Abstract
Inactivation of Cdx2 leads to preimplantation embryonic lethality. Rescue of the implantation defect by tetraploid fusion established that Cdx2 is necessary for trophoblastic development, vasculogenesis in the yolk sac mesoderm, allantoic growth, and chorioallantoic fusion. “Rescued” Cdx2 mutants die at late gastrulation stages because of failure of placental development. Cdx2 is also needed for the completion of the normal process of gastrulation and tail bud elongation. Presegmental paraxial mesoderm is severely restricted in amount and somites posterior to somite 5 are abnormal. The Cdx2 mutation, like mutations impairing Wnt and Fgf signaling, causes posterior truncations and disturbs axial patterning of the embryonic structures, indicated by changes in the Hox expression domains. The gene appears to be important in the integration of the pathways controlling embryonic axial elongation, and anterior-posterior patterning.
The Drosophila homeotic gene caudal (cad) determines the fly's most posterior body segment (1). The three mouse cad homologues, Cdx1, Cdx2 and Cdx4, (2-4) have developmental roles with overlap of expression patterns.
Cdx2 is expressed (5) at 3.5 days postcoitum (dpc) in the trophectoderm but not in the inner cell mass. At 7.5 dpc (Theiler stage 11), it is present in the chorion, ectoplacental cone, mesoderm of the developing allantoic bud, and posterior primitive streak. At 8.5 dpc (Theiler stage 13), expression is seen in all three germ layers at the posterior end of the embryo extending into the mesodermal root of the allantois, in the endodermal epithelium of the hindgut rudiment, and in the neural tube; the presegmented paraxial mesoderm expresses the gene, but the somites, lateral plate, and intermediate mesoderm do not. By 9.5 dpc (Theiler stage 15), the caudal pole of the embryo remains positive, as does the posterior neural tube and posterior gut endoderm. At 12.5 dpc (Theiler stage 20), expression is confined exclusively to the gut endoderm posterior to the foregut/midgut junction (5). Cdx1 (6) is not expressed in the trophectoderm, but is expressed along the primitive streak at 7.5 dpc. It is demonstrable in the neural tube and somitic and lateral plate mesoderm, but not the early definitive endoderm. Cdx4 (3) appears at 7.5 dpc in the posterior part of the primitive streak and allantois and persists until 10 dpc. It has been identified in posterior neurectoderm, presomite and lateral plate mesoderm, and hindgut endoderm. There is no information concerning expression at later stages.
In 8.5 dpc (Theiler stage 13) embryos the appearance is of a nested expression of the three Cdx genes in both the neural tube and mesoderm, suggesting a possible role in patterning the entire anterior-posterior axis. Cdx1 expression extends most anteriorly, followed by Cdx2 and Cdx4, respectively, with all three genes expressed posteriorly.
Cdx2-null mutant embryos die between 3.5 and 5.5 dpc, and heterozygotes have tail abnormalities and exhibit anterior homeotic shifts involving the cervical and upper thoracic vertebrae, ribs, and midgut endoderm (7). Subramanian et al. (8) reported similar homeosis in the anterior regions of the cervical spine in homozygous Cdx1 mutants, but without an apparent gut phenotype.
We used Cdx2-/- embryonic stem (ES) cells derived from heterozygote intercrossing to “rescue” the Cdx2-/- phenotype by using tetraploid aggregation. Cdx2-null mutants developed to the point when a chorio-allantoic placenta normally begins to function, then died because of underdevelopment of the allantois, which failed to fuse with the chorion. The mutant embryos were grossly retarded posteriorly, showing a truncated body and a deficient yolk-sac circulation. Altered expression patterns of cognate genes involved in controlling developmental processes in these mutants showed that Cdx2 is important in the constellation of genes essential for posterior tissue generation and axial patterning during gastrulation.
Materials and Methods
Tetraploid Aggregation. Morulae from Cdx2 heterozygote intercrossess were aggregated with 160 tetraploid embryos generated by electrofusion at the two-cell stage. This technique has been used to rescue lethal mouse mutants caused by defective extraembryonic tissues (9). We show that it overcomes implantation block caused by defective trophoblast development. Tetraploid embryos were derived from two mouse strains (253 and B5/EGFP) expressing HMG-CoA-LacZ (10) and β-actin-CMV-EGFP (11) transgenes, respectively.
To increase numbers, tetraploid embryos were also aggregated with Cdx2-null ES cells to generate completely ES cell-derived embryos (12). Four Cdx2-null mutant ES cell lines were isolated from blastocysts of intercrossed Cdx2+/- heterozygotes of mixed background (129SV × C57BL/6J) using the protocol of Abbondanzo et al. (13). Homozygous mutant blastocysts did not form trophoblastic outgrowths, but development of the inner cell mass (ICM) made it possible to pick the colonies and culture ES cell lines. The tetraploid cells (marked by LacZ staining) did not contribute significantly to embryonic tissues, but they developed normally along the trophoblastic lineage.
Whole-Mount in Situ Hybridization. Whole-mount in situ hybridization was performed essentially as described (14) using probes for Cdx1 (6), Cdx4 (3), Hoxb1 (15), Hoxb8 (16), Hoxd4 (17), Wnt3a (18), Brachyury T (19), Mox1 (20), Paraxis (21), Tbx6 (22), mShh (23), Fgf8 (24), and Hes5 (25).
Whole-Mount Immunohistochemistry. Material was fixed overnight in 4% paraformaldehyde and stained with rat anti-PECAM-1 antibody (clone MEC13.3, Pharmingen). Anti-rat IgG horseradish peroxidase (Biosource) was the secondary antibody.
Results
Cdx2-/- Blastocysts Do Not Form Trophoblastic Outgrowths: Postimplantation Development Can Be Rescued by Aggregation with Wild-Type Tetraploid Embryos. Homozygous null mutant embryos do not implant in vivo (7) and when explanted for the purpose of isolating Cdx2-/- ES cells, did not form trophoblastic outgrowths, indicating that the implantation defect is associated with defective trophoblastic development. We have taken advantage of the rescue of the trophoblastic defect to study the role of Cdx2 in later embryonic development. Embryos produced by aggregation of diploid morulae from heterozygote intercrosses with wild-type tetraploid morulae were transferred to pseudopregnant females, which were killed at 7 days of nominal embryonic development. Forty-six resulting embryos were photographed and genotyped by using the PCR primers described in ref. 7. Of the 46 embryos, 19 were wild type, 20 were heterozygotes, and 7 were homozygous mutants (Fig. 4, which is published as supporting information on the PNAS web site). The null mutant/tetraploid embryo aggregates implanted in a similar manner to the wild-type and heterozygote aggregates. At 7 days, the embryos from null mutants were viable and appeared morphologically normal (Fig. 4), having reached Theiler stage 10 of development with clear evidence of mesoderm formation posteriorly. These results indicate that failure to implant in Cdx2-/- embryos is not the result of failure of the inner cell mass, as suggested by Tamai et al. (26)
Because no gross defects were observed at a nominal age of 7 dpc, we next examined null mutants at later developmental stages.
Phenotype of Cdx2-Null Mutants. For these experiments `rescue' was performed by tetraploid aggregation with null mutant ES cells. Cdx2-/- embryos were generated by using two lines of mutant ES cells both of which yielded similar phenotypes. Tetraploid aggregation controls, generated by using wild-type ES cells did not exhibit any morphological abnormalities.
Mutant embryos only survived to a nominal age of 11.5 days, the time at which a functional chorio-allantoic placenta becomes the principal organ of maintenance. However, the developmental stage reached by the most advanced mutant embryos was 36-48 h behind their nominal age, whereas tetraploid aggregation controls were up to 24 h less developed than their nominal age.
Defects were first apparent at the late gastrulation/early somite stages at a nominal age of 8.5 dpc. At ≈7.5 dpc, the allantois of normal embryos forms as a bud of extraembryonic mesoderm continuous with the posterior end of the primitive streak. Allantoic growth up to the three-somite stage is effected by the addition of mesoderm from the posterior primitive streak. As the bud extends into the exocoelomic cavity, its outer cells differentiate into a mesothelium, making the tip of the allantois competent for fusion with the chorion. The allantois of rescued null mutants at 8.5 dpc was severely underdeveloped and never extended enough to fuse with the chorion, thus preventing the formation of a functional chorio-allantoic placenta (Fig. 1 A and B and Fig. 5 A and B, which is published as supporting information on the PNAS web site). Almost all mutants developed to between 15 and 17 somites (Theiler, stage14), and in every case development of the allantois was rudimentary.
Fig. 1.
Section through the placentae of mutant (A) and wild-type (B) mice at a nominal age of 11.5 dpc created by injection of ES cells into tetraploid wild-type blastocysts bearing a LacZ transgene. The allantoic component of the wild-type placenta is clearly seen (arrow) but is absent from the mutant specimen. YS, yolk sac; D, maternal decidua. The section was stained for β-galactosidase and with hematoxylin and eosin. (Bar = 100 μmin A and 125 μmin B.) The diffuse blue staining on the fetal side of the mutant placenta is an artefact due to leaching of β-galactosidase stain from the trophoblastic nuclei into the surrounding cytoplasm. The tetraploid nuclei are dark due to β-galactosidase staining, whereas those of the allantoic mesoderm and of the decidua (D) of maternal host origin do not have LacZ activity.
The yolk sac circulation was abnormal in null mutant embryos (Fig. 5 C and D). At a nominal age of 8.5 dpc, the fine honeycomb appearance of vessels seen in wild-type embryos (Fig. 2C) was replaced by a coarse plexus of enlarged channels, suggesting failure in vascular remodelling in the yolk sac (Fig. 2D). In the more mature embryos, the circulation was sluggish and the pericardial cavity was usually dilated.
Fig. 2.
Wild-type (A) and Cdx2-/- (B) mouse embryos at a nominal age of 10.5 dpc. The rostral region of the mutant is normal, though somewhat retarded in development when compared with the wild-type embryo. Caudally there is gross truncation distal to the forelimb bud (marked) in the mutant embryo. Arrows mark the tail tip in both mutant and wild-type. (Bar = 250 μmin A and 225 μmin B.) (C and D) An 8.5-dpc yolk sac from wild-type (C) and mutant (D) embryos immunologically stained with a PECAM-1 antibody. A coarse plexus of enlarged vessels is visible in the mutant (D), compared with the fine honeycomb appearance in the control (C). (Bar = 500 μm.) (E-J) Expression of Hox and Cdx genes in wild-type and mutant embryos. Whole-mount in situ hybridization of Hoxb1 (E) and Hoxd4 (F) transcripts at the five-somite stage in control and Cdx2-/- embryos. Note, in the Hoxd4 hybridized mutant embryo, the reduced amount of unsegmented mesoderm, the last somite being close to the anterior part of the streak. Expression of the more 5′ Hox gene, Hoxb8 (G and H) shows a posterior shift of the anterior boundary of the expression domain in the mesoderm (S13 instead of S11) and in the neural tube (level of S7 instead of S5) in the mutant. Expression levels of Cdx1 (I) appear unchanged in Cdx2 mutants at the early somite stage, whereas the expression level of Cdx4 (J) is much reduced compared to wild-type embryos. (Bar = 200 μm.) ov, optic vesicle; r, rhombomere; nsm, nonsegmented mesoderm; Ps, primitive streak; S, somite.
In embryos that survived to day 10 or 11, the cranial region developed normally, but the posterior region was grossly curtailed. The hind limb bud was not developed and the maximum number of somites was 17 (Figs. 2 A and B and 5 A and B). From the fifth onwards, the somites became smaller and irregularly shaped compared with controls at the same stage of brain development. At this level, the neural tube often became irregular.
The oldest mutant embryos, which according to other developmental landmarks should have 20+ (Theiler stage 14/15) but only had 17 somites, showed that many were beginning to exhibit widespread diffuse necrosis probably due to the disturbance of extraembryonic membrane function. Some embryos, however, were not yet moribund, and in these the phenotype was constant. They were truncated posteriorly beyond the forelimb bud, but the neural tube, notochord, and endodermal gut tube extended well toward the posterior tip. There was evidence of cell death in the posterior gut lining and in the surrounding splanchnopleuric mesoderm (Fig. 6, which is published as supporting information on the PNAS web site) and more general mesenchymal death toward the posterior tip of the specimen. The neural tube, notochord, and somites were reasonably well preserved. Somites extended to almost the posterior end of the embryo, and unsegmented paraxial mesoderm was hardly present.
Mesoderm and Neurectoderm Are Properly Specified in Cdx2 Null Embryos but Are Grossly Deficient Posteriorly. Transcript distribution of various genes normally expressed in mesoderm and neurectoderm was investigated by whole mount in situ hybridization starting at stages preceding the inception of externally manifest abnormalities (Theiler stage 11, head fold stages).
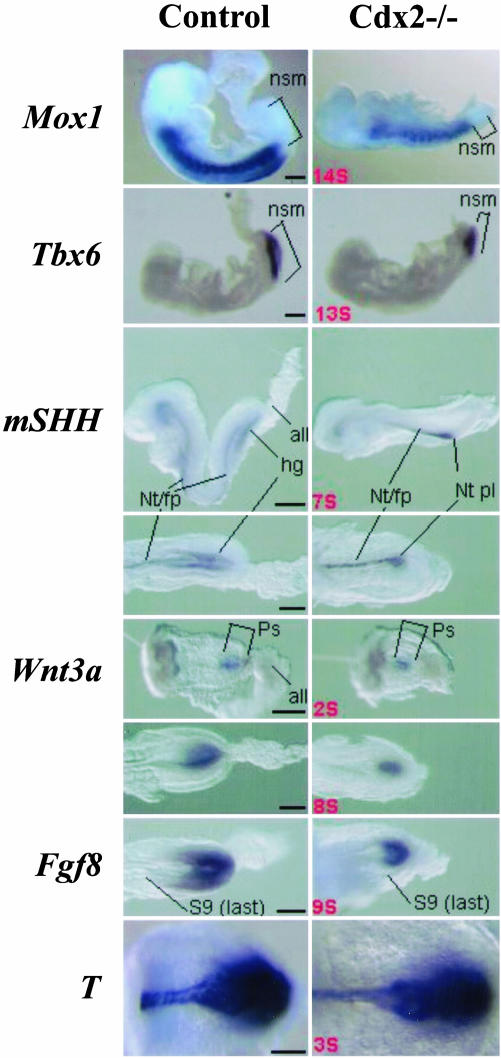
Mox1 and Paraxis are markers of somitic mesoderm. Their expression levels at Theiler stages 13 and 14 (≈10 and 15 somites) was weaker in mutants and confirmed observations that somites caudal to somite 5 are smaller and irregular in mutants (Mox1, Fig. 3; Paraxis, not shown). Because these somite abnormalities and the arrest of axial development at ≈15 somites are rostral to tissues derived from the tail bud, it seems that somites anterior to and preceding in time of development those produced by axial extension of tail bud origin are also affected in mutant embryos. Mox1-labeled somites were apparent to the caudal extremity of the paraxial mesoderm in mutant embryos, indicating a reduction in amount of presomitic mesoderm.
Fig. 3.
Expression of mesoderm, neurectoderm, and endoderm markers, and of Wnt and Fgf signals in Cdx2 mutant embryos and controls. Mox1 marks segmented paraxial mesoderm; note the important reduction in the amount of unsegmented mesoderm in the caudal aspect of the 14-somite Cdx2-/- embryo. The expression domain of Tbx6, marking nonsegmented mesoderm, is reduced in Cdx2-/- compared with controls. This pattern is complementary to that of Mox1. Shh is a marker of midline structures and of hindgut endoderm; a lateral view above, and a ventral view below both show delayed hindgut diverticulum formation in the mutant leading to an absence of Shh endodermal signal posteriorly in Cdx2-/- embryos. Wnt3a was assayed at a stage earlier (two somites, above), or later (eight somite embryos, below) than the first manifestation of an altered phenotype in Cdx2 mutant embryos. Expression of Fgf8 is shown in a nine-somite mutant and a control. Brachyury T was expressed in the posterior mesoderm of both mutant and control at the three- and two-somite stage, respectively. all, allantois; nsm, nonsegmented mesoderm; ps, primitive streak; Nt/fp, Notochord, floorplate; Nt pl, notochordal plate; hg, hindgut; S9, somite 9, the last formed somite. (Bar = 200 μm.)
Tbx6 is expressed in the primitive streak and nascent paraxial mesoderm, and is down-regulated at somite formation (22). Expression of Tbx6 in mutants is reduced antero-posteriorly and is complementary to that of Mox1, in agreement with the reduction in the amount of unsegmented paraxial mesoderm (Fig. 3).
The early neural marker Hes5 labeled the central nervous system to the caudal end of the spinal cord. No ectopic Hes5 was detected in mutants (not shown), indicating that, in contrast to other caudally truncated phenotypes such as T, Wnt3a, FgfrI, and CYP26 mutants (27-30), Cdx2 null mutants do not exhibit ectopic neural tube development.
Endodermal Development in Rescued Cdx2-Null Embryos. Posterior underdevelopment of mutant embryos is associated with delayed appearance of the hindgut diverticulum from four-somite (Theiler stage 12) to eight-somite (Theiler stage 13) stages. However, a distinct hindgut does eventually form (Figs. 6 and 7, which are published as supporting information on the PNAS web site). Sonic hedgehog (Shh) is endodermally expressed at the earliest stages of hindgut development (31), and mutants at seven somites (Theiler stage 12), which had not yet initiated hindgut invagination, did not exhibit Shh expressing endoderm in the caudal region, compared to control specimens (Fig. 3). By contrast, Shh was expressed in the notochord and floor plate of both mutants and controls (Fig. 3 and data not shown). The territories of Hoxd11/13 expression are not generated in Cdx2 mutants, and we cannot therefore draw definitive conclusions concerning the effect of Cdx2 on expression of these genes.
Disturbance of Posterior Elongation at Late Primitive Streak Stages. Signaling by Wnt and Fgf genes, and of the T box-containing transcription factors Tbx6 and Brachyury (T) are essential for posterior axial elongation at late primitive streak and tail bud stages (27, 32-34)
Wnt3a-null mutants exhibit a posteriorly truncated phenotype similar to Cdx2 null embryos (35). Wnt3a is similarly expressed in the primitive streak region of both controls and mutants at the beginning of somite formation (Fig. 3). Later, a decrease in the antero-posterior extent of the Wnt3a expression domain at Theiler stage 13 (seven to nine somites) (Fig. 3) reflects reduced posterior development of the mutants. We conclude that Cdx2-null mutation does not prevent early Wnt3a expression and that Cdx2 is not required upstream of Wnt3a.
FgfR1 (the principal Fgf8 receptor) hypomorph mutations also lead to posteriorly truncated embryos (36). Fgf8 is expressed in the unsegmented posterior region of the paraxial mesoderm of wild-type embryos, and Fgf signaling has an important role in convergence extension movements and exit of paraxial mesodermal cells from the primitive streak (28, 32). Expression of Fgf8 in early somite Cdx2-null embryos is unaltered compared to controls, but at the seven- to nine-somite stage (Fig. 3) it is less extended antero-posteriorly in mutants, reflecting reduced posterior development but indicating that Cdx2 is not required for Fgf8 expression.
Brachyury (T) is down-regulated in the paraxial mesoderm precursor cells of Wnt3a mutants (27) and a feedback loop may exist between Wnt and T (reviewed in ref. 33). Although an apparently normal notochord develops in Cdx2-null embryos, the phenotype has much in common with that of Brachyury embryos. At early stages (between one and four somites, Theiler, early stage 12), T expression in the primitive streak and notochord of wild-type and mutant embryos was similar, although it seemed to be less widely extended laterally in the posterior part of its expression domain (Fig. 3). Importantly, specimens stained for T showed that anterior-posterior patterning at these early stages was similar in wild-type, Cdx2+/-, and Cdx2-/- embryos (Fig. 8, which is published as supporting information on the PNAS web site). On comparing T expression in 10 somite Cdx2-null mutants with 10- and 5-somite wild-type controls the posterior T expression domain in mutants was again restricted laterally (data not shown). In sections posterior to the node, neurectoderm, and mesoderm were considerably less abundant in the mutant (thus confirming the findings obtained by inspection of whole mounts) and hindgut invagination was rudimentary in 10 somite mutants, whereas hindgut was present in 5- and 10-somite controls.
Effect of Cdx2 Mutation on Hox and Other Cdx Genes. Homeotic shifts in the skeletons of Cdx2 heterozygotes (7), potentiation of this effect when in combination with the Cdx1 mutation (37), and the reported regulatory activity of Cdx proteins on Hox gene expression (8, 38) suggested that Cdx2-null mutants undergo changes in the expression patterns of Hox genes.
Expression of Hoxb1 at 11 somites (Theiler stage 13) showed normal anterior distribution in the neural tube, reaching the rhombomere 3/4 boundary in the neurectoderm (Fig. 2E) and a mesodermal level rostral to the first somite (Fig. 2E), though these rostral expression boundaries were somewhat fuzzy in the mesoderm in both mutants and controls. A reduced level of expression of Hoxb1 and Hoxd4 was apparent caudally in mutants (Fig. 2F; data not shown for Hoxb1). This contrasts with the strong notochordal expression of T seen at this level (see above), and therefore does not appear to be the result of a general decrease in caudal gene expression.
In the most advanced Cdx2-/- mutant embryos (equivalent to ≥20 somites, Theiler stage 15) anterior Hoxb8 expression boundaries were shifted posteriorly from somite 11 to 13 in the mesoderm and from the level of somite 5 to 7 in the neurectoderm (Fig. 2 G and H).
Cdx genes are subject to feedback inhibition (39), and cross-inhibitory regulation has been shown to occur. Thus, overexpression of Cdx2 down-regulates expression of Cdx1 in vitro (40) and Cdx2 has been shown to inhibit β-catenin stimulated expression of Cdx1 in human colon cancer cell lines (41). We therefore examined expression levels of the other two Cdx genes in Cdx2-/- embryos. Expression was monitored at 7-10 somites (Theiler stages 12 and 13). Cdx1 levels were not significantly altered in the trunk and along the primitive streak, although poor posterior development resulted in reduction posteriorly (Fig. 2I). The anterior extent of expression appeared unchanged, but the rostral boundaries were somewhat fuzzy (Fig. 2I), making the anterior limit of expression difficult to localize.
Unlike many of the cognate genes examined, near complete loss of Cdx4 expression (Fig. 2 J) is not caused by posterior truncation of the embryo and represented a significant finding. Taken together with the nested expression pattern of the Cdx genes described above at 8.5 dpc, there is a strong possibility that direct cross-regulatory activity exists between Cdx2 and Cdx4.
Discussion
Cdx2 Belongs to the Gene Network Playing a Pivotal Role in Embryonic Axial Elongation. Early gastrulation in rescued homozygous Cdx2 mutants takes place normally, but disturbances appear during the later development of the primitive streak. The first defects seen involve abnormalities of extraembryonic mesodermal development. Deficient allantoic outgrowth prevents fusion with the chorion so that a chorio-allantoic placenta fails to form. Furthermore, abnormal yolk sac vasculogenesis may be caused by defective extra-embryonic mesoderm produced by the posterior part of the streak. The longest surviving Cdx2-/- embryos show failure in extension of the body axis beyond forelimb levels (about somite 17) and an alteration in somite morphology posterior to somite 5. The observation that the first five somites are unaffected accords with the fact that expression of Cdx2, spreading from posterior streak regions, only reaches the node region at late head fold stages (Fig. 9, which is published as supporting information on the PNAS web site), when cells making up the first five somites have left the node region (42). This is also consistent with the finding that Wnt3a and Fgf8 expressions are only affected after the completion of the early, presomite stages of development in mutants and specifically in areas that normally express Cdx2 in wild types.
It has been reported that cad homologues are downstream of Fgf in numerous vertebrate species (43-45), and our observations suggest that Cdx2 operates as a transducer of Fgf and Wnt signals in all germ layers. However, the existing interaction between the Wnt and Fgf signaling cascades (32, 46), and the possible feedback loops between the genetic steps involved, complicate the elucidation of the hierarchical cascades impaired by the Cdx2 mutation.
The Cdx2-/- phenotype is somewhat similar to Brachyury (T), suggesting that both are involved (perhaps cooperatively) in controlling mesoderm formation at gastrulation, downstream of Fgf and Wnt signaling. However, the Cdx2-null mutant differs from T, Tbx6, Wnt3a, and Fgfr1 mutants in several respects. First, in all of the mutants exhibiting caudal truncations so far described, the posterior paraxial mesoderm is partially transformed into ectopic neural tissue (27, 29, 30, 32, 47), and no such structures were detected in Cdx2-null embryos. Thus mesoderm specification is not disturbed in the Cdx2-null mutants. Second, the gene seems to be necessary for processes originating not only from the more anterior part of the streak (paraxial mesoderm, neurectoderm, endoderm), but also from derivatives of the posterior part of the streak (extraembryonic mesoderm), which is unaffected in Wnt3a and FgfR1 mutants but is compromised in T mutants. Wnt3a, FgfR1, and Cdx2 mutants are not impaired in axial mesoderm generation for, unlike Brachyury T, they generate a normal notochord. This indicates that there is some degree of specificity in the involvement of each of the participants in the constellation orchestrating posterior morphogenesis and patterning.
Cdx2 Function and Axial/Paraxial Progenitors in Caudalmost Regions of Mouse Embryos. Posterior morphogenesis and balanced tissue generation making up the trunk and tail depend on the integrated activity of the Fgf and Wnt pathways and on the subsequent activity of T-box proteins. Our observations indicate that Cdx2 is also centrally involved. Dubrulle et al. (48) and Vasiliaukas and Stern (49) suggest that high Fgf signaling around the anterior part of the primitive streak and anterior to the node maintains a population of self-renewing immature (nonsegmented) paraxial mesoderm cells. High Fgf signaling has also been shown to maintain an immature population of neural progenitors in a stem cell-like state in the growth region of the caudal neural plate (50). A feature accompanying posterior truncation in the Cdx2-null mutant phenotype is the reduced amount of unsegmented paraxial mesoderm present at late gastrulation. At Theiler stage 13, somites are present as far posteriorly as the node, whereas in wild-type controls a region of unsegmented mesoderm equivalent to seven somites is present rostral to the node (51).
The reduction in the amount of newly formed nonsegmented paraxial mesoderm, and the slowing down and arrest of mesoderm and neurectoderm formation in the posterior region of Cdx2-null mutants suggests a role for Cdx2 in the mechanism of tissue generation. Because formation of endoderm also seems to be affected in posterior development of Cdx2 mutant embryos, all three germ layers are posteriorly truncated “in register.” Cdx2 might thus be necessary for continued maintenance of proliferation at and around the anterior primitive streak, regulating tissue production as the axis elongates.
Cdx Genes and Integration of Antero-Posterior Patterning and Caudal Morphogenesis. Cdx mutations produce homeotic-like transformation accompanied by changes in the distribution of Hox gene expression (8, 37, 44). In addition to causing axial truncation, loss of Cdx2 also causes homeotic alterations along the body axis (7) and posterior shifts in Hox expression domains (this work). Cdx genes, as a class, may have a homeotic function of their own, being phylogenetically related to the Hox genes (52); for example, Cdx2 deficiency produces an anterior homeotic alteration in the specification of midgut endoderm (53), a tissue in which Hox genes are not expressed (54).
Supplementary Material
Acknowledgments
We are grateful to S. Gibblett, G. Elia, J. Korving, and S. Forlani for assistance and the transgenic Core Facility at The Samuel Lunenfeld Research Institute for tetraploid aggregation. We thank the following for probes: E. Olson for Paraxis, D. Duboule for Hoxd4, R. Krumlauf for Hoxb1, B. Meyer for Cdx1, C. Wright for Cdx4 and Mox1, A. McMahon for mShh and Wnt3a, G. Martin for Fgf8, V. Papaioannou for Tbx6, and B. Herrmann for Brachyury T. We thank K. Lawson, Y. Yanamaka, and H. Lickert for discussion and criticism. F.B. is grateful to the Medical Research Council and the Anti Cancer Council Victoria for research grants and to the Leverhulme Trust for an Emeritus Fellowship. K.C. is a CJ Martin Fellow. J.D. was supported by the Netherlands Organization for Scientific Research/Aard-en Levenswetenschappen. J.R. is a distinguished Investigator of the Canadian Institute of Health Research (CIRH) and is grateful to CIRH for financial support.
Abbreviations: dpc, days postcoitum; ES, embryonic stem.
References
- 1.Moreno, E. & Morata, G. (1999) Nature 400, 873-877. [DOI] [PubMed] [Google Scholar]
- 2.Duprey, P., Chowdhury, K., Dressler, G. R., Balling, R., Simon, D., Guenet, J. L. & Gruss, P. (1988) Genes Dev. 2, 1647-1654. [DOI] [PubMed] [Google Scholar]
- 3.Gamer, L. W. & Wright, C. V. (1993) Mech. Dev. 43, 71-81. [DOI] [PubMed] [Google Scholar]
- 4.James, R. & Kazenwadel, J. (1991) J. Biol. Chem. 266, 3246-3251. [PubMed] [Google Scholar]
- 5.Beck, F., Erler, T., Russell, A. & James, R. (1995) Dev. Dyn. 204, 219-227. [DOI] [PubMed] [Google Scholar]
- 6.Meyer, B. I. & Gruss, P. (1993) Development (Cambridge, U.K.) 117, 191-203. [DOI] [PubMed] [Google Scholar]
- 7.Chawengsaksophak, K., James, R., Hammond, V. E., Kontgen, F. & Beck, F. (1997) Nature 386, 84-87. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian, V., Meyer, B. I. & Gruss, P. (1995) Cell 83, 641-653. [DOI] [PubMed] [Google Scholar]
- 9.Rossant, J. & Spence, A. (1998) Trends Genet. 14, 358-363. [DOI] [PubMed] [Google Scholar]
- 10.Tam, P. P. & Tan, S. S. (1992) Development (Cambridge, U.K.) 115, 703-715. [DOI] [PubMed] [Google Scholar]
- 11.Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. (1998) Mech. Dev. 76, 79-90. [DOI] [PubMed] [Google Scholar]
- 12.Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W. & Roder, J. C. (1993) Proc. Natl. Acad. Sci. USA 90, 8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbondanzo, S. J., Gadi, I. & Stewart, C. L. (1993) Methods Enzymol. 225, 803-823. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson, D. (1992) in In Situ Hybridization: A Practical Approach (IRL Press, Oxford), pp. 75-83.
- 15.Wilkinson, D. G., Bhatt, S., Cook, M., Boncinelli, E. & Krumlauf, R. (1989) Nature 341, 405-409. [DOI] [PubMed] [Google Scholar]
- 16.Charite, J., de Graaff, W., Shen, S. & Deschamps, J. (1994) Cell 78, 589-601. [DOI] [PubMed] [Google Scholar]
- 17.Featherstone, M. S., Baron, A., Gaunt, S. J., Mattei, M. G. & Duboule, D. (1988) Proc. Natl. Acad. Sci. USA 85, 4760-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada, S., Stark, K. L., Shea, M. J., Vassileva, G., McMahon, J. A. & McMahon, A. P. (1994) Genes Dev. 8, 174-189. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson, D. G., Bhatt, S. & Herrmann, B. G. (1990) Nature 343, 657-659. [DOI] [PubMed] [Google Scholar]
- 20.Candia, A. F., Hu, J., Crosby, J., Lalley, P. A., Noden, D., Nadeau, J. H. & Wright, C. V. (1992) Development (Cambridge, U.K.) 116, 1123-1136. [DOI] [PubMed] [Google Scholar]
- 21.Burgess, R., Cserjesi, P., Ligon, K. L. & Olson, E. N. (1995) Dev. Biol. 168, 296-306. [DOI] [PubMed] [Google Scholar]
- 22.Chapman, D. L., Agulnik, I., Hancock, S., Silver, L. M. & Papaioannou, V. E. (1996) Dev. Biol. 180, 534-542. [DOI] [PubMed] [Google Scholar]
- 23.Echelard, Y., Epstein, D. J., St-Jacques, B., Shen, L., Mohler, J., McMahon, J. A. & McMahon, A. P. (1993) Cell 75, 1417-1430. [DOI] [PubMed] [Google Scholar]
- 24.Crossley, P. H. & Martin, G. R. (1995) Development (Cambridge, U.K.) 121, 439-451. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuka, T., Sakamoto, M., Guillemot, F. & Kageyama, R. (2001) J. Biol. Chem. 276, 30467-30474. [DOI] [PubMed] [Google Scholar]
- 26.Tamai, Y., Nakajima, R., Ishikawa, T., Takaku, K., Seldin, M. F. & Taketo, M. M. (1999) Cancer Res. 59, 2965-2970. [PubMed] [Google Scholar]
- 27.Yamaguchi, T. P., Takada, S., Yoshikawa, Y., Wu, N. & McMahon, A. P. (1999) Genes Dev. 13, 3185-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciruna, B. G., Schwartz, L., Harpal, K., Yamaguchi, T. P. & Rossant, J. (1997) Development (Cambridge, U.K.) 124, 2829-2841. [DOI] [PubMed] [Google Scholar]
- 29.Sakai, Y., Meno, C., Fujii, H., Nishino, J., Shiratori, H., Saijoh, Y., Rossant, J. & Hamada, H. (2001) Genes Dev. 15, 213-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Abed, S., Dolle, P., Metzger, D., Beckett, B., Chambon, P. & Petkovich, M. (2001) Genes Dev. 15, 226-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riddle, R. D., Johnson, R. L., Laufer, E. & Tabin, C. (1993) Cell 75, 1401-1416. [DOI] [PubMed] [Google Scholar]
- 32.Ciruna, B. & Rossant, J. (2001) Dev. Cell 1, 37-49. [DOI] [PubMed] [Google Scholar]
- 33.Pourquie, O. (2001) Annu. Rev. Cell Dev. Biol. 17, 311-350. [DOI] [PubMed] [Google Scholar]
- 34.Xu, X., Li, C., Takahashi, K., Slavkin, H. C., Shum, L. & Deng, C. X. (1999) Dev. Biol. 208, 293-306. [DOI] [PubMed] [Google Scholar]
- 35.Ikeya, M. & Takada, S. (2001) Mech. Dev. 103, 27-33. [DOI] [PubMed] [Google Scholar]
- 36.Partanen, J., Schwartz, L. & Rossant, J. (1998) Genes Dev. 12, 2332-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Akker, E., Forlani, S., Chawengsaksophak, K., de Graaff, W., Beck, F., Meyer, B. I. & Deschamps, J. (2002) Development (Cambridge, U.K.) 129, 2181-2193. [DOI] [PubMed] [Google Scholar]
- 38.Charite, J., de Graaff, W., Consten, D., Reijnen, M. J., Korving, J. & Deschamps, J. (1998) Development (Cambridge, U.K.) 125, 4349-4358. [DOI] [PubMed] [Google Scholar]
- 39.Prinos, P., Joseph, S., Oh, K., Meyer, B. I., Gruss, P. & Lohnes, D. (2001) Dev. Biol. 239, 257-269. [DOI] [PubMed] [Google Scholar]
- 40.Lorentz, O., Duluc, I., Arcangelis, A. D., Simon-Assmann, P., Kedinger, M. & Freund, J. N. (1997) J. Cell Biol. 139, 1553-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domon-Dell, C., Wang, Q., Kim, S., Kedinger, M., Evers, B. M. & Freund, J. N. (2002) Gut 50, 525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forlani, S., Lawson, K. A. & Deschamps, J. (2003) Development (Cambridge, U.K.) 130, 3807-3819. [DOI] [PubMed] [Google Scholar]
- 43.Pownall, M. E., Tucker, A. S., Slack, J. M. & Isaacs, H. V. (1996) Development (Cambridge, U.K.) 122, 3881-3892. [DOI] [PubMed] [Google Scholar]
- 44.Isaacs, H. V., Pownall, M. E. & Slack, J. M. (1998) EMBO J. 17, 3413-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bel-Vialar, S., Itasaki, N. & Krumlauf, R. (2002) Development (Cambridge, U.K.) 129, 5103-5115. [DOI] [PubMed] [Google Scholar]
- 46.Aulehla, A., Wehrle, C., Brand-Saberi, B., Kemler, R., Gossler, A., Kanzler, B. & Herrmann, B. G. (2003) Dev. Cell 4, 395-406. [DOI] [PubMed] [Google Scholar]
- 47.Yoshikawa, Y., Fujimori, T., McMahon, A. P. & Takada, S. (1997) Dev. Biol. 183, 234-242. [DOI] [PubMed] [Google Scholar]
- 48.Dubrulle, J., McGrew, M. J. & Pourquie, O. (2001) Cell 106, 219-232. [DOI] [PubMed] [Google Scholar]
- 49.Vasiliauskas, D. & Stern, C. D. (2001) Cell 106, 133-136. [DOI] [PubMed] [Google Scholar]
- 50.Mathis, L., Kulesa, P. M. & Fraser, S. E. (2001) Nat. Cell Biol. 3, 559-566. [DOI] [PubMed] [Google Scholar]
- 51.Tam, P. P. (1986) J. Embryol. Exp. Morphol. 92, 269-285. [PubMed] [Google Scholar]
- 52.Brooke, N. M., Garcia-Fernandez, J. & Holland, P. W. (1998) Nature 392, 920-922. [DOI] [PubMed] [Google Scholar]
- 53.Beck, F., Chawengsaksophak, K., Waring, P., Playford, R. J. & Furness, J. B. (1999) Proc. Natl. Acad. Sci. USA 96, 7318-7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck, F., Tata, F. & Chawengsaksophak, K. (2000) BioEssays 22, 431-441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.