Abstract
Background and Objective: Prolyl endopeptidase (PEP) (EC 3.4.21.26) is a serine peptidase involved in differentiation, development and proliferation processes of several tissues. Recent studies have demonstrated the increased expression and activity of this cytosolic enzyme in colorectal cancer (CRC). However, there are no available data about the impact of this peptidase in the biological aggressiveness of this tumor in patient survival.
Methods: The activity of PEP in tissue (n=80) and plasma (n=40) of patients with CRC was prospectively analyzed by fluorimetric methods. Results were correlated with the most important classic pathological data related to aggressiveness, with 5-year survival rates and other clinical variables.
Results: 1) PEP is more active in early phases of CRC; 2) Lower levels of the enzyme in tumors were located in the rectum and this decrease could be related with preoperative chemo-radiotherapy; 3) PEP activity in tissue was higher in patients with better overall and disease-free survival (log-rank p<0.01, Cox analysis p<0.01); 4) Plasmatic PEP activity was significantly higher in CRC patients than in healthy individuals and this was associated with distant metastases and with worse overall and disease-free survivals (log-rank p<0.05, Cox analysis p<0.05).
Conclusions: PEP activity in tissue and plasma from CRC patients is an independent prognostic factor in survival. The determination of PEP activity in the plasma may be a safe, minimally invasive and inexpensive way to define the aggressiveness of CRC in daily practice.
Keywords: Prolyl endopeptidase, PEP, colorectal cancer, prognosis, survival.
Introduction
CRC is the third commonest malignancy in males and females in the United States, with more than 142,000 estimated new cases and almost 51,000 estimated deaths in 2013 1. Europe and other developed regions show similar rates of incidence and mortality 2. Traditionally related to dietary habits 3, its frequency has steadily increased in the last decades in Western countries as a result of modern style of life to become a health problem of major concern. Huge resources are being invested in prevention and early diagnosis of this disease. Population-based screening campaigns try to discover as much early tumors and precursor lesions as possible, aiming to decrease the incidence of the disease, to simplify the clinical management of patients once the lesion develops, and to improve survival.
The recent molecular classification of CRC proposed by Jass in 2007 4 has been of much help in the understanding of the diverse etiopathogenetic mechanisms of this disease. From a clinical perspective, adenomatous lesions in the large bowel are fully accepted precursors of CRC 5 and the adenoma-carcinoma sequence still provides a solid model for research on carcinogenesis.
Peptidases play a key role in carcinogenesis in several ways, for instance regulating bioactive peptides that are crucial in neoplastic growth, degrading the extracellular matrix, acting as adhesion molecules or participating in intracellular signalling 6. Besides, some studies have demonstrated that the activity and expression of these enzymes vary in different tumors depending on the several clinicopathological parameters like histological Grade, Stage, and patient survival 7-12. For this reason, peptidases are useful tools in the development of clinical strategies for treatment and follow up of cancer patients.
Prolyl endopeptidase (EC 3.4.21.26) (PEP), also known as prolyl oligopeptidase (POP or PREP), is a member of the serine peptidase group classified under the prolyl oligopeptidase family, a set of enzymes which have the ability to cleave peptides at internal proline residues 13. PEP has been classically described as a cytosolic enzyme, although a membrane-bound fraction has also been described in several tissues and in human body fluids 14-16. Most studies of the physiological role of PEP have been performed in the central nervous system 17, however this enzyme is widely expressed throughout most human peripheral tissues such as the epithelial cell of the digestive tract and the urinary system 18,19.
Several authors have demonstrated that PEP plays a role in cell differentiation and development 15, 17, 20, 21. There are evidences supporting the implication of PEP in proliferative disorders, cancer included. In this sense, higher expression and activity levels of this enzyme have been detected in carcinomas from lung, thyroid, ovary, kidney and head and neck when compared with the non-neoplastic respective samples 14, 19, 22-24. Additionally, changes in circulating PEP activity of prostate cancer patients have also been reported 14. Regarding CRC and its precursors, very recent studies have demonstrated high PEP expression in CRC 19 and in early stages of the adenoma-carcinoma sequence 24. The objective of these studies was to analyze the expression and activity of PEP in tumors and in their respective adjacent non tumor tissues, trying to define the role of this enzyme in carcinogenesis 14, 19, 22-24. However, the relationship between PEP activity and cancer survival has been studied only in the kidney in a recent study published by our own research group 24.
The aim of this study is to explore the role of PEP in the clinical behaviour of CRC. For such a purpose, a prospective analysis of PEP activity in tissue and plasma of patients with CRC has been performed. The obtained data have been correlated with classic clinical and pathological parameters and with 5-year survival.
Material & Methods
The authors declare that all the experiments carried out in this study comply with current Spanish laws and conform to the principles outlined in the Declaration of Helsinki.
Patients
A total of 80 patients with CRC have been prospectively included in the study. All patients received partial colectomies. Males predominated in the series (50M/30F), the average age being 70 years for males and 67 years for females. Mean follow-up was 50.2 months (range 3-80). Follow-up was closed by December 31, 2012. At that time, 25 patients had died of disease. AJCC system 25 has been applied to assign Stage and Grade. Clinical data included in the study were retrieved from the patient clinical records and are summarized in Table 1. Plasma was also collected preoperatively and analyzed in 40 of these patients. Table 2 shows the clinico-pathological data in this subset of patients. Plasma from 24 healthy volunteers with no clinical history of neoplastic diseases was used as control sample.
Table 1.
PEP activity in CRC tissue according to clinicopathologic characteristics. Values are means ± SE of peptidase activity recorded as pmol of units of peptidase (UP) per milligram of protein.
| Variables | n = | UP/mg prot (Media ± S.E ) | T student (p =) |
|---|---|---|---|
| Grade | |||
| Low (G1-G2) | 62 | 14337 ± 1062 | 0.284 |
| High (G3-G4) | 18 | 11776± 1914 | |
| Stage | |||
| Low (T1-T2) | 11 | 12282 ± 1963 | 0.721 |
| High (T3-T4) | 69 | 13964 ± 1026 | |
| Nodal invasion | |||
| No | 49 | 14825 ± 1311 | 0.213 |
| Yes | 31 | 12442 ± 1269 | |
| Distant Metastases | |||
| No | 73 | 13651 ± 988 | 0.527 |
| Yes | 7 | 15680 ± 3062 | |
| Lymphatic invasion | |||
| No | 71 | 14058 ± 1012 | 0.531 |
| Yes | 9 | 12146 ± 2820 | |
| Vascular invasion | |||
| No | 65 | 14983 ± 1058 | 0.014 |
| Yes | 15 | 9134 ± 1685 | |
| Perineural invasion | |||
| No | 75 | 14013 ± 962 | 0.461 |
| Yes | 5 | 10952 ± 4314 | |
| Grouped stage | |||
| Low (0-IIC) | 45 | 14605 ± 1372 | 0.389 |
| High (IIIA-IV) | 35 | 12975 ± 1255 |
Table 2.
PEP activity in plasma from CRC patients according to clinicopathologic characteristics. Values are means ± SE of units of peptidase per liter of plasma (UP/L).
| Variables | n = | UP/L (Media ± S.E ) | Mann-Whitney (p =) |
|---|---|---|---|
| Grade | |||
| Low (G1-G2) | 33 | 12.3 ± 0.88 | 0.676 |
| High (G3-G4) | 7 | 10.7 ± 0.75 | |
| Stage | |||
| Low (T1-T2) | 9 | 13.5 ± 2.04 | 0.532 |
| High (T3-T4) | 31 | 11.6 ± 0.78 | |
| Nodal invasion | |||
| No | 29 | 12.3 ± 0.9 | 0.493 |
| Yes | 11 | 11.1 ± 1.38 | |
| Distant Metastases | |||
| No | 37 | 11.5 ± 0.73 | 0.038 |
| Yes | 3 | 17.56 ± 2.9 | |
| Lymphatic invasion | |||
| No | 34 | 11.7 ± 0.8 | 0.389 |
| Yes | 6 | 13.5 ± 2.5 | |
| Vascular invasion | |||
| No | 33 | 12.2 ± 0.89 | 0.701 |
| Yes | 7 | 10.7 ± 1.03 | |
| Perineural invasion | |||
| No | 38 | 12.1 ± 0.77 | 0.852 |
| Yes | 2 | 10.1 ± 1.63 | |
| Grouped stage | |||
| Low (0-IIC) | 28 | 12.2 ± 0.91 | 0.712 |
| High (IIIA-IV) | 12 | 11.5 ± 1.31 |
Tissue Specimens
Surgical resections were submitted in fresh to the Pathology Lab within a period of 30 minutes after removal. Handling of specimens was performed following conventional protocols for the management of surgical resections of colon and rectum 26. Tumor characteristics were recognized on gross examination and selected fragments of tumor were frozen in isopentane and stored at -80ºC. Routine procedures in the Pathology Lab included formalin fixation of the surgical specimen and paraffin embedding of the tissue fragments selected for histopathological examination. Pathological data included in the study are summarized in Table 1. Besides, peripheral venous blood samples from 40 of these patients were collected prior to surgery in EDTA tubes and centrifuged at 1500 rpm during 15 minutes. The obtained plasma was also stored at -80ºC. Enzyme assays have also been performed in plasma obtained from 24 healthy volunteers (matched by sex and age).
Sample preparation
Explanted tumor samples were homogenized in 10 mM Tris-HCl buffer at pH 7.4, for 30 seconds at 800 rpm using a Heidolph PZR 50 Selecta homogenizer, and ultracentrifuged in a Centrikon T-2070 Kontron Instruments apparatus at 100,000 g for 35 minutes. The resulting supernatants were used to measure PEP activity. Previously collected plasma samples were used to determine plasmatic PEP activity. All the above-described steps were carried out at 4 °C.
Prolyl endopeptidase activity measurements
PEP activity was fluorimetrically measured using a modified version of the method described by Olivo et al. 27, using Z-Gly-Pro-β-naphthylamide (0.125mM) as substrate. The assay is based on the fluorescence of β-naphthylamine generated from the substrate by PEP. The components of the assay mixture (total volume 2ml) included the following: 50mM of sodium phosphate buffer (pH 7.4), 2 mM of DL-dithiothreitol and 0.15mg/ml of bovine serum albumin. The reaction was initiated by adding 50μL of tissue or plasma sample to 1mL of the assay mixture. This was incubated at 37ºC for 30 minutes and the reaction was stopped by addition of 1 mL of 0.1M sodium acetate buffer (pH 4.2). The excitation and emission wavelengths were 345 and 412 nm, respectively. Blanks were used to determine background fluorescence. Relative fluorescence was converted into picomoles of product using a standard curve constructed with increasing concentrations of β-naphthylamine.
To verify that the formation of β-naphthylamine (β-NA) was due to the action of PEP and not due to other enzymes, we performed inhibition assays in CRC tissue and plasma with a specific inhibitor of PEP (KYP-2047). The releasing of β-NA was completely inhibited in tumor tissues (100%) and mainly inhibited (%78) in plasma samples.
Protein concentration was measured in triplicate by the Bradford method 28, using BSA (1 mg/mL) as the calibrator. Results from the CRC tissues and from plasma samples were recorded as units of peptidase per milligram of protein (UP/mg prot) and per liter of plasma (UP/L), respectively. One unit of peptidase activity (UP) is the amount of enzyme required to release one pmol of β-naphthylamine per minute. Fluorogenic assays were linear with respect to hydrolysis time and protein content.
Statistical analysis
Kolmogorov-Smirnov and Shapiro-Wilk tests were applied to data obtained from tissue and plasma samples respectively to know if the numbers followed or not a normal distribution. Based on this information, PEP activity differences in tissue and plasma were analyzed with parametric (Student t) and non-parametric (Mann-Whitney test) probes, respectively. ANOVA and Scheffé post-hoc test was applied to detect differences in PEP activity of tumors from different topographies (colon vs. rectum), and Spearman Rho to correlate tumor size, plasmatic and tissue PEP activity. Kaplan-Meier curves and log-rank test were performed to evaluate the association between PEP and overall and disease-free survival, comparing groups created by cut-off points based on median PEP activity values. Receiver-operating characteristic (ROC) curves were also performed to test the sensitivity and specificity of the selected cut-off values of plasmatic PEP. A Cox regression model was used to test the independent effects of clinical and pathological variables and PEP activity on survival. SPSS® 19.0 software was used for the statistical analysis.
Results
PEP activity in tissue according to clinicopathologic variables
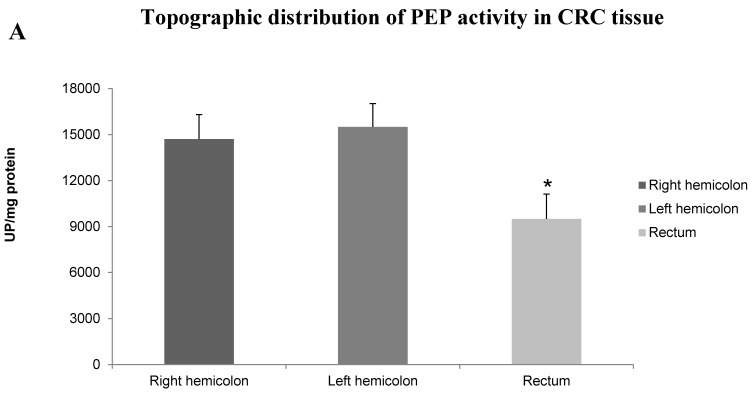
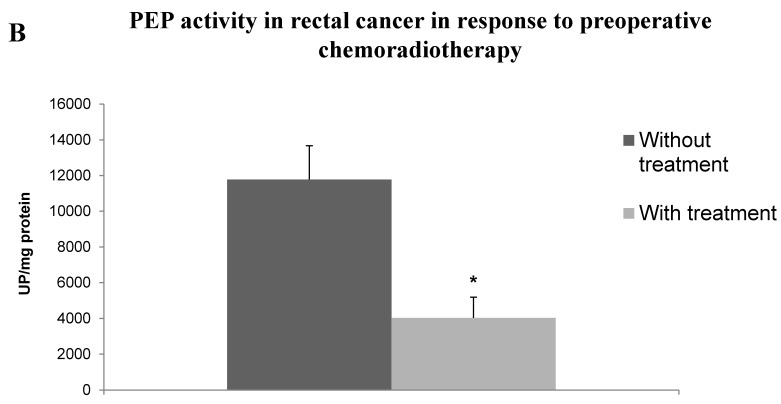
PEP activity was decreased in colorectal adenocarcinomas showing vascular invasion (Student t, p<0.05). Tumors with high Grade, advanced Stage, lymph node metastases, and lymphatic and perineurial invasion showed also a non-significant tendency to have decreased activity levels in this enzyme. Results are summarized in Table 1. Topographic distribution of tumors also displayed significant differences in PEP activity (Fig. 1A), with higher activity in tumors located in the colon than in the rectum (ANOVA, p<0.05; Scheffé test p<0.05). To elucidate if these significant differences between the two topographies, colon and rectum, were related with the chemo-radiotherapy regimes previously administered to some patients with rectal adenocarcinoma, we compared the enzyme activity in cases with and without this previous treatment (Fig. 1B). Interestingly, PEP activity was decreased threefold in the group who received chemo-radiotherapy (Student t, p<0.05).
Figure 1.
A. Topographic distribution of PEP activity in CRC tissue. Columns compare PEP activity of tumor tissues from right hemicolon (between cecum and the end of transverse colon), left hemicolon (between the splenic flexure and the end of sigmoid colon) and rectum. Values are means ± SE of peptidase activity recorded as pmol of units of peptidase (UP) per milligram of protein. One-way ANOVA p<0.05. (*) Scheffé Test, p<0.05, when comparing left hemicolon and rectum. B. PEP activity in rectal cancer in response to preoperative chemo-radiotherapy. Columns compare PEP activity in neoplastic tissue from rectal cancer patients without and with preoperative chemo-radiotherapy. Values are means ± SE of peptidase activity recorded as pmol of units of peptidase (UP) per milligram of protein. (*) Student's T test, p<0.05.
PEP activity in tissue according to overall and disease-free survival
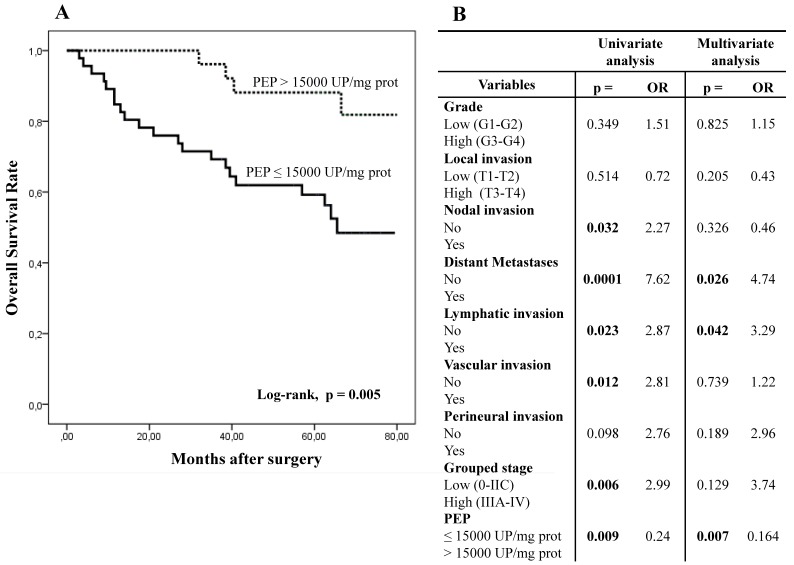
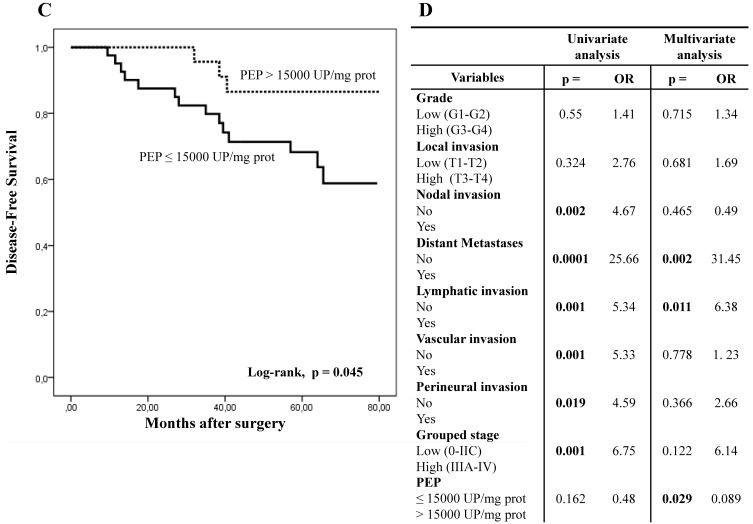
Five-year overall survival (OS) and disease-free survival (DFS) of patients with CRC was better when PEP activity was higher than 15,000 UP/mg of protein (Figs. 2A and 2C).
Figure 2.
Kaplan-Meier curves and Multivariate Analysis (Cox regression model) with PEP activity in CRC tissues. Overall survival (A) and disease-free survival (C) of 80 CRC patients according to their tumor PEP activity pattern. Multivariate analysis of clinicopathologic variables and tumor PEP activity in predicting overall survival (B) and disease-free survival (D) of patients with CRC.
Fig.2B and 2D show results from the Cox regression model. By univariate analysis, OS and DFS correlated with distant metastases, grouped stage and nodal and lymphatic, vascular. OS also correlated with PEP activity while DFS also correlated with perineural invasion. Multivariate analysis showed that PEP activity, distant metastases and lymphatic invasion were independent prognostic factors for OS and DFS.
PEP activity in plasma samples
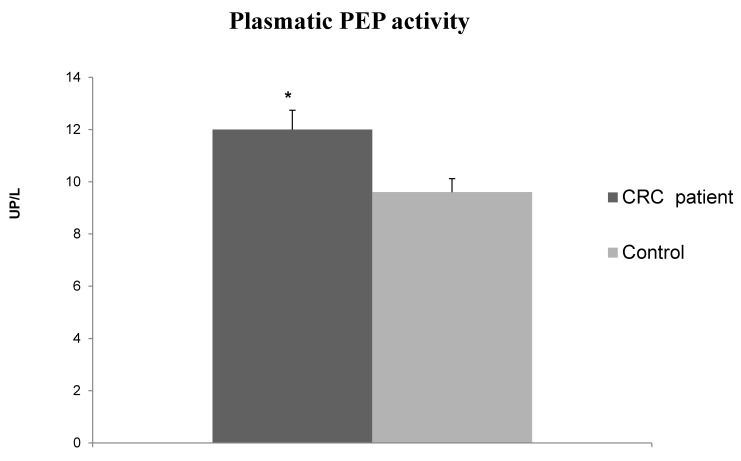
PEP activity in plasma samples of CRC patients was significantly higher than in healthy individuals (12 ± 0.74 UP/L vs. 9.6 ± 0.62 UP/L, Mann-Whitney test p<0.05) (Fig. 3). The stratification of data according to clinicopathological variables (Table 2), showed that CRC with distant metastases showed higher PEP activity than tumors without metastases.
Figure 3.
Plasmatic PEP activity in CRC patients and healthy subjects (controls). Values are means ± SE of units of peptidase per liter of plasma (UP/L). (*) Student's T test, p<0.05.
Spearman Rho test did not showed any significant correlation between plasmatic and tissue PEP activity (r = -0.278; p<0.1) and tumor size (r= -0.111; p<0.494).
PEP activity in plasma according to overall and disease-free survival
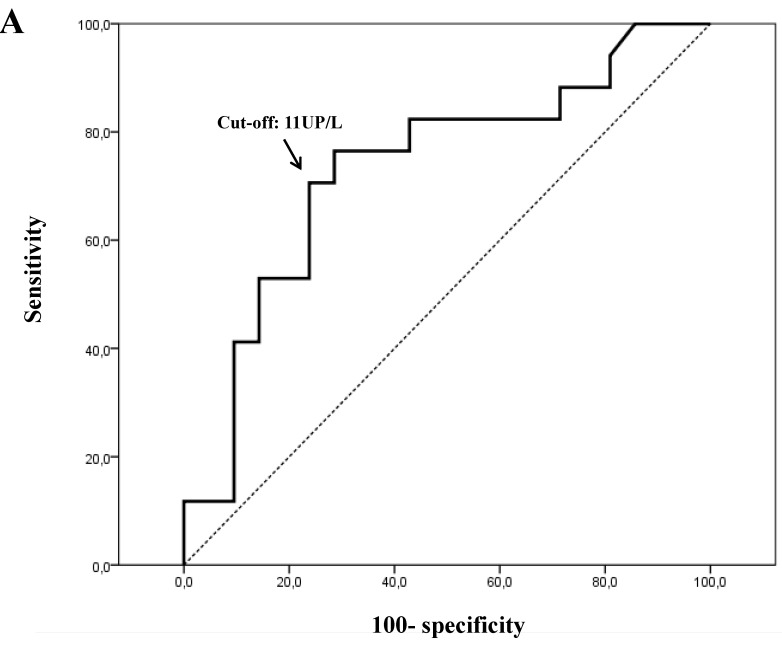
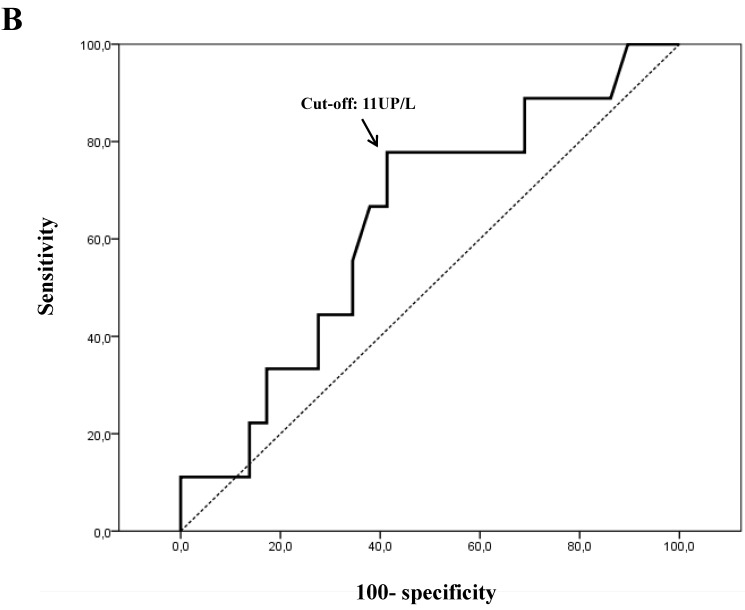
For plasmatic activity, the 11 UP/L cut-off value showed the most optimal sensitivity (Se) and specificity (Sp) ratios (Se=77% and Sp=72% for OS, and Se=78% and Sp=59% for DFS) (Figs. 4A and 4B).
Figure 4.
Receiver-operating characteristic (ROC) curves for plasmatic PEP activity. Optimal sensitivity and specificity ratios were observed using the following cut-off value: 11 UP/L, both for overall survival (A) and disease-free survival (B).
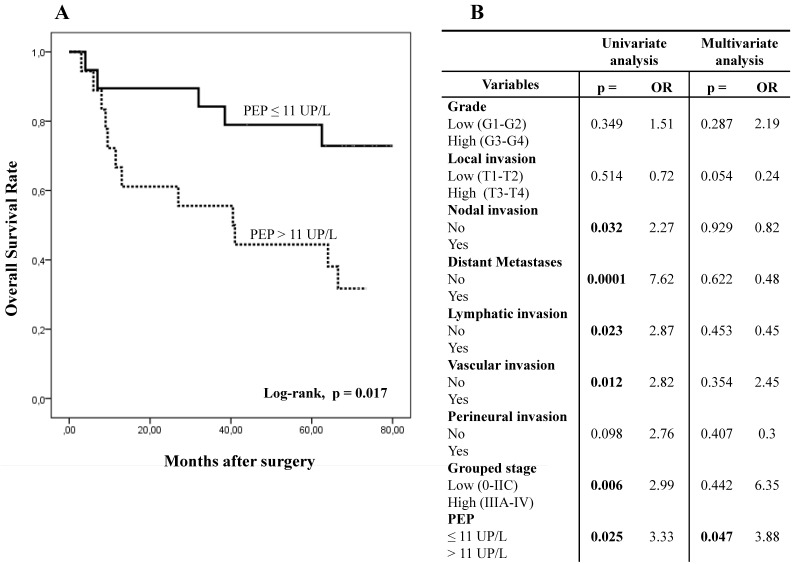
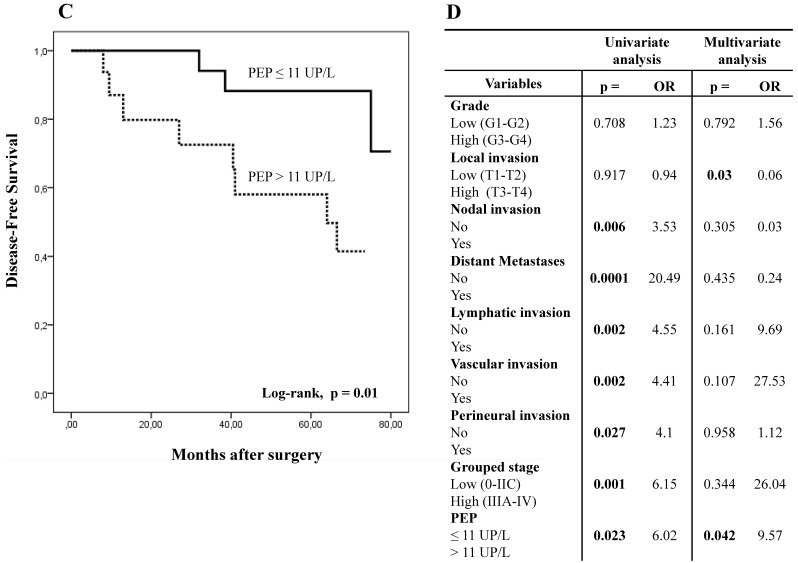
With this cut-off value, the analysis of PEP activity in plasma samples displayed opposite results to those obtained in tissue. Thus, when plasmatic PEP activity was higher than 11 UP/L the overall and disease-free survivals were significantly worse in Kaplan-Meier curves (Figs. 5A and 5C).
Figure 5.
Kaplan-Meier curves and Multivariate Analysis (Cox regression model) with PEP activity in plasma from CRC patients. Overall survival (A) and disease-free survival (C) of 40 CRC patients according to their plasmatic PEP activity pattern. Multivariate analysis of clinicopathologic variables and plasmatic PEP activity in predicting overall survival (B) and disease-free survival (D) of patients with CR.
Using univariate analysis (Cox regression model), OS and DFS correlated with plasmatic PEP activity, distant metastases, grouped stage and nodal, lymphatic and vascular invasion. DFS also correlated with perineural invasion. Multivariate analysis demonstrated that plasmatic PEP activity was an independent prognostic factor in OS and DFS (Figs. 5B and 5D).
Discussion
PEP is a serine peptidase involved in differentiation, development and carcinogenesis of several tissues 14, 16, 17, 19, 22, 24. Recent studies have demonstrated that PEP expression and activity increase in CRC 19, 24, thus suggesting the participation of this enzyme in the neoplastic transformation of the intestinal mucosa. However, there is no data supporting the impact of this peptidase in CRC prognosis. For this reason, we designed a prospective analysis of PEP activity in plasma and in tissue samples in a series of CRC patients aiming to define the role of this enzyme in the prognosis and survival of these patients.
Several studies have shown that peptidases may have a predictive value in the assessment of survival in patients with cancer 6-12. We have shown in this study that the quantification of PEP activity may be used as an additional prognostic parameter when evaluating life expectancy and clinical management of CRC patients.
The adenoma-carcinoma sequence in the large bowel describes that the gradual progression from normal to dysplastic epithelium, and hence to carcinoma, is the result of the successive accumulation of genetic mutations 29. We have previously shown that PEP activity shows a distinct pattern in this sequence 24. On one hand, PEP activity was significantly increased in colonic and rectal preneoplastic adenomatous lesions but, on the other, PEP did not significantly increase in CRC when compared with the uninvolved mucosa. These unexpected and apparently discordant findings have had support in the present study because CRC without blood vessel invasion showed significantly higher levels of PEP activity than CRC with invasion. A similar tendency has been observed in low grade, organ confined, and not invading nodal, lymphatic vessels and nerves CRC. In addition, 5-year overall and disease-free survival of patients were significantly better when PEP activity from CRC tissues was higher. All these findings taken together suggest that PEP activity in tissue may be a marker of early and/or less aggressive forms of the disease.
PEP activity levels in tissue samples varied depending on the topography. So, rectal carcinomas displayed a significantly decreased PEP expression compared with colonic adenocarcinomas. Some patients with rectal cancer received chemo-radiotherapy preoperatively for a better control of disease and in this subgroup of patients PEP activity was significantly decreased compared with those who did not receive this treatment. The sensitivity of PEP to chemo-radiotherapy shown in the present study has also been observed in other proteases like matrix metalloproteinases, and indicates that these enzymes could be of potential use in clinical practice to predict the response to preoperative chemo-radiotherapy 30, 31.
Membrane-bound and cytosolic peptidases may be secreted to the extracellular space and appear in diverse body fluids 6, 32. Several authors have demonstrated significant variations of circulating peptidases in cancer patients 7, 10, 14, 32-35, and this minimally invasive approach to analyze these enzymes has gained some acceptance to define the prognosis of the disease and to monitor the patient follow-up. For instance, the analysis of the serine peptidase DPPIV/CD26 in the plasma of patients with colorectal cancer has proved to be a reliable method in its early detection, being complementary to the classic fecal occult blood exam 7, 32-34.
We have detected in this study that PEP activity increases significantly in the plasma of CRC patients when compared with healthy individuals. What is more, patients with distant metastases and with poorer overall and disease-free survivals had even higher plasma PEP activities. This finding is exactly the opposite of that observed with PEP activity in tissue samples (the higher the worse in plasma and the higher the better in tissue).
The origin of circulating peptidases in patients with cancer is a controversial issue. The main hypothesis places its origin in the tumor cells 10,36, but other authors also point to the immune cells 32. From our data it could be inferred that, as the CRC progresses and becomes more aggressive, there is a release of the enzyme from the tumor microenvironment to the plasma. However, statistical analysis of correlation between tumor and plasma PEP activity, or between tumor size and plasma PEP, did not yielded significant results. This point might be helpful to validate these proteins as reliable markers of diagnosis and prognosis in cancer patients, and further studies are needed to clarify it.
PEP is a peptidase enabled to regulate several bioactive peptides 13. Assays in cell cultures and in animal models show that some of its natural substrates, like angiotensins and thymosin β4, are implicated in cell growth, angiogenesis and apoptosis 23, 37, 38. Some authors suggest that potentially coordinated actions of this enzyme with matrix metalloproteinases are at the base of some inflammatory conditions 39, and still others advocate that PEP regulates the angiogenesis and cell growth independently from its catalytic action 17,40,41. As stated in other peptidases 6, 42, these data suggest that PEP is a multifunctional protein. Therefore, the role of this enzyme in neoplasia most probably is the result of the sum of its different actions 17.
The high PEP expression and activity levels detected in urological, laryngeal, ovarian and thyroidal tumors point to a hypothetical stimulating role of this enzyme in cancer 14, 19, 22-24. However, it is largely debatable what is the meaning of PEP in early phases of CRC, or which is the meaning of PEP activity decreases in the aggressive behaviour of this neoplasm, because the lesser the PEP activity in tumor tissue the worse the patient survival. In this sense, some authors stress that peptidases may either promote or impede development depending on the specific type of tumor or on the phase of development the tumor is 6,43-45.
Phenomena of specificity set forth practical difficulties when applying peptidase inhibitors in cancer therapies 46, and this point is being actively investigated nowadays. A new trend in this topic consists in the use of cytotoxic prodrugs enabled to be activated by specific peptidases only in where these peptidases are more active or highly expressed, aiming to damage the neoplastic cell without modifying the enzyme functionality 46-48. Some investigators are working with prodrugs with the fibroblast activation protein (FAP) as a target 48. FAP is a cell-surface serine peptidase with an enzymatic activity similar to PEP that is expressed in early phases of CRC which has been proposed as a prognostic marker of this disease 35,49. Following the same argument, our results suggest that PEP could be the target of similar prodrugs in early phases of CRC in which this enzyme is more active 24.
In conclusion
PEP activity is an independent prognostic factor in the survival of patients with CRC.
PEP activity shows an opposite pattern in plasma and in tissue in CRC patients. So, poor survival rates had low PEP activity in the tissue and high activity in the plasma. The underlying reasons explaining this finding still remain unknown.
The determination of PEP activity in the plasma is a safe, minimally invasive and inexpensive way to define the aggressiveness of CRC in daily practice.
Further studies are needed to use PEP as a target in CRC therapy.
Acknowledgments
We wish to thank Arantza Pérez (UPV/EHU) for her technical contribution to this study and to Professor Juan Bilbao (UPV/EHU) for his statistical support. This work was supported by grants from the Basque Government (IT8-11/13 and S-PE12UN042), the University of the Basque Country UPV/EHU (UFI 11/44) and the Gangoiti Barrera Foundation.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract. 2012;27:613–23. doi: 10.1177/0884533612454885. [DOI] [PubMed] [Google Scholar]
- 4.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–30. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim EC, Lance P. Colorectal polyps and their relationship to cancer. Gastroenterol Clin North Am. 1997;26:1–17. doi: 10.1016/s0889-8553(05)70280-6. [DOI] [PubMed] [Google Scholar]
- 6.Carl-McGrath S, Lendeckel U, Ebert M. et al. Ectopeptidases in tumor biology: A review. Histol Histopathol. 2006;21:1339–53. doi: 10.14670/HH-21.1339. [DOI] [PubMed] [Google Scholar]
- 7.Cordero OJ, Ayude D, Nogueira M. et al. Preoperative serum CD26 levels: diagnostic efficiency and predictive value for colorectal cancer. Br J Cancer. 2000;80:1139–46. doi: 10.1054/bjoc.2000.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashida H, Takabayashi A, Kanai M. et al. Aminopeptidase N is involved in cell motility and angiogenesis: its clinical significance in human colon cancer. Gastroenterology. 2002;122:376–86. doi: 10.1053/gast.2002.31095. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda N, Nakajima Y, Tokuhara T. et al. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin Cancer Res. 2003;9:1503–08. [PubMed] [Google Scholar]
- 10.Murakami H, Yokoyama A, Kondo K. et al. Circulating aminopeptidase N/CD13 is an independent prognostic factor in patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:8674–79. doi: 10.1158/1078-0432.CCR-05-1005. [DOI] [PubMed] [Google Scholar]
- 11.Tokuhara T, Hattori N, Ishida H. et al. Clinical significance os aminopeptidase N in non-small cell lung cancer. Clin Cancer Res. 2006;12:3971–78. doi: 10.1158/1078-0432.CCR-06-0338. [DOI] [PubMed] [Google Scholar]
- 12.Larrinaga G, Blanco L, Sanz B. et al. The impact of peptidase activity in clear cell renal cell carcinoma survival. Am J Physiol Renal Physiol. 2012;303:F1584–91. doi: 10.1152/ajprenal.00477.2012. [DOI] [PubMed] [Google Scholar]
- 13.Szeltner Z, Polgár L. Structure, Function and Biological Relevance of Prolyl Oligopeptidase. Curr Protein Pept Sci. 2008;9:96–107. doi: 10.2174/138920308783565723. [DOI] [PubMed] [Google Scholar]
- 14.Goossens F, De Meester I, Vanhoof G. et al. Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur J Clin Chem Clin Biochem. 1996;34:17–22. doi: 10.1515/cclm.1996.34.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Irazusta J, Larrinaga G, González-Maeso J. et al. Distribution of prolyl endopeptidase activities in rat and human brain. Neurochem Int. 2002;40:337–45. doi: 10.1016/s0197-0186(01)00078-x. [DOI] [PubMed] [Google Scholar]
- 16.Tenorio-Laranga J, Venäläinen JI, Männistö PT. et al. Characterization of membrane-bound prolyl endopeptidase from brain. FEBS J. 2008;275:4415–27. doi: 10.1111/j.1742-4658.2008.06587.x. [DOI] [PubMed] [Google Scholar]
- 17.García-Hornsman JA, Männistö PT, Venäläinen JI. On the role of prolyl oligopeptidase in health and disease. Neuropeptides. 2007;41:1–24. doi: 10.1016/j.npep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Myöhänen TT, Venäläinen JI, García-Hornsman JA. et al. Distribution of prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues. Histochem Cell Biol. 2008;130:993–1003. doi: 10.1007/s00418-008-0468-x. [DOI] [PubMed] [Google Scholar]
- 19.Myöhänen TT, Pyykkö E, Männistö PT. et al. Distribution of prolyl oligopeptidase in human peripheral tissues and in ovarian and colorectal s. J Histochem Cytochem. 2012;60:706–15. doi: 10.1369/0022155412453051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agirregoitia N, Casis L, Gil J. et al. Ontogeny of prolyl endopeptidase and pyroglutamyl peptidase I in rat tissues. Regul Pept. 2007;139:52–8. doi: 10.1016/j.regpep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Moreno-Baylach MJ, Felipo V, Männisto PT. et al. Expression and traffic of cellular prolyl oligopeptidase are regulated during cerebellar granule cell differentiation, maturation, and aging. Neuroscience. 2008;156:580–5. doi: 10.1016/j.neuroscience.2008.06.072. [DOI] [PubMed] [Google Scholar]
- 22.Sedo A, Krepela E, Kasafírek E. Dipeptidil peptidase IV, prolyl endopeptidase and cathepsin B activities in primary human lung tumors and lung parenchyma. J Cancer Res Clin Oncol. 1991;117:249–53. doi: 10.1007/BF01625433. [DOI] [PubMed] [Google Scholar]
- 23.Liu JL, Kusinski M, Ilic V. et al. Overexpression of the angiogenin tetrapeptide AcSDKP in human malignant s. Anticancer Res. 2008;28:2813–18. [PubMed] [Google Scholar]
- 24.Larrinaga G, Perez I, Blanco L. et al. Increased prolyl endopeptidase activity in human neoplasia. Regul Pept. 2010;163:102–106. doi: 10.1016/j.regpep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Edge SB, Byrd DR, Compton CC, AJCC Cancer Staging Manual, 7th Edition. New York: Springer; 2010. [Google Scholar]
- 26.Washington MK, Berlin J, Branton P. et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–51. doi: 10.5858/133.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivo RA, Pereira Teixeira PT, Silveira PF. Representative aminopeptidases and prolyl endopeptidase from murine macrophages: comparative activity levels in resident and elicited cells. Biochem Pharmacol. 2005;69:1441–50. doi: 10.1016/j.bcp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Leslie A, Carey FA, Pratt NR. et al. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845–60. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 30.Unsal Kilic D, Uner A, Akyurek N. et al. Matrix metalloproteinase-9 expression correlated with response in patients with locally advanced rectal cancer undergoing preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:196–203. doi: 10.1016/j.ijrobp.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Nishioka M, Shimada M, Kurita N. et al. Gene expression profile can predict pathological response to preoperative chemoradiotherapy in rectal cancer. Cancer Genomics Proteomics. 2011;8:87–92. [PubMed] [Google Scholar]
- 32.Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother. 2009;58:1723–47. doi: 10.1007/s00262-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Chiara L, Rodríguez-Piñeiro AM, Rodríguez-Berrocal FJ. et al. Serum CD26 is related to histopathological polyp traits and behaves as a marker for colorectal cancer and advanced adenomas. BMC Cancer. 2010;10:333. doi: 10.1186/1471-2407-10-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bünger S, Haug U, Kelly M. et al. A novel multiplex-protein array for serum diagnostics of colon cancer: a case-control study. BMC Cancer. 2012;12:393. doi: 10.1186/1471-2407-12-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Javidroozi M, Zucker S, Chen WT. Plasma seprase and DPP4 levels as markers of disease and prognosis in cancer. Dis Markers. 2012;32:309–20. doi: 10.3233/DMA-2011-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Hensbergen Y, Broxterman HJ, Hanemaaijer R. et al. Soluble aminopeptidase N/CD13 in malignant and nonmalignant effusions and intraal fluid. Clin Cancer Res. 2002;8:3747–54. [PubMed] [Google Scholar]
- 37.Liu JM, Bignon J, Ilic V. et al. Evidence for an association of high levels of endogenous Acetyl-Ser-Asp-Lys-Pro, a potent mediator of angiogenesis, with acute myeloid leukemia development. Leuk Lymphoma. 2006;47:1915–20. doi: 10.1080/10428190600688131. [DOI] [PubMed] [Google Scholar]
- 38.Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29:1675–84. doi: 10.1093/carcin/bgn171. [DOI] [PubMed] [Google Scholar]
- 39.Gaggar A, Jackson PL, Noerager BD. et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–9. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno-Baylach MJ, Puttonen KA, Tenorio-Laranga J. et al. Prolyl endopeptidase is involved in cellular signalling in human neuroblastoma SH-SY5Y cells. Neurosignals. 2011;19:97–109. doi: 10.1159/000326342. [DOI] [PubMed] [Google Scholar]
- 41.Myöhänen TT, Tenorio-Laranga J, Jokinen B. et al. Prolyl oligopeptidase induces angiogenesis both in vitro and in vivo in a novel regulatory manner. Br J Pharmacol. 2011;163:1666–78. doi: 10.1111/j.1476-5381.2010.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol Med. 2008;14:361–71. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ino K, Shibata K, Kajiyama H. et al. Regulatory role of membrane-bound peptidases in the progression of gynecologic malignancies. Biol Chem. 2004;385:683–90. doi: 10.1515/BC.2004.084. [DOI] [PubMed] [Google Scholar]
- 44.Kikkawa F, Kajiyama H, Shibata K. et al. Dipeptidyl peptidase IV in progression. Biochim Biophys Acta. 2005;1751:45–51. doi: 10.1016/j.bbapap.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Varona A, Blanco L, Perez I. et al. Expression and activity profiles of DPPIV/CD26 and NEP/CD10 glycoproteins in the human renal cancer are -type dependent. BMC Cancer. 2010;10:193. doi: 10.1186/1471-2407-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–66. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wickström M, Larsson R, Nygren P. et al. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011;102:501–08. doi: 10.1111/j.1349-7006.2010.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brennen WN, Rosen DM, Wang H. et al. Targeting carcinoma-associated fibroblasts within the stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012;104:1320–34. doi: 10.1093/jnci/djs336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry LR, Lee HO, Lee JS. et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–41. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]