Significance
The innate immune system is the first line of defense against invading pathogens. Activation of the innate immune response by infection and suppression during steady state are stringently controlled to eliminate pathogens and prevent inflammation. We found that host glycosylation plays an important role in the activation of immune responses and in maintaining innate immune homeostasis. In the steady state, a high amount of galactose-containing glycan suppresses undesirable activation of the immune response; however, activation of immune responses leads to reduced levels of galactose-containing glycan, which is needed for raising immune responses to an adequate level. Our finding suggests a novel mechanism for the regulation of innate immune quiescence and activation via changes in glycosylation status.
Keywords: innate immunity, glycosylation, Toll pathway, nucleotide sugar transporter, galactose
Abstract
The innate immune system is the first line of defense encountered by invading pathogens. Delayed and/or inadequate innate immune responses can result in failure to combat pathogens, whereas excessive and/or inappropriate responses cause runaway inflammation. Therefore, immune responses are tightly regulated from initiation to resolution and are repressed during the steady state. It is well known that glycans presented on pathogens play important roles in pathogen recognition and the interactions between host molecules and microbes; however, the function of glycans of host organisms in innate immune responses is less well known. Here, we show that innate immune quiescence and strength of the immune response are controlled by host glycosylation involving a novel UDP-galactose transporter called Senju. In senju mutants, reduced expression of galactose-containing glycans resulted in hyperactivation of the Toll signaling pathway in the absence of immune challenges. Genetic epistasis and biochemical analyses revealed that Senju regulates the Toll signaling pathway at a step that converts Toll ligand Spatzle to its active form. Interestingly, Toll activation in immune-challenged wild type (WT) flies reduced the expression of galactose-containing glycans. Suppression of the degalactosylation by senju overexpression resulted in reduced induction of Toll-dependent expression of an antimicrobial peptide, Drosomycin, and increased susceptibility to infection with Gram-positive bacteria. These data suggest that Senju-mediated galactosylation suppresses undesirable Toll signaling activation during the steady state; however, Toll activation in response to infection leads to degalactosylation, which raises the immune response to an adequate level and contributes to the prompt elimination of pathogens.
The innate immune system is the first line of defense against invading pathogens. In the steady state, immune responses are restrained; however, the immune response is activated rapidly on pathogen recognition. The Toll/TLR (Toll-like receptor) pathway plays a conserved role in the innate immune system of insects and vertebrates (1–3). In Drosophila, pathogens are not directly recognized by the Toll transmembrane receptor; rather, they are recognized by several specific pattern recognition receptors. The binding of gram-positive bacteria and fungi by these pattern recognition receptors triggers the activation of the serine protease cascade. Finally, a precursor form of Spatzle (pro-Spz), an endogenous ligand for Toll, is converted to an active form, which binds Toll and triggers activation of the NF-κB−related transcription factor, Dorsal-related immunity factor (Dif), leading to transcription of genes encoding antimicrobial peptides (AMPs) including Drosomycin (Drs). Many of the extracellular components required for Toll activation and suppression have been identified and are well understood; however, it is less known whether the posttranslational modifications in host organisms, such as glycosylation, regulate innate immune responses, despite the known significant roles of glycosylation in various other biological processes (4, 5).
Most cell surface and secreted proteins are glycosylated in the endoplasmic reticulum (ER) and Golgi apparatus. Glycosylation requires glycosyltransferases and nucleotide sugar substrates that are synthesized in the cytosol and nucleus. The nucleotide sugars must be transported into the ER and Golgi lumens by nucleotide sugar transporters (NSTs) (6). On arrival, they are used by glycosyltransferases. The Drosophila genome encodes at least 10 NSTs, which transport different subsets of nucleotide sugars. Studies of mutated NST genes show that protein glycosylation regulates intercellular signaling events. For example, Fringe-connection (a UDP-sugar transporter) (7, 8) and the two GDP-fucose transporters Gfr (9) and Efr (10) all affect Notch glycosylation and activity. Drosophila Gfr has a mammalian ortholog called SLC35C, which is responsible for the congenital disorder of glycosylation IIc (11, 12). These reports suggest that mutant NSTs in Drosophila may be useful tools for investigating the roles of glycosylation in regulation of signaling and in human diseases.
Here, we report that flies harboring a mutation of a novel UDP-galactose transporter named Senju showed hyperactivation of immune signal transduction via the Toll, JAK/STAT, and JNK signaling pathways in the absence of immune challenge. We focused on identifying the mechanism underlying Toll pathway activation and found that Spz was activated in the senju mutant. Moreover, we found that activation of the Toll pathway reduced the amount of galactose-containing glycan. Accordingly, senju overexpression increased galactose-containing glycan levels and repressed activation of the immune response against pathogens. These results suggest that the immune response is dynamically regulated by the amount of galactose-containing glycan, which is high during steady state to maintain immune homeostasis and low in infection to favor prompt elimination of pathogens.
Results
Senju KO Flies Have Abnormal Immune Organs.
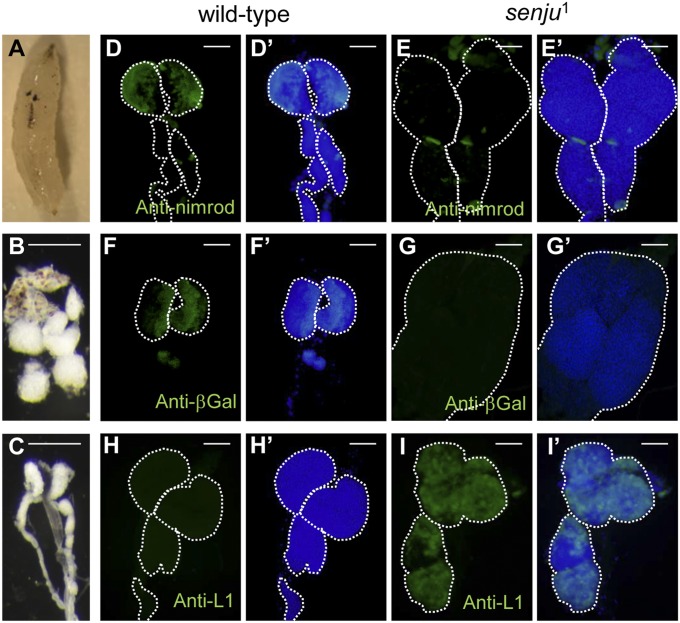
To analyze the biological role of Drosophila NSTs, we generated a series of knock out (KO) flies by homologous recombination (SI Materials and Methods and Fig. S1). Of these, CG14040 KO flies, which are called senju1 in the present study, showed various abnormalities in their immune organs. These abnormalities included melanotic cells in the hemocoel and melanotic tumors in the lymph glands, which are a specialized larval hematopoietic organ in Drosophila (Fig. 1 A–C). The lymph glands were also enlarged (Fig. 1 B, E, G, and I). Therefore, we investigated whether normal hematopoiesis occurs in senju1. Normally, at the late third-instar larval stage, progenitor hemocytes mature into plasmatocytes, crystal cells, and lamellocytes in the lymph gland (13); thus, lymph glands from WT and senju1 larvae were stained with anti-Nimrod C (a marker of plasmatocytes) (14) and anti-L1 (a marker of lamellocytes) (15) antibodies. In immunologically unchallenged WT larvae, most of the differentiated hemocytes were plasmatocytes, which were located along the outer edge of the lymph gland (Fig. 1D). Progenitor cells, which were located within the inner core of the lymph gland, were identified by their expression of domeless (dome) (Fig. 1F) (16), which encodes a receptor that functions in the JAK/STAT signaling pathway. Because lamellocytes are only induced by immune challenge, no lamellocyte staining was observed in the WT larvae (Fig. 1H) (13). By contrast, in immunologically unchallenged senju1 larvae, the numbers of progenitor cells and plasmatocytes were reduced, and we noted a significant degree of differentiation into lamellocytes in the primary and secondary lobes (Fig. 1 E, G, and I). Constitutive melanization and hyperdifferentiation of lamellocytes were also observed in Toll gain-of-function (Tl10B) (17) and JAK gain-of-function (hoptum-l) (18, 19) mutants of Drosophila. Therefore, we next investigated whether the Toll and JAK/STAT signaling pathways were activated in senju1. Because homozygous senju1 died at the late larval or early pupal stages, the majority of experiments (except for some that used adult flies) were performed using late-stage larvae.
Fig. 1.
Loss of the novel UDP-galactose transporter, Senju, causes abnormalities in immune organs. (A–C) Spontaneous melanization in the hemocoel and lymph glands in third-instar larvae of senju1. senju1 (B) and WT (C) lymph glands at high magnification. (Scale bars, 250 μm.) (D–I) Lymph glands from WT (D, F, and H) and senju1 (E, G, and I) late third-instar larvae carrying the Dome-MESO transgene (16) were stained with antibodies against the plasmatocyte marker, Nimrod (D and E); the undifferentiated cell marker, Dome-lacZ (F and G); and the lamellocyte marker, L1 (H and I) (green). Nuclei are stained with DAPI (blue). D′–I′ show merged images. (Scale bars, 200 μm.) The white dashed lines outline the lymph glands.
Signaling Pathways Involved in Innate Immunity Are Activated in senju1 in the Absence of Immune Challenge.
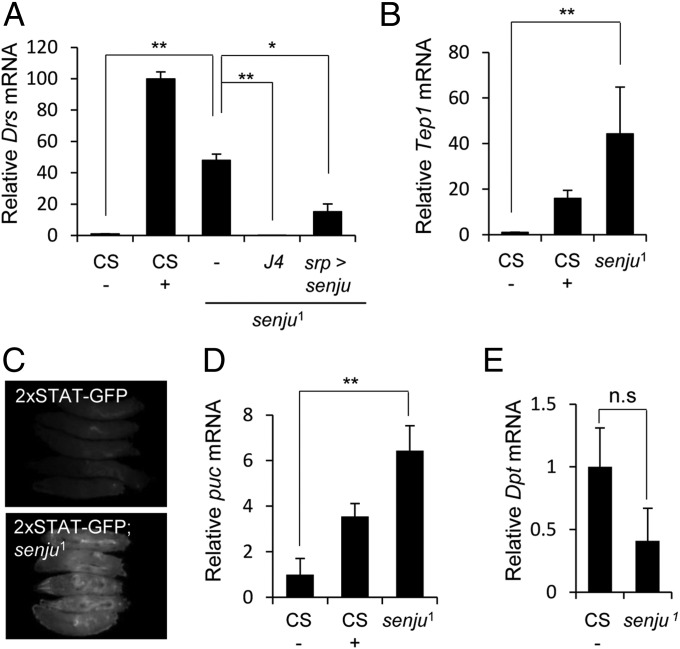
The Toll signaling pathway is the most important pathway in innate immunity against gram-positive bacteria and fungi (2, 20). We found that the Toll pathway was activated in senju1 by quantifying the expression of Drs. In the absence of immune challenge, senju1 larvae expressed ∼50-fold higher Drs mRNA levels than unchallenged WT larvae. The Drs mRNA levels in senju1 were 50% of the Drs mRNA levels of control larvae collected 12 h after infection with Micrococcus luteus (M. luteus), a gram-positive bacterium (Fig. 2A). The up-regulation of Drs in senju1 was significantly reduced by the overexpression of Myc-tagged senju using srp-Gal4 (Fig. 2A), confirming that senju was the gene responsible for the constitutive up-regulation of Drs mRNA levels in senju1. We also observed Drs induction in senju1 larvae cultured under germ-free conditions (Fig. S2), indicating that up-regulation of Drs mRNA in senju1 is likely to be due to host factors rather than commensal bacteria.
Fig. 2.
Loss of senju induces hyperactivation of the innate immune response. (A) qRT-PCR analysis of Drs expression served as a readout for Toll signaling. Drs expression in challenged (+) and unchallenged (−) WT (CS) larvae [which was set at 1 (control)] and in the unchallenged mutant larvae senju1, senju1 J4, and srp-Gal4 > UAS-senjumyc senju1. (B and D) qRT-PCR analysis of other innate immunity genes, namely Tep1 (used as a readout for the JAK/STAT pathway) (B) and puc (used as a readout for the JNK pathway) (D) in immune-challenged (+) and unchallenged (−) WT larvae (CS) and in the unchallenged mutant larvae, senju1. The values in unchallenged WT were set at 1 (control). (C) senju1;2×STAT-GFP larvae also showed constitutive JAK/STAT pathway activation. (E) qRT-PCR analysis of Dpt (used as a readout for the Imd pathway) in senju1 larvae in the absence of immune challenge. Larvae were challenged with M. luteus (A, B, and D). *P < 0.05 and **P < 0.01 (Student t test).
We then tested whether this constitutive expression of Drs in senju1 depended on dorsal (dl) and Dif, the NF-κB–related transcription factors in the Toll pathway. Indeed, a dl and Dif double mutation (J4) suppressed the induction of Drs mRNA in senju1 (Fig. 2A), supporting the notion that the Toll pathway is activated in senju1. Taken together, the Toll signaling pathway is most likely to be negatively regulated by Senju.
JAK/STAT signaling also plays a crucial role in regulating immune responses in Drosophila (21). Thioester-containing protein 1 (Tep1) is one gene regulated by the JAK/STAT pathway (22). Therefore, we quantified the expression of Tep1 mRNA. Unchallenged senju1 expressed 44-fold higher levels of Tep1 mRNA than unchallenged control larvae (Fig. 2B). The Tep1 mRNA levels in senju1 were even higher than the Tep1 mRNA levels in control larvae collected 12 h after infection with M. luteus (Fig. 2B). We also examined the activation of JAK/STAT signaling by using GFP reporter flies that harbor a GFP gene downstream of two STAT-binding sequences (2×STAT-GFP; Fig. 2C) (23). The larvae of 2×STAT-GFP–bearing senju1 showed constitutive activation of the JAK/STAT pathway.
Previous studies showed that the JNK signaling pathway also contributes to immune responses in insects and mammals. Activation of the JNK signaling pathway is induced by pathogens (24, 25) and is required for the expression of AMPs (26). Therefore, we examined whether the JNK signaling pathway is also activated in senju1 by measuring the levels of puckered (puc) mRNA, which are increased by JNK pathway activation (27). Unchallenged senju1 had a 6.6-fold higher puc transcription compared with the unchallenged control larvae (Fig. 2D). The puc transcription was also higher than that observed in challenged control larvae.
We next used Drs-GFP (a readout for the Toll pathway), 10×STAT-GFP (a readout for the JAK/STAT pathway), and puc-lacZ (a readout for the JNK pathway) to examine which tissues are involved in activating these signals in senju1. As shown in Fig. S3, a strong Drs-GFP signal was observed in the fat body but not in the lymph gland. By contrast, the JAK/STAT and JNK pathways were activated in the lymph gland rather than in the fat body.
The Imd pathway is another NF-κB–dependent signaling pathway that is involved in innate immunity (28). We tested whether the Imd signaling pathway was affected in senju1 by measuring the expression of Diptericin (Dpt), which is a major target gene of the Imd pathway. senju1 expressed similar, or lower, Dpt mRNA levels to the unchallenged WT larvae (Fig. 2E), which suggests that the Imd signaling pathway is not activated in unchallenged senju1.
From these results, we conclude that, with the exception of the Imd pathway, Senju-dependent glycosylation negatively regulates the key signaling pathways involved in innate immunity. We named the gene senju, which means “Buddha with a thousand hands manipulating tools to protect us,” because it seemed to protect against excessive activation of these signaling pathways.
Spz Is Activated in senju1 in the Absence of Immune Challenge.
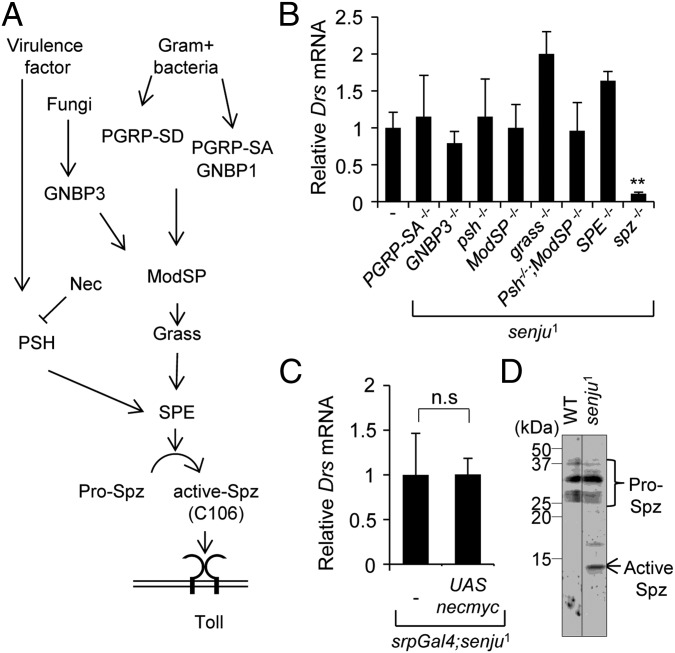
We focused on the mechanisms underlying the hyperactivation of the Toll pathway in senju1. The Golgi localization of Senju (29) (Fig. S4) suggests that Senju-dependent glycosylation of the membrane and/or secretory proteins of the Toll pathway regulate Toll activation (Fig. 3A). To identify which Toll pathway protein is activated in senju1, we performed epistatic analysis with senju1 and mutations in genes that encode extracellular components of the Toll pathway. Mutations in peptide glycan recognition proteins (PGRP-SA and GNBP3), serine proteases (Grass and Psh), and the modulator protein ModSP did not prevent the induction of Drs expression in senju1 (Fig. 3B). In addition, overexpression of the serine protease inhibitor nec did not affect the Drs mRNA levels in senju1 (Fig. 3C). Spatzle-processing enzyme (SPE) is activated by ModSP-dependent and Psh-dependent pathways. When both signaling pathways were inhibited in senju1 by mutations in ModSP and psh, the level of Drs transcription remained high (Fig. 3B). Because the SPE mutant has no null allele, we generated a new SPE mutant allele called SPESK6 by using the Cas9-mediated gene targeting system (30). The SPESK6 allele has an 8-bp deletion mutation (from nt 116 to nt 123) in the coding region that results in the loss of the major protease domain (amino acids 135–400) of SPE (Fig. S5). SPESK6 also did not block the up-regulation of Drs in senju1. By contrast, the senju1 and spzrm7 double mutant exhibited markedly reduced expression of Drs mRNA. This epistatic analysis placed Senju function at the level of Spz because Senju activated the Toll pathway independently of the cascade upstream of Spz.
Fig. 3.
Spz is activated in senju1 in the absence of immune challenge. (A) Schematic depiction of the extracellular Toll pathway. (B) Epistatic analysis of senju1 and Toll activator mutants (PGRP-SAseml, GNBP3hades, psh1, ModSP1, grassHarrade, SPESK6, and spzrm7). Drs mRNA expression was measured by qRT-PCR, normalized, and expressed relative to that in senju1 (which was set at 1). **P < 0.01 (Student t test). (C) Expression of Drs mRNA in srp-Gal4 > UAS-necmyc; senju1 larvae. (D) Immunoblot analysis of hemolymph from WT and senju1 larvae using an anti-Spz C106 antibody. The image was taken in the same blotted membrane under the same conditions, and unnecessary lanes were removed.
We next examined whether Spz is activated in senju1. Spz is mainly expressed in hemocytes and secreted to hemolymph in an inactive form designated as pro-Spz. When the immune system is stimulated, pro-Spz is cleaved by activated SPE to yield a short active form called Spz C106. Immunoblot analysis with an anti-Spz C106 antibody revealed markedly increased active Spz levels in the senju1 hemolymph (Fig. 3D); thus, Spz is activated in senju1 in the absence of immune challenge. These data demonstrated that Senju-dependent glycosylation prevents the aberrant activation of Spz and suppresses the Toll signaling pathway during the steady state.
Synthesis of Galactose-Containing N- and O-Glycans Is Impaired in senju1.
Because senju encodes a protein that is homologous to a NST, we investigated whether Senju is actually involved in glycosylation. First, we used a yeast cell-free system to identify the nucleotide sugar transported by Senju. We measured nucleotide sugar incorporation by microsomes extracted from yeasts that expressed senju or mock vector. The results clearly showed that the Senju protein selectively transports UDP-galactose, i.e., Senju is a UDP-galactose transporter (Fig. 4A).
Fig. 4.
Senju, a UDP-galactose transporter, is required for the synthesis of galactose-containing glycans. (A) Transportation of various nucleotide sugar substrates by Senju. *P < 0.05 (Student t test). (B) Lectin microarray analysis of lymph glands from dl/Dif mutants (J4, control) and senju1 with a dl/Dif mutant background (J4, senju1). The binding of senju1 lysates to less and more reactive lectins is shown relative to that of control fly lysates. The specificities of the lectins are shown. The galactose moieties are highlighted in red. (C) Synthesis of N-linked glycans on Myc-tagged Spz and SPE in senju1. Immunoblot analysis using an anti-Spz C106 antibody (Upper) or an anti-Myc antibody (Lower), respectively. The genotypes of larvae used in this study were srp-Gal4 > UAS-spz-myc and srp-Gal4 > UAS-spz-myc;senju1, or srp-Gal4 > UAS-SPE-myc and srp-Gal4 > UAS-SPE-myc;senju1. The SPE image was taken in the same blotted membrane under the same conditions, and the left half was flipped horizontally. (D) Drs expression in WT flies after the transfer of native or heat-denatured senju1 or WT hemolymph [which was set as 1 (control)]. *P < 0.05 (Student t test).
Next, we examined the glycan structures that were altered in senju1. To avoid feedback effects from the activated Toll signaling pathway, we compared the glycan profiles of the dl/Dif mutant and the senju1 larvae in a dl/Dif mutant background by using a lectin array (31); thus, lymph gland lysates prepared from control and senju1 larvae were labeled with Cy3 and applied to the lectin array (which comprised 45 different lectins fixed on a glass slide). Because the coefficient of variation (CV) associated with this method is 10% (Glycotechnica), changes in binding intensity of >20% (twice the CV) were considered significant. As shown in Fig. 4B and Dataset S1, five of the six lectins (RCA120, SNA, BPL, UEA-I, and MAL-I) that were less reactive with the senju1 lysate than with the control lysate recognized N-linked and O-linked glycans that contained galactose. This reduction of galactose-containing glycans is consistent with the finding that Senju acts as a UDP-galactose transporter. Among the five lectins, BPL and RCA120 lectins provided higher signal intensities than the other three lectins (Dataset S1). By contrast, the lectins that were more reactive with the senju1 lysates recognized mannose, GlcNAc, and GalNAc. These results indicate that some components of N-linked and O-linked glycans, which are distal to the galactose residue, are not synthesized in senju1. Therefore, GlcNAc and GalNAc are exposed at the ends of these aberrant glycans, which may explain why the lectins recognizing GlcNAc and GalNAc were more reactive with senju1 lysates.
The next question was whether the glycosylation of components of the Toll signaling pathway is actually impaired in senju1. We examined the glycosylation of Spz because, of all of the known extracellular components of the Toll pathway, Spz was the only protein that was required for the Toll hyperactivation in senju1 (Fig. 3B). A Myc-tagged Spz protein was expressed using srp-Gal4 in the immune organs of WT and senju1 larvae. The protein was then immunopurified and subjected to immunoblot analysis with an anti-Myc antibody (Fig. 4C, Upper). Two bands were detected, both corresponding to expressed Spz. The difference in the molecular weights was due to heterogeneity of N-glycosylation: the proteins had the same molecular weight after treatment with PNGase, which specifically removes N-linked glycans from proteins. The higher-molecular-weight band (as indicated by the arrow in Fig. 4C, Upper), which is likely to represent Spz-bearing N-linked glycans with additional branching or extensions on galactose residues, was absent in senju1. This result indicates that the N-linked glycans distal to galactose residues on Spz are not fully synthesized in senju1 due to the low levels of UDP-galactose present in the Golgi lumen. The low-molecular-weight form of Spz (as indicated by the arrowhead in Fig. 4C, Upper) in senju1 may also carry small but significant defects in glycosylation. Although defects in N-linked glycans could be detected by this electrophoretic analysis, it was hard to determine whether O-linked glycans were also impaired in senju1 because O-linked glycans, apart from the glycosaminoglycan and mucin types, are generally smaller than N-linked glycans. We also investigated the glycosylation of SPE, which is not involved in Toll activation in senju1 (Fig. 3B). Immunoblot analysis showed that the molecular weight of Myc-tagged SPE expressed in the immune organs of WT and senju1 were the same and that treatment with PNGase F reduced the molecular weights of both proteins such that they were the same size. These results indicate that glycosylation of SPE is not impaired in senju1 (Fig. 4C, Lower).
The data presented above suggested two possibilities: (i) that Senju-dependent glycosylation of Toll pathway proteins negatively regulates the Toll pathway and (ii) that the altered glycan structure in senju1 is recognized as non-self, thereby leading to activation of the immune response. To distinguish between these two possibilities, we performed an experiment in which the hemolymph of WT and senju1 larvae was transferred to WT flies. As expected, transfer of senju1 hemolymph up-regulated Toll activation to a greater extent than transfer of WT hemolymph (Fig. 4D). If a Toll pathway protein is activated in senju1, then heat-denatured senju1 hemolymph would not up-regulate Toll activation to a greater extent than heat-denatured WT hemolymph. By contrast, if altered glycans activate the Toll pathway, heat-denatured senju1 hemolymph (which contains altered glycans) would up-regulate the Toll pathway. The results clearly showed that the level of Drs mRNA in WT flies injected with heat-denatured senju1 hemolymph was comparable with that in WT flies injected with heat-denatured WT hemolymph (Fig. 4D). These data suggest that Toll activation in senju1 is likely caused by hyperactivation of a pathway protein rather than by the recognition of altered glycans as non-self.
Toll Activation Reduces Galactose-Containing Glycan Levels, Which Is Needed to Raise the Immune Response to an Adequate Level Following Infection.
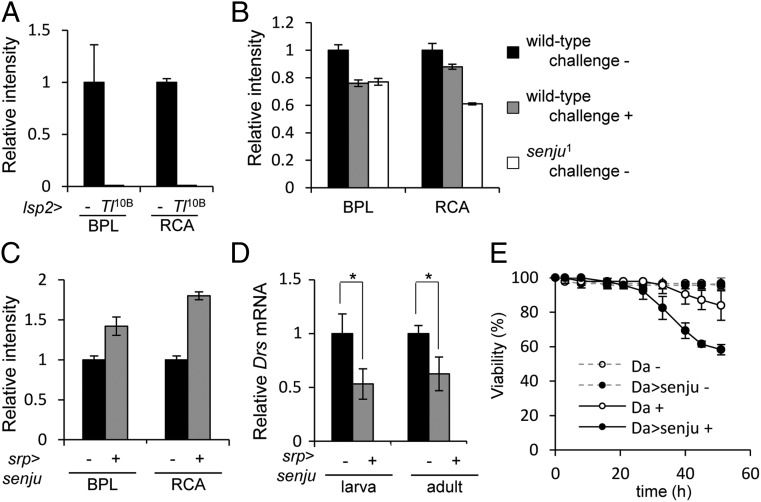
The data above suggested that galactose-containing glycans negatively regulate the Toll signaling pathway. This regulation is conserved at both the larval and adult stages because a few adult senju flies (escaper) exhibited significantly higher levels of Drs expression than adult control flies (Fig. S6A). We next asked whether infection alters the amount of these glycan structures in adult flies, as infection experiments yielded more reliable results at the adult stage. To mimic pathogen infection, we first expressed the activated form of Toll, Tl10B, in the fat body, an organ in which the Toll pathway is mainly activated after infection, and examined glycan expression in the hemolymph (which contains hemocytes and Spz) using a lectin array. The hemolymph of Tl10B-expressing flies showed almost no reactivity with BPL and RCA120 lectins (Fig. 5A). These results suggest that immune challenge causes degalactosylation of glycans. We further confirmed that infection by pathogens actually alters the amount of these glycan structures. Hemolymph from WT flies was collected 24 h after infection with Bacillus subtilis (B. subtilis). Lectin array analysis using BPL and RCA120 lectins showed that immune challenge reduced the level of reactivity with these lectins (Fig. 5B). Interestingly, the size of the reduction in reactivity with the BPL lectin after immune challenge was comparable with that observed in senju1.
Fig. 5.
Toll activation results in reduced levels of galactose-containing glycans in the hemolymph, which raises the immune response to an adequate level. (A) BPL and RCA120 lectin reactivity of the control (lsp2-Gal4, −) and Toll-activated (lsp2-Gal4 > UAS-Tl10B, Tl10B) hemolymph. (B) BPL and RCA120 lectin reactivity of immune-challenged (with B. subtilis) (+), unchallenged (−) WT, and unchallenged (−) senju1 hemolymph. (C) BPL and RCA120 lectin reactivity of senju-overexpressing (srp-Gal4 > UAS-senjumyc) (+) and control (srp-Gal4, −) hemolymph. (D) Drs expression in immune-challenged (with B. subtilis) larvae and adult flies. (+) indicates senju-overexpressing strains and (–) indicates control srp-Gal4 strains (set at 1). *P < 0.05 (Student t test). (E) Survival of senju-overexpressing (daGal4 > UAS-senjumyc, Da > senju) and control (daGal4>, Da) flies after immune challenge with B. subtilis (+) or no challenge (−).
We next investigated whether the amounts of galactose-containing glycans affect the strength of the immune response to infection. The hemolymph of flies overexpressing Myc-tagged senju in immune organs (driven by srp-Gal4) showed markedly increased reactivity with BPL and RCA120 lectins, which recognize typical galactose residues, suggesting that senju-overexpressing flies have large amounts of galactose-containing glycans (Fig. 5C). Next, we examined whether senju-overexpressing flies exhibited reduced Drs mRNA expression after infection with gram-positive bacteria. Indeed, larvae and flies overexpressing Senju in immune organs (driven by srp-Gal4) showed 53% and 62% of the Drs mRNA levels observed in WT larvae and flies, respectively (Fig. 5D). Finally, we used the ubiquitously expressed driver, da-Gal4, to test susceptibility to microbial infection in senju-overexpressing flies. Following immune challenge with B. subtilis, the survival rate of senju-overexpressing flies was lower (61.5% at 45 h after infection) than that of WT flies (87.1%) (Fig. 5E). A similar result was obtained when we used srp-Gal4 to overexpress Senju in immune organs (Fig. S6B). This reduced resistance of senju-overexpressing flies to B. subtilis infection was consistent with the low-level induction of Drs mRNA. Taken together, we conclude that high levels of galactose-containing glycans reduce the strength of the immune response following infection.
Discussion
Proper regulation of immune responses is critical for health. Inappropriate activation of inflammatory responses or lack of immune signaling lead to serious conditions such as inflammatory diseases or immunodeficiency, respectively. Recent studies reveal that innate immunity is regulated by multiple factors, including sumoylation and the retromer complex, which fine tune immune activity (32, 33). The present study clearly showed that glycosylation plays an important role in maintaining innate immune homeostasis and activation of immune responses.
Several studies show that pathogen glycans are involved in pathogen recognition and the interactions between host molecules and microbes (34, 35); however, the mechanisms by which host glycans regulate innate immune signaling pathways in vivo remain to be identified. The present study provides in vivo evidence for the essential role of host glycosylation in innate immune quiescence. Drosophila immunity is controlled by various signaling pathways such as Toll, Imd, JNK and JAK/STAT pathways. In senju mutants, Toll, JNK, and JAK/STAT pathways were activated in the absence of infection. Because these pathways can activate each other by complex cross-talk, it is difficult to determine whether only the Toll pathway or all or some of the other pathways are also directly activated in senju1.
Our epistatic analysis suggested that the constitutive activation of the Toll pathway in senju1 depends on Spz. The precursor form of Spz is N-glycosylated, and the Spz molecules that are expressed in senju1 exhibited impaired glycan synthesis (Fig. 4C). By contrast, no glycan defects were observed in the SPE expressed in senju1 (Fig. 4C). SPE is not necessary for Toll pathway activation in senju1 (Fig. 3B); thus, Spz is a candidate protein whose inadequate glycosylation may be responsible for the hyperactivation of the Toll pathway in senju1. It remains possible that the Senju-dependent glycan regulates an unknown pathway that can activate Spz.
Genetic remodeling of protein glycosylation in mice reveals that glycans play important roles in autoimmune disease and inflammation (36). Conversely, infection and inflammation lead to changes in host glycan structure (37). Although it is well known that pathogens express enzymes that directly alter host glycans and thereby facilitate their life cycle (e.g., Haemophilus influenzae), it is unclear whether glycan changes that are achieved by the host cellular machinery (e.g., by regulating glycosyltransferase or other pathways) are beneficial for host defense. Our results show that host-driven glycan changes in response to Toll activation may be an efficient mechanism by which the host combats microbes. As mentioned above, galactose-containing glycan is a negative regulator of Toll signaling. When the Toll pathway is activated by infection, the galactose-containing glycan levels decrease (Fig. 5B). This effect is independent of pathogen-derived enzymes because these glycan changes were observed in Tl10B-expressing flies that had not been challenged with pathogen (Fig. 5A). The degalactosylation may result in up-regulation of the Toll signaling pathway because the analysis of senju mutants showed that deletion of galactose residues increased Toll signaling. These glycan changes induced by Toll activation may contribute to the rapid elimination of pathogens in the acute phase in cooperation with the increased expression of spz, Tl, and SPE. Senju-overexpressing flies, which have large amounts of galactose-containing glycan (Fig. 5C), showed reduced Drs expression and viability after infection (Fig. 5 D and E and Fig. S6B), which suggests that the reduction in the levels of galactose-containing glycan is important for generating adequate immune responses to infections. Taken together, the results of the present study indicate the presence of a novel mechanism that regulates the immune response through changes in glycan structure.
Degalactosylation is thought to be caused by two possible mechanisms. One is activation of the degradation of galactose-containing glycans associated with Toll activation. Another mechanism is suppression of synthesis of galactose-containing glycans. Recent reports showed that treatment with proinflammatory cytokines (IL-1 and TNFα) affects the expression of glycosyltransferases and leads to glycan changes in mammals (38, 39). Therefore, the activation of Toll signaling by infection may also involve regulation of the activity of as-yet-unidentified galactosyltransferases and/or enzymes that are required for UDP-galactose biosynthesis, although the level of senju mRNA in the Tl10B-expressing flies was unchanged (Fig. S7). Further studies are needed to fully understand the mechanisms underlying the changes in glycan structure that occur in response to Toll pathway activation.
In summary, we identified a novel mechanism that regulates innate immune quiescence and activation via changes in glycosylation status during steady state and infection, respectively.
Materials and Methods
Generation of senju KO Flies.
senju KO flies were generated using ends-out technology. A targeting vector was designed to replace the entire senju gene with the white gene. See SI Materials and Methods for a detailed description.
Quantitative RT-PCR.
Real-time PCR was performed by using a 7500HT Fast Real-Time PCR system (Applied Biosystems). The amounts of amplified transcript were normalized against an internal control (ribosomal protein 32). See SI Materials and Methods for a detailed description. The primers used are listed in Table S1.
Lectin Array.
Extracted proteins (1 μg) from lymph glands or hemolymph were labeled with Cy3 Monoreactive Dye (GE Healthcare) and subjected to glycan profiling analyses using their LecChip lectin array chips and an evanescent-field fluorescence scanner (Glycostation Reader 1200; SC-Profiler; Glycotechnica Ltd.; www.glycotechnica.com/). The net signal intensity values from three spots were then averaged. See SI Materials and Methods for a detailed description.
Statistical Analysis.
Statistical analysis was performed by the Student t test using Microsoft Excel.
Supplementary Material
Acknowledgments
We thank Ms. Wakae Awano for excellent technical support and peoples and stock centers listed in Table S2 for providing fly strains. This work was supported by grants-in-aid for scientific research from Ministry of Education, Culture, Sports, Science and Technology, and Japan Society for the Promotion of Science and a grant from The Sumitomo Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424514112/-/DCSupplemental.
References
- 1.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 2.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7(11):862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 4.Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6(7):530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto-Hino M, Okano H, Kanie O, Goto S. Structure, function and formation of glycans in Drosophila. In: Mora Montes HM, editor. Glycans: Biochemistry, Characterization and Applications. Nova Science Publishers; New York: 2012. [Google Scholar]
- 6.Liu L, Xu YX, Hirschberg CB. The role of nucleotide sugar transporters in development of eukaryotes. Semin Cell Dev Biol. 2010;21(6):600–608. doi: 10.1016/j.semcdb.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selva EM, et al. Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events. Nat Cell Biol. 2001;3(9):809–815. doi: 10.1038/ncb0901-809. [DOI] [PubMed] [Google Scholar]
- 8.Goto S, et al. UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat Cell Biol. 2001;3(9):816–822. doi: 10.1038/ncb0901-816. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa HO, et al. Notch deficiency implicated in the pathogenesis of congenital disorder of glycosylation IIc. Proc Natl Acad Sci USA. 2005;102(51):18532–18537. doi: 10.1073/pnas.0504115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa HO, et al. Two pathways for importing GDP-fucose into the endoplasmic reticulum lumen function redundantly in the O-fucosylation of Notch in Drosophila. J Biol Chem. 2010;285(6):4122–4129. doi: 10.1074/jbc.M109.016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lühn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 2001;28(1):69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- 12.Lübke T, et al. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet. 2001;28(1):73–76. doi: 10.1038/ng0501-73. [DOI] [PubMed] [Google Scholar]
- 13.Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9(5):1117–1126. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 14.Kurucz E, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17(7):649–654. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Kurucz E, et al. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol Hung. 2007;58(Suppl):95–111. doi: 10.1556/ABiol.58.2007.Suppl.8. [DOI] [PubMed] [Google Scholar]
- 16.Krzemień J, et al. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446(7133):325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- 17.Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125(10):1909–1920. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- 18.Luo H, Hanratty WP, Dearolf CR. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 1995;14(7):1412–1420. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14(12):2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetru C, Hoffmann JA. NF-kappaB in the immune response of Drosophila. Cold Spring Harb Perspect Biol. 2009;1(6):a000232. doi: 10.1101/cshperspect.a000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 22.Lagueux M, Perrodou E, Levashina EA, Capovilla M, Hoffmann JA. Constitutive expression of a complement-like protein in toll and JAK gain-of-function mutants of Drosophila. Proc Natl Acad Sci USA. 2000;97(21):11427–11432. doi: 10.1073/pnas.97.21.11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bach EA, et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7(3):323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Sluss HK, et al. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10(21):2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 25.Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3(5):711–722. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 26.Delaney JR, et al. Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J. 2006;25(13):3068–3077. doi: 10.1038/sj.emboj.7601182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Blanco E, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12(4):557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemaitre B, et al. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92(21):9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto-Hino M, et al. Cisterna-specific localization of glycosylation-related proteins to the Golgi apparatus. Cell Struct Funct. 2012;37(1):55–63. doi: 10.1247/csf.11037. [DOI] [PubMed] [Google Scholar]
- 30.Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195(3):715–721. doi: 10.1534/genetics.113.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirabayashi J, Yamada M, Kuno A, Tateno H. Lectin microarrays: Concept, principle and applications. Chem Soc Rev. 2013;42(10):4443–4458. doi: 10.1039/c3cs35419a. [DOI] [PubMed] [Google Scholar]
- 32.Anjum SG, et al. Regulation of Toll signaling and inflammation by β-arrestin and the SUMO protease Ulp1. Genetics. 2013;195(4):1307–1317. doi: 10.1534/genetics.113.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou B, et al. Retromer promotes immune quiescence by suppressing Spätzle-Toll pathway in Drosophila. J Cell Physiol. 2014;229(4):512–520. doi: 10.1002/jcp.24472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7(6):424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royet J, Gupta D, Dziarski R. Peptidoglycan recognition proteins: Modulators of the microbiome and inflammation. Nat Rev Immunol. 2011;11(12):837–851. doi: 10.1038/nri3089. [DOI] [PubMed] [Google Scholar]
- 36.Orr SL, et al. A phenotype survey of 36 mutant mouse strains with gene-targeted defects in glycosyltransferases or glycan-binding proteins. Glycobiology. 2013;23(3):363–380. doi: 10.1093/glycob/cws150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreisman LS, Cobb BA. Infection, inflammation and host carbohydrates: A Glyco-Evasion Hypothesis. Glycobiology. 2012;22(8):1019–1030. doi: 10.1093/glycob/cws070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higai K, Miyazaki N, Azuma Y, Matsumoto K. Interleukin-1beta induces sialyl Lewis X on hepatocellular carcinoma HuH-7 cells via enhanced expression of ST3Gal IV and FUT VI gene. FEBS Lett. 2006;580(26):6069–6075. doi: 10.1016/j.febslet.2006.09.073. [DOI] [PubMed] [Google Scholar]
- 39.Colomb F, et al. TNF regulates sialyl-Lewisx and 6-sulfo-sialyl-Lewisx expression in human lung through up-regulation of ST3GAL4 transcript isoform BX. Biochimie. 2012;94(9):2045–2053. doi: 10.1016/j.biochi.2012.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.