggesting that mitochondria, not NADPH oxidases, are the source of ROS formation in the absence of IEX-1 (17).
ROS increase Il17 transcription in the presence of RORγt
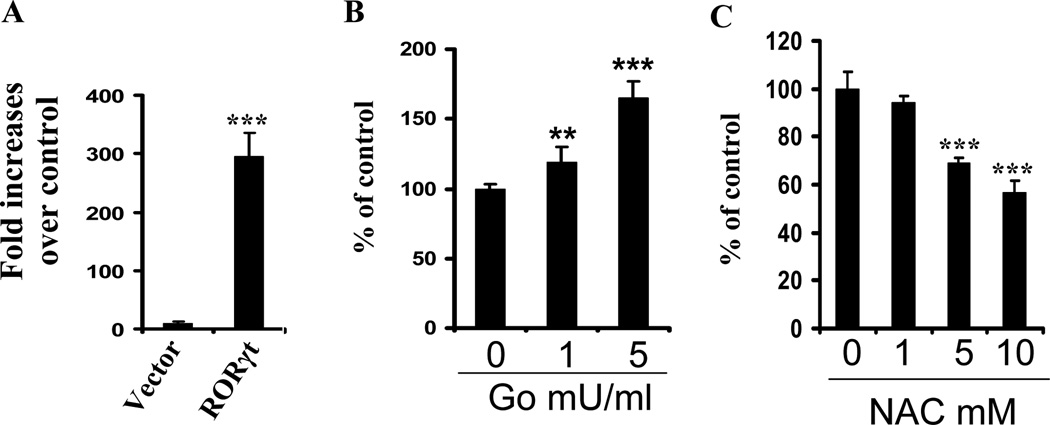
In Il17 gene promoter, the CNS2 site contains two ROREs that can be recognized and bound by RORγt, the Th17-lineage-specific transcription factor and is indispensable for Il17 transcription (19,26). To address whether ROS had any effects on Il17 transcription, a luciferase reporter directed under the Il17- CNS2 minimal promoter was transfected into HEK293 cells. The Il17 minimal promoter alone displayed little luciferase activity (figure 3A), which was not affected by inclusion of 1 or 5 mU/ml of glucose oxidase (GO) in the cell culture (data not shown). Glucose oxidase is an enzyme that is used as a continuous generator of H2O2 and thus a suitable model for chronic exposure of cells to low levels of H2O2 (27,28). However, when an expression vector encoding RORγt was cotransfected with the Il17 minimal promoter-luciferase reporter, the Il17 minimal promoter was drastically activated over a control (Figure 3A). Importantly, Il17 transcription was further elevated by glucose oxidase in a dose-dependent manner (Figure 3B ). In contrast, the presence of antioxidant NAC in the culture attenuated the luciferase activity, also in a dose-dependent manner (Figure 3C). The data suggest that ROS facilitate Th17 differentiation at least in part through enhancing RORγt transcriptional activity in the Il17 promoter.
Figure 3.
ROS increase IL17 transcription in the presence of RORγt. HEK293 cells were transfected with a RORγt expression vector (RORγt) or empty vector (Vector) along with a minimal CNS2-Il17 promoter-luciferase reporter (A). The cells transfected with the RORγt plasmid and Il17 promoter-CNS2 luciferase reporter were treated with 0, 1 or 5 mU/ml of glucose oxidase (GO) (B) or 0, 1, 5, or 10 mM of NAC (C). The resultant cells were lysed in 24 hr and luciferase activities were assayed in which renilla luciferase activity was used for the normalization of transfection efficiency and background luciferase activities in control cells transfected with empty vector alone were set to 1. The data are expressed as mean fold increases ± SD (A) or mean percentages ± SD (B and C) of luciferase activity values relative to control cells from three separated experiments each performed in duplicate. * and ***, p<0.05 or 0.001, respectively, compared to the values in control cells.
The enhancement of Th17 differentiation is not ascribed to STAT3 activation or RORγt/RORα expression
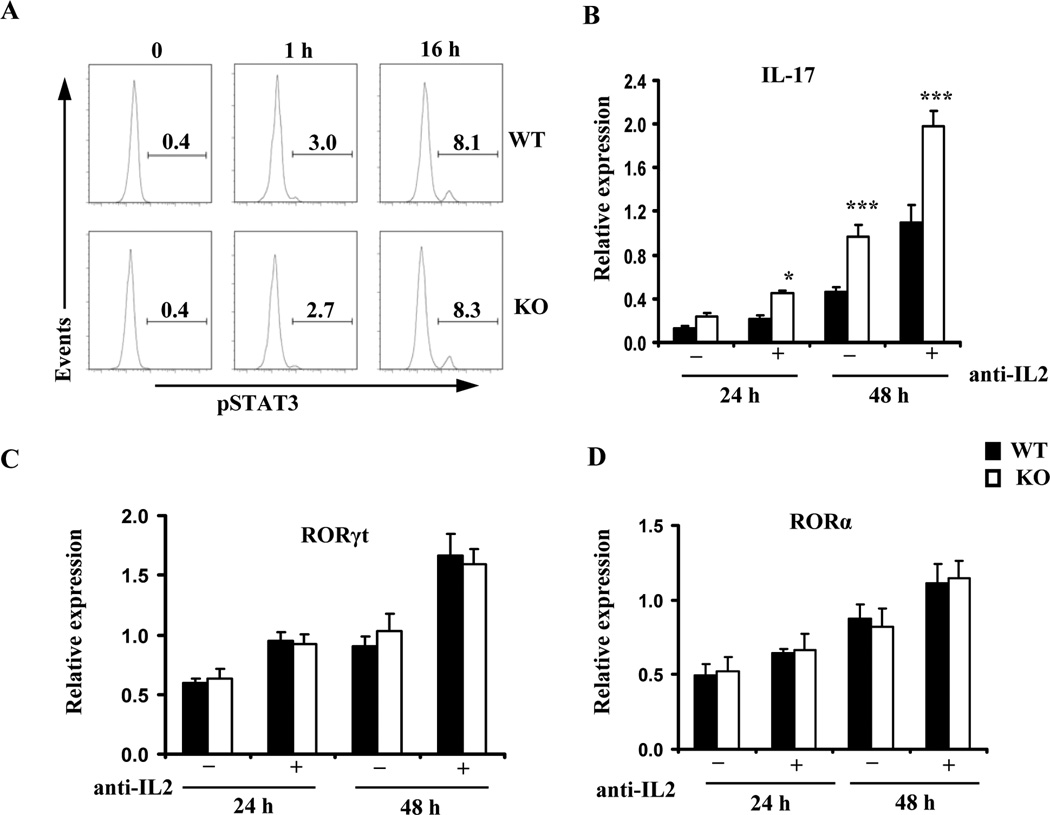
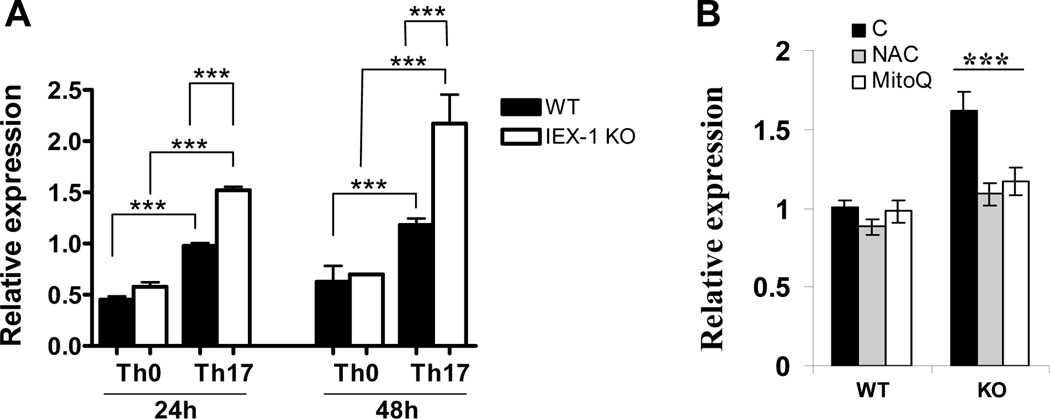
We went on to determine the signaling pathways augmenting Il17 transcription in the absence of IEX-1. STAT3 is a redox-sensitive transcription factor and activated in response to IL-6, and it can directly enhance Il17 transcription through binding to the promoter (29). Moreover, ROS have been shown to trigger STAT3 tyrosine phosphorylation and nuclear translocation in lymphocytes (30,31). This, in line with increased sensitivity of IEX-1 deficient T cells to IL-6 (Figure 1C), promoted us to evaluate STAT3 phosphorylation at varying times after polarization of the CD4+ T cells in Th17-conditioins. Intracellular staining for phosphorylated STAT3 (pSTAT3) revealed an increase in the percentages of cells containing pSTAT3 after activation of Th cells (Figure 4A). However, the levels and kinetics of STAT3 phosphorylation were comparable between WT and IEX-1 KO Th cells and with or without anti-IL2 Ab (Figure 4A, data not shown). Consistent with an unaltered level of STAT3 expression and phosphorylation in the presence or absence of IEX-1, the mRNA levels of RORγt and RORα, two STAT3-regulated transcription factors that are crucial in directing Th17 differentiation, did not differ significantly in WT and IEX-1 KO CD4+ T cells in 24 or 48 hr culture under Th17-polarizing conditions (Figure 4C and 4D). Notably, at these two time points, a higher level of IL17 expression was already detected in IEX-1 KO Th cells compared with their WT counterparts (Figure 4B), ruling out that the enhanced Th17 differentiation in IEX-1 KO CD4+ T cells is associated with increased expressions of RORγt or RORα.
Figure 4.
Enhanced Th17 differentiation in IEX-1 KO T cells is independent on STAT3 phosphorylation or RORγt/RORα expression. A. Unaltered STAT3 phosphorylation in the presence or absence of IEX-1. WT or IEX-1 KO CD4+ T cells were treated with Th17 inducers for 0, 1, and 16 hr, respectively and subjected to intracellular staining for phosphorylated STAT3 (pSTAT3) followed by flow cytometric analysis. The numbers indicate the percentages of pSTAT3+CD4+ T cells relative to total CD4+ T cells. Similar results were obtained from three independent experiments. B, C, and D. RORγt or RORα expression is not elevated by null mutation of IEX-1 in spite of increasing IL-17 production in the cells. WT and IEX-1 KO CD4+ T cells were polarized under Th17 conditions with or without anti-IL-2 Ab for 24 or 48 hr. Total RNA was extracted from the resultant cells. The expression level of IL-17 (B), RORγt (C), and RORα (D) was measured by quantitative RT-PCR and normalized to the expression level of β-actin. The data are the mean values ± SD of three independent experiments each performed in duplicate. * and ***, p<0.05 or 0.001, respectively, in presence or absence of IEX-1.
Increased expression of Batf in IEX-1-deficient cells
The observation of similar levels of RORγt and RORα expression in the presence or absence of IEX-1, in spite of a significantly higher level of IL-17 production in IEX-1 deficient Th17 cells, suggested that other transcription factor(s) were involved in the increased transcription activity of Il17 promoter. Transcription factors such as Batf, Runx1, Irf4, IκBζ can directly enhance the transcription activity of IL-17 in the presence of RORγt (32). Among them, Batf and IκBζ are the member of the AP-1 or NF-κB family, respectively, both sensitive to redox-mediated regulation (33). We found that Batf was up regulated significantly in Th17 cells as compared to Th0 cells (figure 5A), confirming previous investigation (16). The expression was correlated with IL-17 production in the cells with a significantly higher level in IEX-1 KO Th17 cells than WT Th17 cells at each time point tested (figure 5A), in a good agreement with synergistic effects of Batf with RORγt on induction of IL-17 production previously demonstrated (16,34). There was no significant difference in Batf expression in the presence or absence of IEX-1 when the cells were differentiated under a Th0-polarizing condition. Batf expression in IEX-1 deficient Th17 cells was brought down to a WT level in the presence of anti-oxidant NAC or MitoQ in the culture (Figure 5B), corroborating regulation of Batf expression by redox-sensitive signaling in the absence of IEX-1. Unlike Batf, IκBζ was increased in Th0 cells in the absence compared to the presence of IEX-1 and no significant difference was found in Th17 cells regardless of IEX-1 expression (data not shown).
Figure 5.
Increased expression of Batf in the absence of IEX-1 in a ROS-dependent fashion. WT and IEX-1 KO CD4+ T cells were polarized for 24 or 48 hr in Th0 as figure 1 or Th17 conditions as Figure 2 (A). The cells were also differentiated under a Th17-polarizing condition in the presence or absence (B) of either 5 mM NAC or 200 nM MitoQ for 24 hr. Total RNA was extracted from the differentiated cells and Batf expression was measured by quantitative RT-PCR and normalized to the expression level of β-actin. The data are the mean values ± SD of three independent experiments each performed in duplicate. ** and ***, p<0.01 or 0.001, respectively, in presence or absence of IEX-1 or between Th0 and Th17 cells for A or in the presence or absence of indicated oxidants (B).
IEX-1 deficiency increases incidence and severity of collagen-induced arthritis
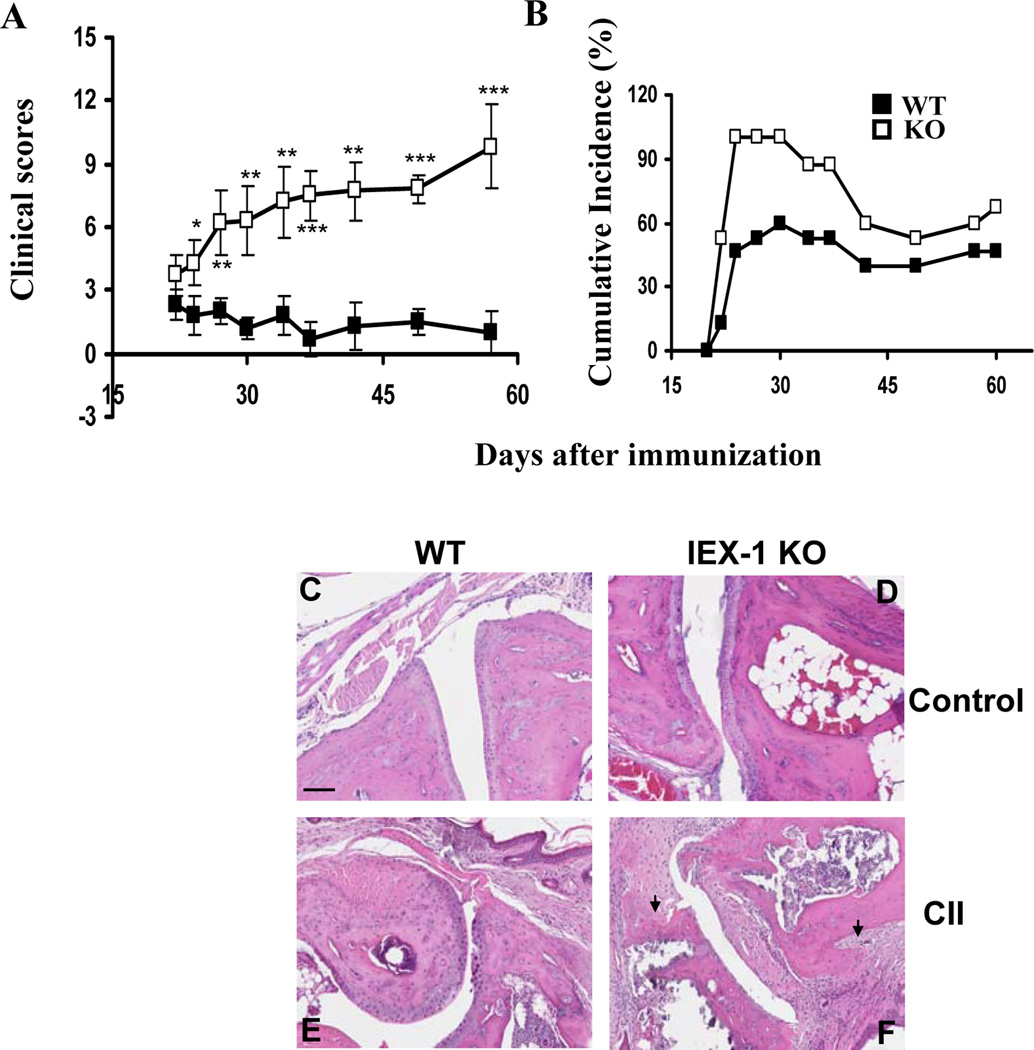
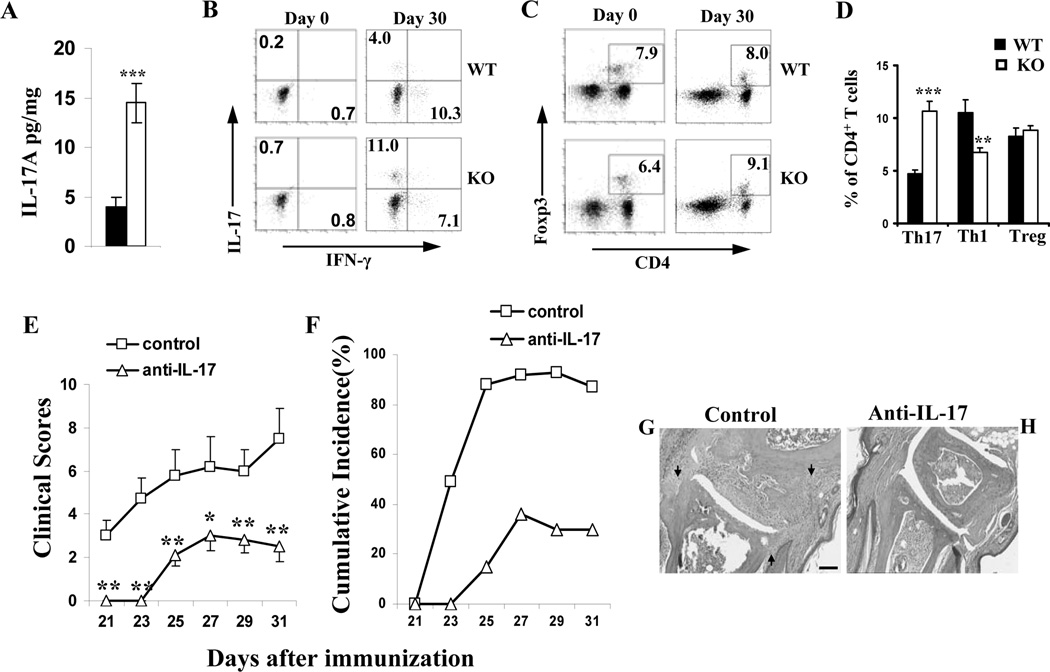
Our previous study showed an increased number of Th17 cells in Leishmanial infection and in DSS-induced colitis (14,15). However these animal models are not typical models for validating a role for Th17 cells in autoimmune diseases. In contrast, collagen-induced arthritis (CIA) is one of the several organ-specific autoimmune disease models initially used to demonstrate the importance of Th17 cells in autoimmunity (35). CIA susceptibility was thus assessed in IEX-1 KO mice per a published protocol (18). IEX-1 null mutation exaggerated collagen-induced autoimmunity in the paw (Figure 6A–B), manifested by significantly greater levels of both the clinical scores and cumulative incidence of arthritis in the absence than in the presence of IEX-1 (p<0.01). No arthritis was found in control mice receiving CFA alone as previously described (data not shown) (18). The clinic scores were correlated with histological analysis in which lack of IEX-1 caused widespread infiltration of inflammatory cells throughout the joint tissue, synovial hyperplasia, and cartilage and bone erosion (figure 6F). Pannus (arrow) was frequently seen invading from the marginal zone into the cartilage or the medulla of the subcondral bone (Figure 6F). In contrast, WT mice displayed a low level of inflammation mainly in the bone marrow and cartilage (figure 6E) as compared to non-arthritic control mice (Figure 6C and 6D). Despite increased severity of arthritis in the absence of IEX-1, total anti-CII IgG or anti-CII IgG isotypes were not significantly different in the presence or absence of IEX-1 (data not shown), which was not unexpected because similar levels of anti-CII Ab production were previously described in CII-immunized mice regardless of arthritis development (18) or IL-17 levels (36). The result stresses that lack of IEX-1 may preferentially affect cellular or innate immune responses over humoral immune responses. In accordance with a role for IL-17 in aggravation of CIA in IEX-1 KO mice, the proportions of Th17 cells in the draining lymph nodes were significantly elevated in IEX-1 KO mice, concurrent with a diminished level of Th1 cells when compared to WT mice (Figure 7B and 7D). There was no difference in the level of T regulatory (Treg) populations in the draining lymph nodes between WT and IEX-1 KO mice (Figure 7C and 7D). IL-17 production was also significantly higher in inflamed paws in the absence relative to the presence of IEX-1 (Figure 7A). Furthermore, treatment of IEX-1 KO mice with anti-IL-17 Ab for 4 consecutive weeks during CIA development significantly suppressed clinic score (Figure 7E) and cumulative incidence of CIA (Figure 7F), and histopathology (figure 7G) in IEX-1 KO mice as compared to the mice treated with a control Ab (Figure 7E, 7F and 7H). These results argue strongly for an importance of IL-17 in the pathogenesis of CIA in the absence of IEX-1, but other cell types or factors may be also involved because anti-IL-17 Ab did not completely abolish the disease in the mice.
Figure 6.
Lack of IEX-1 aggravates collagen-induced arthritis (CIA). CIA was induced in WT and IEX-1 KO mice and the clinical scores (A) and cumulative incidence of arthritic mice (B) were assessed in the mice every other day after the booster immunization. Shown in (C–F) are representative macroscopic images of H&E stained joint sections prepared from WT (C&E) and IEX-1 KO (D&F) mice immunized with adjuvant only (C and D) or a mixture of CII and adjuvant (E and F). *, ** and ***, p<0.05, 0.01 and 0.001, respectively, in presence or absence of IEX-1 and n = 12. Arrow indicates pannus and scale bar, 100 µm.
Figure 7.
Th17-biased immune responses in IEX-1 deficient mice. Diseased mice were killed on day 30 after primary immunization, the hind paws were dissected and homogenized, and IL-17A levels in the homogenate were determined by ELISA (A). Draining lymph nodes were isolated from WT and IEX-1 KO mice on day 0 or 30 after primary immunization. The percentages of Th17, Th1, and Treg cells were analyzed by intracellular staining for IL-17 and IFN-γ (B) or Foxp3 (C), respectively. Representative flow cytometric profiles are shown in B and C, and mean percentages ± SD of Th17, Th1, and Treg cells on day 30 are summarized in D. IEX-1 KO mice were immunized with CII in CFA as Figure 6, and anti-IL-17 Ab or control Ab (control) was i.p. administered for 4 consecutive weeks starting on day 0. The clinical scores (E) and cumulative incidence of arthritis (F) were assessed in the mice every other day up to 10 days after the booster immunization. Shown in G and H are representative macroscopic images of H&E stained joint sections prepared from IEX-1 KO mice treated with control Ab (G) or anti-IL-17 Ab (H). *, ** and ***, p<0.05, 0.01 and 0.001, respectively, in presence or absence of IEX-1; n = 6, arrows, pannus; and scale bar, 100 µm.
Discussion
Differentiation of Th17 cells is primarily directed by a lineage-specific transcription factor RORγt (19,37). The current study shows for the first time that the transcriptional activity of the IL-17 promoter can be potentially regulated by a redox-sensitive mechanism through up-regulation of Batf. This finding is in a good agreement with the ability of resveratrol, a plant-derived compound, to suppress CIA in part by inhibition of Th17 cell differentiation (38). It may not be coincident that resveratrol suppresses Th17 differentiation by up-regulation of super oxide dismutase at mitochondria that modulates mitochondrial oxidative stress (39). These observations stress a role for mtROS in tuning a balance of Th1/1h17 immune responses. Our data showed that while IL-6 promoted Th17 polarization in both WT and IEX-1 KO Th cells in a dose-dependent manner, it had a significantly greater impact in the absence of IEX-1. IL-6 stimulated transcription of RORγt/RORα via phosphorylation of STAT3, but we failed to find any significant difference in STAT3 phosphorylation or RORγt/RORα expression in Th17 cells in the presence or absence of IEX-1, despite an importance of ROS in the regulation of STAT3 phosphorylation and transactivation (30,40). Won et. al. showed that glutathione peroxidase 1(GPx1)-deficient Th cells produced higher levels of intracellular ROS and IL-2 than WT Th cells, which suppressed Th17 cell development (41). This was apparent not the case for IEX-1 KO T cells in which IL-2 production was not altered significantly in the absence compared to the presence of IEX-1, probably because the effect of ROS varies with the level and location of ROS production and cell type or tissues involved. In addition to RORγt and RORα, transcription factors such as Batf, Runx1, Irf4, IκBζ can directly enhance the transcription activity of IL-17 in the presence of RORγt (32). Among them, Batf but not IκBζ was up-regulated at a level significantly higher in IEX-1 KO T cells than in WT T cells when differentiated under Th17-polarizing conditions.
Previous investigation showed normal TGF-β signaling and STAT3 phosphorylation in Batf-deficient T cells but altered expression of a subset of IL-6-induced genes and compromised RORγt expression in Th17-differentiation conditions (16). As a result, the mice have a defect in Th17 cell differentiation and are resistant to EAE (16). However, retroviral RORγt expression of Batf−/− cells only partially restored IL-17 expression in the cells, suggesting that Batf-regulated RORγt expression is not the only mechanism for its regulation of IL-17 transcription. Indeed, in addition to regulation of RORγt expression, Batf was found to directly bind the regulatory regions that overlapped with RORγt binding site in IL-17 promoter where it interacted with RORγt and synergized the induction of IL-17 expression (16,34). Batf is a member of the basic leucine zipper proteins of the activator protein-1 (AP-1) family and it is sensitive to a redox status of the cells (figure 5B). mtROS induced by IEX-1 deficiency enhance the transcription activity of IL-17 at least in part by up regulation of Batf in light of its important transcription activity on IL-17 promoter, although it cannot rule out that other mechanisms may be also involved such as acetylation of the IL-17 promoter or nuclear translocation of RORγt or RORα transcription factor (26).
The second important finding of this investigation is to demonstrate a role for mitochondria in Th differentiation under a pathological condition. Considerable evidence has demonstrated participation of cell membrane-associated NADPH oxidases in the regulation of inflammation. Lack of a functional NADPH oxidase reduced the level of ROS production in monocytes, macrophages, dendritic cells, and phagocytes, predisposing to severe CD4+ cell-dependent CIA in mice lacking Ncf-1 or gp47, a subunit of the cell membrane-associated NADPH oxidases (42). In contrast the well-established role of NADPH oxidase in regulation of inflammation, our understanding of how mtROS contribute to innate immune responses has just been emerging (43). Mitochondrial antiviral and Jun/MAPK-signaling pathways are two major pathways that can be activated or augmented by mtROS, leading to NF-κB activation and inflammatory responses (44,45). Several redox sensitive proteins involved in cell signaling have been also identified in mitochondria including PKC, Akt, A-Raf and src protein tyrosine kinases, activation of which could augment the transcriptional activity of AP-1, CREB, or NF-κB (46). How the mtROS-mediated signaling is involved in augmentation of Batf expression in the absence of IEX-1 is under current investigation. The present study extends the role of mtROS in the innate immune responses to Th17 differentiation under pathological conditions. The mitochondrial source of ROS generation is concluded by the fact that T cells do not express cell membrane-associated NADPH oxidases (47). Moreover, MitoQ, but not apocynin, blunts the propensity of IEX-1 KO T cells to differentiate into Th17 cells. Interestingly, NAC, a broad-spectrum antioxidant, suppresses Th17 differentiation in both WT and IEX-1 KO Th cells, whereas MitoQ specifically abrogates Th17 differentiation in the absence of IEX-1, suggesting that mtROS-mediated signaling is not required for Th17 differentiation under physiological conditions but it is involved in pathological conditions, making it an attractive target for treatment.
Accumulating evidence suggests that Th differentiation, in particular, Th17 cell differentiation, can be tuned by a variety of environmental cues. For instance, HIF-1α, a sensor of hypoxia, can directly up-regulate RORγt transcription or through a metabolic switch to glycolysis facilitating Th-17 differentiation (48,49). Likewise, the mammalian target of rapamycin complex (mTORC)-1 and mTORC-2 are two well-known metabolic sensors that regulate cellular metabolism in response to environmental cues like acids, insulin, and growth factors (50). mTORC1 signaling is found to be required for Th1 and Th17 differentiation whereas mTORC2 signaling is pivotal for Th2 differentiation (51). Our current and previous investigations show that mtROS also contribute to a balance of Th1 and Th17 cell differentiation, which may be of particular significance to autoimmune diseases in the elderly as mitochondrial function degenerates with age. IEX-1 is highly inducible by a variety of environmental cues and its induction may be necessary to protect Th1 cells from apoptosis but suppress Th17 cell differentiation (14). In accordance with this, over-expression of IEX-1 in T cells predisposed mice to the development of a lupus-like autoimmune disease and T cell lymphoma due to insufficient apoptosis of T cells (12,13). In contrast, lack of IEX-1 reduced the percentage of Th1 cells in inflamed paws. Similarly, lack of IEX-1 increased inflammation cytokine-induced apoptosis in Th1 cells, ameliorating inflammation in DSS-induced acute colitis as well as a wasting disease triggered by adoptive transfer of CD4+CD45RBhigh cells (15). Strikingly, in marked contrast to increased susceptibility to apoptosis of Th1 cells, lack of IEX-1 promoted the differentiation of IL-17-producing T cells in both in vivo and in vitro. The proportions of Th17 cells in the draining lymph nodes and the levels of IL-17 in paw tissues of arthritic IEX-1 KO mice were significantly higher than those in WT arthritic mice, suggesting Th17 dominant in the exacerbated arthritis in IEX-1 KO mice. The ability of differential regulation of the survival of T cells in a T cell-subset specific manner explains how targeting one Th subset may promote the development of the other as well as a failure of many clinical trials using anti-oxidants in treatment of various autoimmune diseases including rheumatoid arthritis (RA), despite an established, inverse association between an overall anti-oxidant index and the development of RA (52).
In conclusion, our investigation suggests that mtROS-mediated signaling serves as another layer of complex regulation of Th17 differentiation in addition to the milieu of cytokines. Although both Th1 and Th17 effector T cells can cause autoimmune-mediated pathology, they are distinct in recruitment of inflammatory leukocytes into the site of inflammation and in the preferential tissue location of the pathology, thereby affecting the clinical disease manifestations and treatment. A better understanding of how Th17 cells can be predominant in some autoimmune diseases whereas Th1 cells in others even in the same autoimmune disease would be essential for developing specific treatment of autoimmune diseases.
Acknowledgments
We thank the members in Dr. Wu groups for stimulating discussions and valuable comments and Dr. Chen Dong from Department of Immunology, M.D. Anderson Cancer Center for RORγt expression plasmid and a luciferase reporter vector containing Il17 minimal promoter with a CNS2 element. We are grateful to Dr Michael Murphy from Medical Research Council Dunn Human Nutrition Unit (Cambridge, United Kingdom) for the generous gift of MitoQ 10.
Funding sources: This work is supported in part by National Institutes of Health (NIH) grants AI050822 and AI070785, and a Senior Research Award from the Crohn’s & Colitis Foundation of America (to M.X.W.).
Footnotes
Author contributions: All authors were involved in the drafting the article and approved for the submission. Study conception and design: Mei X. Wu; performance of the experiments: Liang, Ustyugova, Chen and Zhang; and analysis and interpretation of the data: Liang, Wu, and Ustyugova.
Reference List
- 1.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr.Opin.Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J.Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 4.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J.Exp.Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubberts E, Koenders MI, Oppers-Walgreen B, van den BL, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 6.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J.Exp.Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann.N.Y.Acad.Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J.Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu MX. Roles of the stress-induced gene IEX-1 in regulation of cell death and oncogenesis. Apoptosis. 2003;8:11–18. doi: 10.1023/a:1021688600370. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Zhi L, Hu W, Wu MX. IEX-1 targets mitochondrial F1Fo-ATPase inhibitor for degradation. Cell Death.Differ. 2009;16:603–612. doi: 10.1038/cdd.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Schlossman SF, Edwards RA, Ou CN, Gu J, Wu MX. Impaired apoptosis, extended duration of immune responses, and a lupus-like autoimmune disease in IEX-1-transgenic mice. Proc.Natl.Acad.Sci.U.S.A. 2002;99:878–883. doi: 10.1073/pnas.022326699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Finegold MJ, Porteu F, Kanteti P, Wu MX. Development of Tcell lymphomas in Emu-IEX-1 mice. Oncogene. 2003;22:6845–6851. doi: 10.1038/sj.onc.1206707. [DOI] [PubMed] [Google Scholar]
- 14.Akilov OE, Ustyugova IV, Zhi L, Hasan T, Wu MX. Enhanced susceptibility to Leishmania infection in resistant mice in the absence of immediate early response gene X-1. J.Immunol. 2009;183:7994–8003. doi: 10.4049/jimmunol.0900866. [DOI] [PubMed] [Google Scholar]
- 15.Ustyugova IV, Zhi L, Wu MX. Reciprocal regulation of the survival and apoptosis of Th17 and Th1 cells in the colon. Inflamm.Bowel.Dis. 2011 doi: 10.1002/ibd.21772. [DOI] [PubMed] [Google Scholar]
- 16.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahid M, Shen L, Seldin DC, Lu B, Ustyugova IV, Chen X, Zapol WM, Wu MX. Impaired 3',5'-cyclic adenosine monophosphate-mediated signaling in immediate early responsive gene X-1-deficient vascular smooth muscle cells. Hypertension. 2010;56:705–712. doi: 10.1161/HYPERTENSIONAHA.110.154880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell IK, Hamilton JA, Wicks IP. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur.J.Immunol. 2000;30:1568–1575. doi: 10.1002/1521-4141(200006)30:6<1568::AID-IMMU1568>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 22.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J.Exp.Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy MP. How mitochondria produce reactive oxygen species. Biochem.J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert.Opin.Investig.Drugs. 2010;19:535–554. doi: 10.1517/13543781003727495. [DOI] [PubMed] [Google Scholar]
- 26.Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J.Biol.Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 27.Salazar JJ, Van Houten B. Preferential mitochondrial DNA injury caused by glucose oxidase as a steady generator of hydrogen peroxide in human fibroblasts. Mutat.Res. 1997;385:139–149. doi: 10.1016/s0921-8777(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 28.Gow AJ, Branco F, Christofidou-Solomidou M, Black-Schultz L, Albelda SM, Muzykantov VR. Immunotargeting of glucose oxidase: intracellular production of H(2)O(2) and endothelial oxidative stress. Am.J.Physiol. 1999;277:L271–L281. doi: 10.1152/ajplung.1999.277.2.L271. [DOI] [PubMed] [Google Scholar]
- 29.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O'shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat.Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carballo M, Conde M, El Bekay R, Martin-Nieto J, Camacho MJ, Monteseirin J, Conde J, Bedoya FJ, Sobrino F. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J.Biol.Chem. 1999;274:17580–17586. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- 31.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J.Biol.Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 32.Hwang ES. Transcriptional regulation of T helper 17 cell differentiation. Yonsei Med.J. 2010;51:484–491. doi: 10.3349/ymj.2010.51.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NFkappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997;11:301–312. doi: 10.1006/meth.1996.0424. [DOI] [PubMed] [Google Scholar]
- 34.Martinez GJ, Dong C. BATF: bringing (in) another Th17-regulating factor. J.Mol.Cell Biol. 2009;1:66–68. doi: 10.1093/jmcb/mjp016. [DOI] [PubMed] [Google Scholar]
- 35.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat.Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 36.Kelchtermans H, Schurgers E, Geboes L, Mitera T, Van Damme J, Van Snick J, Uyttenhove C, Matthys P. Effector mechanisms of interleukin-17 in collageninduced arthritis in the absence of interferon-gamma and counteraction by interferongamma. Arthritis Res.Ther. 2009;11:R122. doi: 10.1186/ar2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann.Rheum.Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- 39.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am.J.Physiol Heart Circ.Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol.Cell Biol. 2001;21:7721–7730. doi: 10.1128/MCB.21.22.7721-7730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Won HY, Sohn JH, Min HJ, Lee K, Woo HA, Ho YS, Park JW, Rhee SG, Hwang ES. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid.Redox.Signal. 2010;13:575–587. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]
- 42.George-Chandy A, Nordstrom I, Nygren E, Jonsson IM, Postigo J, Collins LV, Eriksson K. Th17 development and autoimmune arthritis in the absence of reactive oxygen species. Eur.J.Immunol. 2008;38:1118–1126. doi: 10.1002/eji.200737348. [DOI] [PubMed] [Google Scholar]
- 43.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat.Rev.Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J.Exp.Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 46.Felty Q, Roy D. Mitochondrial signals to nucleus regulate estrogen-induced cell growth. Med.Hypotheses. 2005;64:133–141. doi: 10.1016/j.mehy.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 47.Gelderman KA, Hultqvist M, Holmberg J, Olofsson P, Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc.Natl.Acad.Sci.U.S.A. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) Balance by Hypoxia- Inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J.Exp.Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 51.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat.Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hitchon CA, El Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res.Ther. 2004;6:265–278. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]