Abstract
Rationale
The Transforming Growth Factor–β (TGFβ) family member Nodal promotes cardiogenesis, but the mechanism is unclear despite the relevance of TGFβ family proteins for myocardial remodeling and regeneration.
Objective
Determine the function(s) of TGFβ family members during stem cell cardiogenesis.
Methods and Results
Murine embryonic stem cells (mESCs) were engineered with a constitutively active human Type I Nodal receptor (caACVR1b) to mimic activation by Nodal and found to secrete a paracrine signal that promotes cardiogenesis. Transcriptome and gain- and loss-of-function studies identified the factor as TGFβ2. Both Nodal and TGFβ induced early cardiogenic progenitors in ESC cultures at day 0–2 of differentiation. However, Nodal expression declines by day 4 due to feedback inhibition whereas TGFβ persists. At later stages (day 4–6), TGFβ suppresses the formation of cardiomyocytes from multipotent Kdr+ progenitors, while promoting the differentiation of vascular smooth muscle and endothelial cells.
Conclusions
Nodal induces TGFβ, and both stimulate the formation of multipotent cardiovascular Kdr+ progenitors. TGFβ, however, becomes uniquely responsible for controlling subsequent lineage segregation by stimulating vascular smooth muscle and endothelial lineages and simultaneously blocking cardiomyocyte differentiation.
Keywords: Nodal, Cripto, TGFβ2, Kdr, cardiogenesis
INTRODUCTION
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) hold great potential as sources of cardiomyocytes, and as models to understand how cardiomyocytes, vascular smooth muscle and endothelial cells arise from common cardiopoietic progenitors1. Defining the signals that control cardiopoietic differentiation will be important for numerous applications, including regenerative medicine.
The divergent Transforming Growth Factor–β (TGFβ family member Nodal is critical for the formation of the heart and other visceral organs. Nodal activates a heteromeric complex of type I [Acvr1b (Alk4), or Acvr1c (Alk7)] and type II (Acvr2a and b) serine/threonine kinase receptors, leading to phosphorylation of Smad2 and -3 that then activate target genes2. Mouse embryos lacking Acvr1b, Smad2, or Nodal, and double knockout of the two type II receptors (Acvr2a and Acvr2b) fail to gastrulate or form mesendoderm3. Genetic deletion of Cripto, an essential Nodal co-receptor in most contexts, is less severe, such that embryos form mesendoderm but are severely deficient in cardiogenic progenitor cells4, 5. The cardiogenesis deficit inherent in Cripto−/− ESCs can be rescued either by incorporation into chimeric (Cripto−/−:wildtype (WT)) embryos4, or by a constitutively active mutant human ACVR1b receptor6, demonstrating the existence of yet unknown paracrine effectors that propagate the signal from cell to cell.
We used mESCs to model cardiogenesis and found that TGFβ2 is induced by Nodal and propagates the cardiogenic signal. The essential nature of TGFβ for cardiogenesis is based on resistance to the feedback inhibitors Lefty1, Lefty2 and Cerberus1 (Cer1) that block Nodal. Consequently, both Nodal and TGFβ induce early cardiogenic progenitors, but Nodal expression declines due to feedback inhibition while TGFβ expression persists in Kdr+ cardiopoietic precursors. In this population, TGFβ suppresses cardiomyocyte differentiation, while promoting vascular smooth muscle and endothelial cell formation. Thus, a Nodal to TGFβ cascade, including feedback inhibition, provides biphasic control over cardiopoietic cell fate.
METHODS
Protocols and primer sequences are in online supplemental materials.
RESULTS
Cardiogenic rescue implicates a diffusible factor downstream of Nodal/Avcr1b
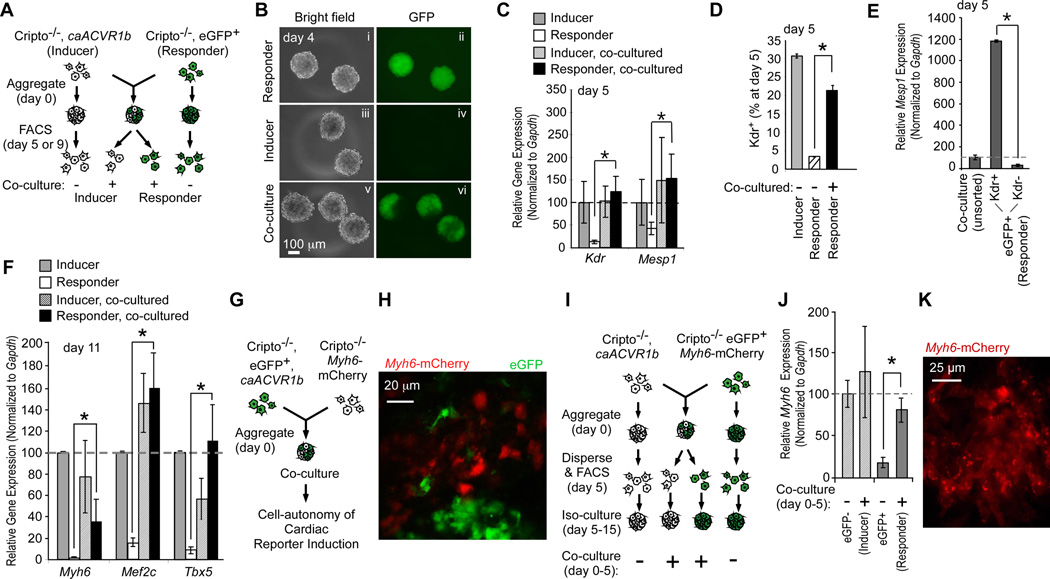
Cripto−/− mESCs are deficient in production of cardiogenic progenitors, exhibiting low Kdr and Mesp1 expression (Online Fig. IA and4, 5), and are thus ideal for a cell-mixing study to identify paracrine factors that initiate cardiogenesis downstream of Nodal/Avcr1b (Fig. 1A). A constitutively active human ACVR1b receptor (caACVR1b) was stably introduced into Cripto−/− mESCs to activate downstream signaling (Cripto−/−caACVR1b, inducers) (Online Fig. IA). Co-culture (Fig. 1A,B) of these cells dramatically restored Kdr and Mesp1 expression in eGFP-labeled Cripto−/− mESCs (responders) (Fig. 1C). Co-culture also increased the number of Kdr+ progenitors among the responder (eGFP+) population, from 3.34% ± 0.06% to 21.28% ± 1.37% after 5 days (Fig. 1D). FACS-isolated GFP+, Kdr+ cells (responders) co-expressed Mesp1 (Fig. 1E). By day 9, the induced cells expressed cardiomyocyte markers (Fig. 1F) and beat rhythmically (Online Movie I). Residual Cripto−/−caACVR1b cells contaminating the responder population after FACS (0.5%) were insufficient to account for this level of rescue (Online Fig. II). Finally, the rescue occurred cell non-autonomously, since mixtures of eGFP-labeled Cripto−/−caACVR1b inducers with Myh6-mCherry responders revealed clearly distinct patterns of eGFP and mCherry expression (Fig. 1G,H and Online Movie II).
Fig. 1. Paracrine signaling downstream of Nodal.
A, B, Schematic (A) and images (B) of the cell mixing experiment. C–E, Kdr and Mesp1 expression (C) and proportion of Kdr+ cells (D) in FACS-isolated populations from co-cultures. Mesp1 expression in FACS-isolated Kdr+/GFP+ cells (E). Note induction by co-culture. F, Myh6, Mef2c, and Tbx5 in FACS-isolated populations from co-cultures; representative of >3 trials (see Online Fig. II). G, H, Cell non-autonomous signaling induced cardiogenesis. Schematic of the experiment (G) and representative confocal image of Myh6-mCherry reporter (H). Movie II shows multiple optical planes. I–K, Co-culture from day 0–5 is sufficient for cardiogenesis in responder cells. Schematic of experiment (I). Quantitative real-time reverse transcription PCR (qRT-PCR) analysis of Myh6 expression (J) and image of Myh6-mCherry reporter (K) after 10 days iso-culture. *P< 0.05, unpaired Student’s T-test. Error bars indicate the S. E. M.; n=3.
To test if the induced Kdr+ progenitors autonomously form cardiomyocytes, aggregated responder (Cripto−/−, Myh6-mCherry, eGFP+) and inducer (Cripto−/− caACVR1b) cells were separated by FACS at day 5, re-aggregated separately, and cultured for an additional 15 days (Fig. 1I). The responder cells expressed Myh6 (Fig. 1J) and mCherry (Fig. 1K), showing that the paracrine factor(s) initiate cardiogenesis prior to day 5. Since Cripto−/− cells negligibly respond to Nodal (Online Fig. IB), the factor is neither Nodal nor a shed version of Cripto.
TGFβ2 acts downstream of Nodal to induce cardiogenic mesoderm
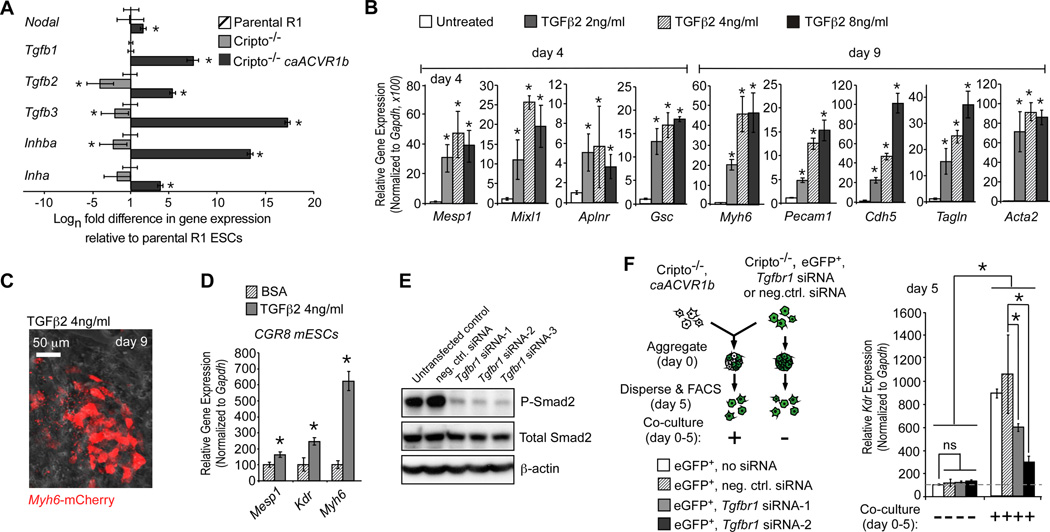
Microarray analysis (not shown) showed that caACVR1b upregulated mRNAs encoding TGFβ1, TGFβ2, TGFβ3 and inhibins. Of these, Tgfb2, Tγϕβ3 and Inhba were greatly upregulated by caACVR1b transfection in Cripto−/− mESCs (Fig. 2A). Since E5.5 to E7.5 mouse embryos express mRNAs encoding Tgfb2, but not Tgfb3 and Inhibins7, TGFβ2 emerged as an attractive candidate for the paracrine factor. Indeed, TGFβ2 treatment from days 0–2 gave a dose-dependent induction of genetic markers of mesoderm (Mesp1, Mixl1 and Gsc) and mesoderm derivatives (Myh6, Pecam1, Aplnr,Tagln, Cdh5 and Acta2), and the Myh6-mCherry reporter in Cripto−/− ESCs (Fig. 2B,C) and even enhanced Mesp1, Kdr and Myh6 in WT cells (Fig. 2D), revealing a functional relationship.
Fig. 2. TGFβ2 acts downstream of Nodal/Acvr1b to induce Kdr+ progenitors.
A, qRT-PCR analysis of TGFβsuperfamily members in R1, Cripto−/− and Cripto−/− caACVR1b ESCs. B–D, Treatment of Cripto−/− (B,C) and WT (D) ESCs treated with TGFβ2 between days 0–2 of differentiation under defined conditions analyzed for gene (B,D) and Myh6-mCherry expression (C). E–F, Effect of siRNA knockdown of Tgfbr1 on Kdr. Western blot of R1 ESCs showing efficacy of Tgfbr1 siRNAs (E). Schematic protocol and qRT-PCR analysis of Kdr in responder cells (F). *P< 0.05, unpaired Student’s T-test. Error bars indicate the S. E. M.; n=3.
To test if TGFβis necessary downstream of Nodal/Acvr1b, Cripto−/− responder ESCs were transfected with siRNA against Tgfbr1 prior to co-culture with Cripto−/−caACVR1b ESCs (Fig. 2F). Tgfbr1 siRNAs blocked induction of Kdr transcripts (to about 20% of negative control siRNA), establishing TGFβ2 as a paracrine mediator of Nodal signaling.
TGFβ2 suppresses cardiomyocyte differentiation during a late stage of differentiation
The preceding showed that TGFβ2 induces cardiogenic progenitors prior to day 5. Tgfb2 mRNA, however, continues to rise between days 4–8 (Fig. 3A) while Nodal mRNA declines, suggesting that TGFβ but not Nodal, plays a role as Kdr+ progenitors differentiate. To understand the basis for the shift from Nodal to Tgfb2, we examined expression of Lefty1, Lefty2 and Cer1, encoding Nodal inhibitors3. All three became expressed concomitantly with the decline in Nodal levels (Fig. 3A) and each was induced by Nodal/TGFβsignaling (Fig. 3B, C). Moreover, Nodal and TGFβboth induced Nodal (Figs. 2A and 3C). The fact that Cer1 and Lefty1,2 do not block TGFβ3 likely accounts for the persistence of Tgfb2 after the decline in Nodal. Interestingly, TGFβ2 does not induce Tgfb1 or Tgfb2, and only minimally induced Tgfb3 (Fig. 3C), making the cascade inherently self-limiting.
Fig. 3. Biphasic role of TGFβ2 in cardiogenesis.
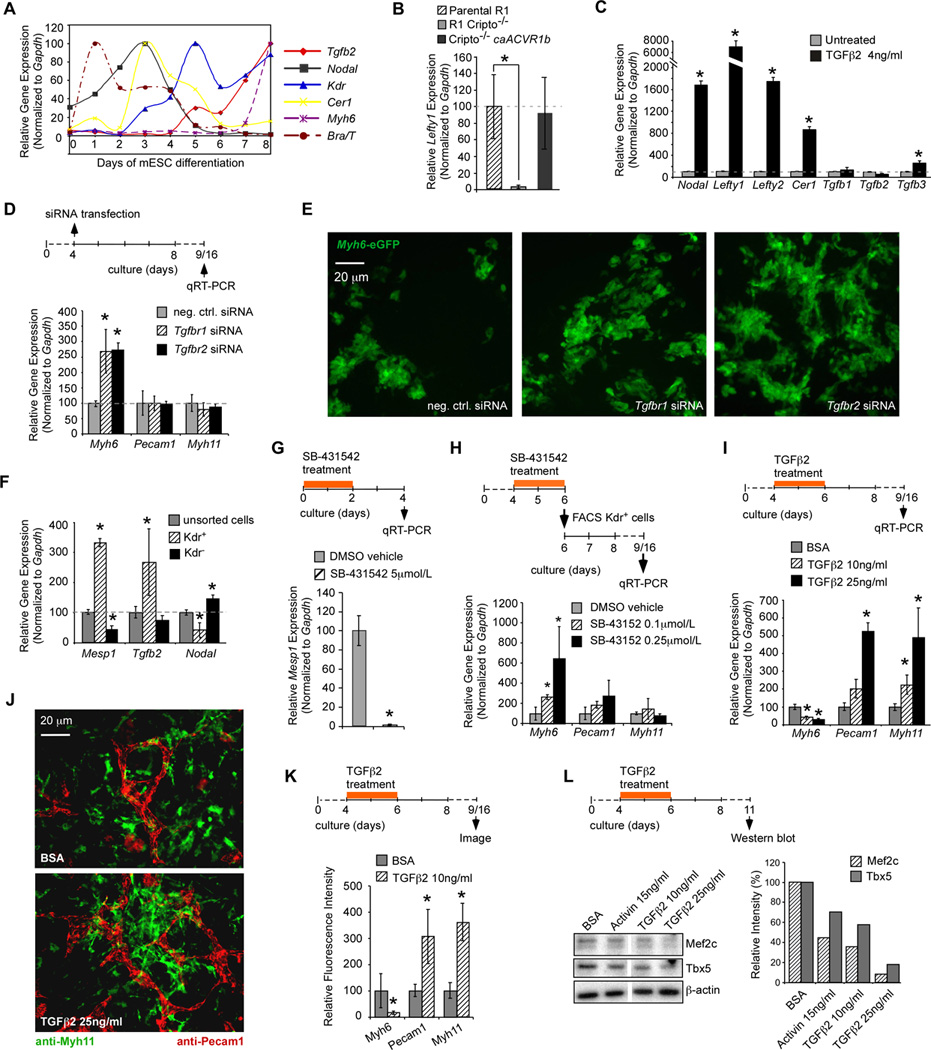
A, Temporal expression profiles of Tgfb2, Nodal, Cer1, Kdr and Myh6 during mESC differentiation. B, Lefty1 expression by qRT-PCR in WT and Cripto−/− mESCs, and induction by caACVR1b. C, Induction profile of Nodal cascade genes in response to recombinant TGFβ2. Cripto−/− EBs were used to provide low basal levels of expression. Note induction of Nodal but not TGFβ. D,E, siRNAs against Tgfbr1 and Tgfbr2 transfected at day 4 enhanced expression of Myh6 mRNA (D), as well as Myh6-GFP reporter (E), without effects on Pecam1 and Myh11 (day 16) (D). F, Kdr+ cells express Tgfb2, by qRT-PCR. G–H, Contrasting effects of SB-431542 treatment of WT CGR8 mESCs at early (days 0–2) (G) and of isolated Kdr+ progenitors at late (days 4–6) (H) stages of differentiation.I–L, TGFβ2 treatment between days 4–6 attenuated expression of cardiomyocyte markers [Myh6 mRNA (I), Tbx5 and Mef2c protein (L), and Myh6 immunostaining (K)], but increased markers of vascular endothelial and smooth muscle [Pecam1 and Myh11 mRNA (I) and immunostaining (J,K)]. *P< 0.05, unpaired Student’s T-test. Error bars indicate the S. E. M.; n=3.
We next asked whether TGFβ influences cardiopoietic differentiation. siRNAs to either Tgfbr1 or Tgfbr2 transfected at day 4 unexpectedly increased expression of Myh6, as well as eGFP driven by the Myh6 promoter (Fig. 3D,E). At this time, Tgfb2 mRNA predominates in Kdr+ cells (Fig. 3F), suggesting autocrine repression of cardiomyocyte differentiation.
To gain further insight into the bimodal function of TGFβ we treated ESC cultures with SB-431542, a small molecule inhibitor of Acvr1b/1c and Tgfbr1, at early and late time windows (Figs 3G and H). Treatment between 0–2 days of culture abolished Mesp1 expression (Fig. 3G). Treatment at 4–6 days, in contrast, markedly enhanced Myh6 levels in Kdr+ derivatives (Fig. 3H). Conversely, recombinant TGFβ2 between days 4–6 suppressed Myh6 mRNA as well as Mef2c and Tbx5 protein, but increased Pecam1 and Myh11 mRNAs and the level of Pecam1 and Myh11 immunostaining (Fig. 3I–L). We conclude that a Nodal to TGFβ2 cascade enhances production of cardiogenic mesoderm prior to day 4, and that TGFβ persists to suppress cardiomyocyte differentiation of Kdr+ cells while biasing their differentiation towards endothelial and smooth muscle lineages.
DISCUSSION
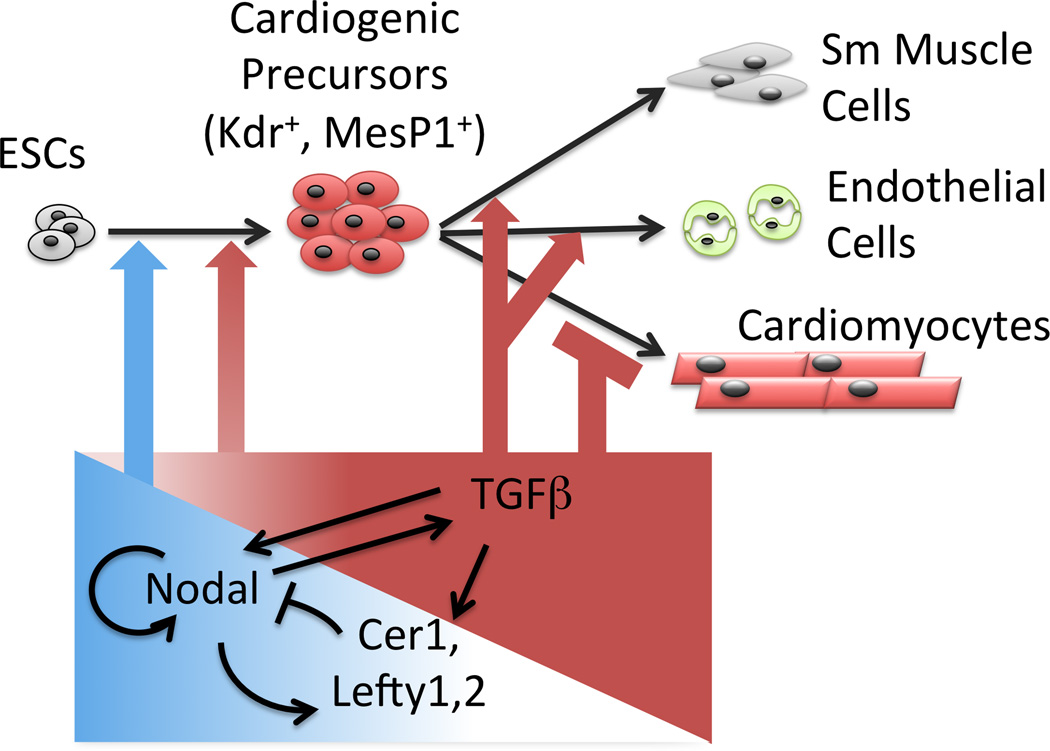
Genetic and stem cell experiments have shown that Nodal acts positively and negatively in cardiogenesis depending on the developmental stage; however, the identity and function of downstream mediators were unknown4, 6, 8, 9. Our results define a Nodal to TGFβ signaling cascade that exerts positive and negative effects on progenitor induction and cardiomyocyte differentiation, respectively (Fig. 4). The biphasic function resembles that of Wnts and BMPs, both of which promote formation of cardiogenic progenitors (e.g. Mesp1+, Kdr+) during the period when mesoderm is induced, but suppress the subsequent formation of cardiac precursors (e.g. Nkx2.5+), and at least BMP acts positively again once Nkx2.5+ progenitors arise1.
Fig. 4. Summary.
Nodal and TGFβ induce early cardiogenic progenitors. Subsequently, feedback inhibition blocks Nodal, allowing persistence of TGFβ which inhibits cardiomyocyte differentiation and promotes formation of vascular smooth muscle and endothelial cells.
Mechanistically, the cascade incorporates auto-induction and inhibition properties that regulate Nodal and TGFβ expression within narrowly delimited developmental times. Nodal is well-known for activating its own transcription, as well that of its antagonists Lefty1, 2 and Cer1, yielding an auto-induction cascade that is feedback inhibited. However, TGFβ cannot auto-induce (Figs. 2A and 3C) nor is inhibited by Cer1 and Lefty. Consequently, Tgfb2 expression is induced by Nodal, and persists after Nodal expression declines.
Considering the possible functions for a time-resolved Nodal-TGFβ cascade led to the finding that TGFβ suppresses cardiomyocyte differentiation while simultaneously enhancing formation of endothelial and smooth muscle lineages (Fig. 3E–L). The only other factors known to apportion cardiopoietic fate are Wnts, which also suppress cardiomyocyte differentiation at the same developmental stage1.
A specific requirement for TGFβ in cardiac differentiation has implications for understanding congenital heart defects. Genetic deletion of Tgfbr1 in mice causes severe cardiovascular defects10, and mutation of the latent TGFβ binding protein 3, which regulates TGFβ bioavailability, impairs differentiation of second heart field (SHF) cells in zebrafish11. It will be important to determine if altered TGFβ signaling at the time of cardiac progenitor specification underlies human congenital heart disease, such as the cardiac defects that can present in Loeys-Dietz syndrome caused by mutated TGFBR1 or TGFBR2.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is Known?
The divergent TGFβ protein Nodal is well-known to play a role in specifying cardiac tissue during early development, and is commonly used to generate cardiac cell types, including cardiomyocytes, from pluripotent stem cells.
The cardiogenic activity of Nodal is propagated from cell to cell by unknown paracrine signals, although a shed version of the Nodal co-receptor Cripto has been suggested to be involved.
What New Information Does This Article Contribute?
Nodal induces TGFβ2, and both induce the formation of cardiogenic progenitors in embryonic stem cell (ESC) cultures.
Nodal expression declines as cardiogenic progenitors form; TGFβ persists and suppresses cardiomyocyte differentiation while simultaneously promoting vascular smooth muscle and endothelial lineages.
TGFβ uperfamily members are important for cardiogenesis, as well as fibrosis and inflammation associated with myocardial injury. Here we describe a regulatory cascade that controls the production of TGFβ. TGFβinitially promotes the formation of multi-potent cardiac progenitors, but subsequently inhibits their differentiation to cardiomyocytes. TGFβ might play a similarly bimodal role in myocardial regeneration.
ACKNOWLEDGEMENTS
We thank Yoav Altman, Joseph Russo and Dr. Ed Monosov (SBMRI) for expert assistance.
SOURCES OF FUNDING
NIH and California Institute for Regenerative Medicine.
Non-standard Abbreviations
- Acvr1b
Alk4, activin A receptor, type IB
- Tgfbr1
Alk5, transforming growth factor, beta receptor I
- Acvr1c
Alk7, activin A receptor, type IC
- Acvr2a
activin receptor IIA
- Acvr2b
type 2 Activin receptorb
- Cer1
cerberus 1 homolog (Xenopus laevis)
- Inhba
inhibin beta-A
- Inha
Inhibin alpha
- Kdr
Flk1, kinase insert domain protein receptor
- Lefty1
left right determination factor 1
- Lefty2
left right determination factor 2
- Mesp1
mesoderm posterior 1
- Myh6
αMHC, myosin, heavy polypeptide 6, cardiac muscle, alpha
- Myh11
sm-MHC, myosin, heavy polypeptide 11, smooth muscle
- Pecam1
CD31, platelet/endothelial cell adhesion molecule 1
- qRT-PCR
quantitative real-time reverse transcription PCR
- T
Bra/T, brachyury
- Tgfbr2
transforming growth factor, beta receptor IIb
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: Lessons from development. Genes & development. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through smads. Annual review of cell and developmental biology. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 3.Schier AF. Nodal signaling in vertebrate development. Annual review of cell and developmental biology. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 4.Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development (Cambridge, England) 1999;126:483–494. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- 5.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 6.Parisi S, D'Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. The Journal of cell biology. 2003;163:303–314. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson MC, Slager HG, Duffie E, Mummery CL, Akhurst RJ. Rna and protein localisations of tgfβ2 in the early mouse embryo suggest an involvement in cardiac development. Development (Cambridge, England) 1993;117:625–639. doi: 10.1242/dev.117.2.625. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura R, Takahashi T, Nakajima N, Isodono K, Asada S, Ueno H, Ueyama T, Yoshikawa T, Matsubara H, Oh H. Stage-specific role of endogenous smad2 activation in cardiomyogenesis of embryonic stem cells. Circulation research. 2007;101:78–87. doi: 10.1161/CIRCRESAHA.106.147264. [DOI] [PubMed] [Google Scholar]
- 9.Foley AC, Korol O, Timmer AM, Mercola M. Multiple functions of cerberus cooperate to induce heart downstream of nodal. Developmental biology. 2007;303:57–65. doi: 10.1016/j.ydbio.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur HM, Bamforth SD. Tgfbeta signaling and congenital heart disease: Insights from mouse studies. Birth Defects Res A Clin Mol Teratol. 2011;91:423–434. doi: 10.1002/bdra.20794. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Cashman TJ, Nevis KR, Obregon P, Carney SA, Liu Y, Gu A, Mosimann C, Sondalle S, Peterson RE, Heideman W, Burns CE, Burns CG. Latent tgf-beta binding protein 3 identifies a second heart field in zebrafish. Nature. 2011;474:645–648. doi: 10.1038/nature10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.