Abstract
Transcription factors play important roles in the control of neuronal function in physiological and pathological conditions. We previously reported reduced levels of transcription factor SP4 protein, but not transcript, in the cerebellum in bipolar disorder and associated with more severe negative symptoms in schizophrenia. We have recently reported phosphorylation of Sp4 at S770, which is regulated by membrane depolarization and NMDA receptor activity. The aim of this study was to investigate SP4 S770 phosphorylation in bipolar disorder and its association with negative symptoms in schizophrenia, and to explore the potential relationship between phosphorylation and protein abundance. Here we report a significant increase in SP4 phosphorylation in the cerebellum, but not the prefrontal cortex, of bipolar disorder subjects (n=10) (80% suicide) compared to matched controls (n=10). We found that SP4 phosphorylation inversely correlated with SP4 levels independently of disease status in both areas of the human brain. Moreover, SP4 phosphorylation in the cerebellum positively correlated with negative symptoms in schizophrenia subjects (n=15). Further, we observed that a phospho-mimetic mutation in truncated Sp4 was sufficient to significantly decrease Sp4 steady-state levels, while a non-phosphorylatable mutant showed increased stability in cultured rat cerebellar granule neurons. Our results indicate that SP4 S770 phosphorylation is increased in the cerebellum in bipolar disorder subjects that committed suicide and in severe schizophrenia subjects, and may be part of a degradation signal that controls Sp4 abundance in cerebellar granule neurons. This opens the possibility that modulation of SP4 phosphorylation may contribute to the molecular pathophysiology of psychotic disorders.
Keywords: Bipolar disorder, schizophrenia, cerebellum, Sp4 transcription factor, phosphorylation, protein stability
1. Introduction
Bipolar disorder (BD) is a debilitating psychiatric disease characterized by mood instability leading to acute episodes of mania and depression (Belmaker, 2004). Despite the prevalence and burden of bipolar disorder, the biological and molecular mechanisms underlying this disorder remain poorly understood. The regulation of gene expression by transcription factors is a key event for the normal development and function of the nervous system; however, the transcriptional programs altered in bipolar disorder are not completely characterized.
Increasing evidence suggests that the transcription factor Specificity protein 4 (SP4) may play a role in bipolar disorder. Polymorphisms at the SP4 transcription factor gene locus have been linked to bipolar disorder and schizophrenia (Zhou et al., 2009). Sp4 is a zinc finger transcription factor that binds to GC/T rich sequences, regulating the expression of a large number of genes implicated in diverse biological functions (Suske, 1999). Sp4 is highly expressed in neurons (Mao et al., 2007), suggesting a role for Sp4 in neuronal function. In fact, Sp4 regulates developmental dendrite patterning, hippocampal long-term potentiation, and animal behaviors including contextual and spatial memory and prepulse inhibition (Ramos et al., 2007; Zhou et al., 2005; Zhou et al., 2007). Some of these processes have been linked to alterations observed in subjects with bipolar disorder and schizophrenia; namely altered connectivity (Hajek et al., 2005; Harrison and Weinberger, 2005; Mahon et al., 2009; Wadehra et al., 2013) and dysfunctional prepulse inhibition as an endophenotype in schizophrenia (Braff, 2011; Braff et al., 2007) and when displaying active manic and acute psychotic symptoms in bipolar disorder (Kohl et al., 2013). Hence, this evidence indicates that regulation of SP4 transcription factor protein levels may be relevant to the pathophysiology of severe psychiatric disorders.
Several lines of evidence indicate that physiological and pathological conditions regulate Sp4 via changes in protein stability. For instance, inhibition of COX-2 has been reported to induce Sp4 degradation by enhancing protein ubiquitination in cancer cells (Abdelrahim and Safe, 2005). In neurons, Sp4 is degraded by calcium-activated proteases in response to glutamate-induced excitotoxicity (Mao et al., 2007), and through the proteasome upon ubiquitination in non-depolarizing conditions as we previously described (Pinacho et al., 2011). Thus activity and calcium-dependent regulation seem to be key modulators of Sp4 protein stability in neurons, however, the post-translational modifications responsible for triggering Sp4 ubiquitin-dependent degradation cascade are yet to be elucidated. We have recently identified a site of phosphorylation on Sp4 at S770 which is important for Sp4-dependent dendritic pruning of developing cerebellar granule neurons (Saia et al., 2014). Sp4 S770 phosphorylation is increased upon inhibition of N-Methyl-D-Aspartate (NMDA) receptor signaling and in non-depolarizing conditions, where Sp4 protein stability is reduced (Pinacho et al., 2011; Saia et al., 2014). These previous observations suggest that Sp4 S770 phosphorylation may participate in the mechanisms leading to Sp4 degradation.
In pathological conditions, such as in bipolar disorder, we have observed a significant reduction in SP4 protein levels in the postmortem cerebellum and prefrontal cortex (PFC) of BD subjects (Pinacho et al., 2011). SP4 reduction in the cerebellum in bipolar disorder was only observed at the protein level and not at the transcript level, as was found in the PFC (Pinacho et al., 2011). We have also previously described reduced SP4 protein levels, but not mRNA, in the cerebellum of schizophrenia subjects with severe negative symptoms (Pinacho et al., 2013). Thus, although other mechanisms may occur in the prefrontal cortex in BD, these data suggest that SP4 protein stability is altered in the cerebellum of both bipolar disorder subjects and in schizophrenia subjects with more severe negative symptoms.
Taken together, we hypothesized that SP4 S770 phosphorylation may contribute, at least in part, to SP4 stability regulation in the cerebellum of both bipolar disorder subjects and schizophrenia subjects with more severe negative symptoms. Thus, the main aim of this study was to investigate whether phosphorylation at SP4 S770 in the cerebellum is increased in bipolar disorder subjects, is associated with negative symptoms in schizophrenia, and correlates with reduced SP4 protein abundance. We included an analysis of the prefrontal cortex of the same BD subjects to test if this SP4 modification is specific of a protein reduction independent of gene expression regulation. We also examined the effect of Sp4 phosphorylation on Sp4 protein levels using point mutants of full-length and truncated Sp4 in rat cultured cerebellar granule neurons.
2. Experimental procedures
2.1. Postmortem human brain tissue samples
Postmortem human brain samples from the cerebellum and prefrontal cortex of subjects with bipolar disorder (n=10) and control subjects with no history of psychiatric episodes (n=10) were obtained from the Universidad del País Vasco / Euskal Herriko Unibertsitatea (UPV/EHU) brain collection, according to the policies set forth by the research and ethical review boards of the Basque Institute of Legal Medicine, Bilbao, Spain. Demographic data of this cohort is detailed in Table 1 (Pinacho et al., 2011). Samples were obtained at autopsy by forensic pathologists under research policies with postmortem samples. All deaths were subjected to retrospective analysis for previous medical diagnosis. Subjects with antemortem criteria for bipolar disorder according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) were matched to control subjects based on gender, age and postmortem delay (PMD). Toxicological screening for antipsychotics, antidepressants, and other drugs was performed at the National Institute of Toxicology, Madrid, Spain.
Table 1.
Demographic characteristics of subjects with bipolar disease (BD) (n=10) and controls (n=10).
| Gender1 |
Age (years)2 |
Postmortem delay (hours)2 |
pH2 |
Cause of death |
Treatment3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pairs | BD | C | BD | C | BD | C | BD | C | BD | C | BD | C |
| 1 | M | M | 72 | 72 | 22 | 43 | 6.5 | 7.1 | Suicide | Accident | Drug-free | Drug-free |
| 2 | F | F | 44 | 49 | 19 | 40 | 6.5 | 6.8 | Suicide | Accident | APS. BZD. Mirtazapine | Drug-free |
| 3 | M | M | 58 | 54 | 10 | 23 | 7.1 | 6.5 | Suicide | Accident | APS. Lamotrigine | BZD |
| 4 | M | M | 40 | 43 | 17 | 11 | 6.8 | 6.5 | Suicide | Accident | BZD | Drug-free |
| 5 | M | M | 27 | 30 | 10 | 11 | 6.8 | 6.5 | Suicide | Accident | Drug-free | Cannabis |
| 6 | M | M | 63 | 60 | 8 | 4 | 6.8 | 7.1 | Suicide | Natural | BZD | Drug-free |
| 7 | M | M | 64 | 61 | 23 | 23 | 7.1 | 6.8 | Suicide | Accident | BZD. Venlafaxine | Drug-free |
| 8 | M | M | 57 | 55 | 22 | 22 | 6.5 | 6.5 | Natural | Natural | Drug-free | Drug-free |
| 9 | M | M | 63 | 67 | 31 | 19 | 6.5 | 7.1 | Natural | Natural | Drug-free | NSAID |
| 10 | F | F | 73 | 74 | 3 | 19 | 6.8 | 6.8 | Suicide | Accident | Drug-free | NSAID |
| Statistic | 0.00; 14 | 48.504 | 36.004 | 47.004 | N/A | N/A | ||||||
BD, Bipolar Disorder; C, control; M, male; F, female; APS, antipsychotics; BZD, benzodiazepines; NSAID, nonsteroidal anti-inflammatory drug.
Chi-square statistic and degrees of freedom are shown for categorical variables.
Mann Whitney U is shown for non-parametric variables.
Treatment indicates results of toxicology analysis performed by the National Institute of Toxicology, Madrid, Spain.
Not significant p value, p>0.05.
Postmortem human brain samples from the cerebellum of subjects with chronic schizophrenia (SZ, n=15) were obtained from the antemortem clinically well-characterized and controlled collection of Sant Joan de Déu Brain Bank (Roca et al., 2008). The demographic and clinical characteristics for these SZ subjects were previously reported (Pinacho et al., 2013) and are summarised in Table 2. All SZ subjects were institutionalized donors with a long duration of the illness (Table 2) and had no history of neurological episodes. Experienced clinical examiners interviewed each donor antemortem to confirm schizophrenia diagnosis according to DSM-IV and International Classification of Diseases (ICD-10) criteria. Our study includes the following schizophrenia diagnoses: chronic residual schizophrenia (73.33%, n=11), chronic paranoid schizophrenia (13.33%, n=2), chronic disorganized schizophrenia (6.67%, n=1), chronic catatonic schizophrenia (6.67%, n=1). Moreover, donor subjects were evaluated antemortem with the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression-Schizophrenia scale (CGI-SCH) (Haro et al., 2003; Kay et al., 1987) with a death to clinical assessment interval shorter than 48 months (Table 2). Our institutionalized donors shared the same environment from the clinical evaluation to their death, reducing external variability on clinical scores. The last mean daily chlorpromazine equivalent dose for the antipsychotic treatment of patients was based on the electronic records of last drug prescriptions administered up to death (Table 2) and was calculated as previously described (Gardner et al., 2010). Four patients were being medicated with first generation antipsychotics (26.67%), nine were medicated with second generation antipsychotics (60%), and two were antipsychotic-free (12.5%).
Table 2.
Demographic, clinical and tissue-related features of chronic schizophrenia cases (n=15)
| Schizophrenia (n=15) | |
|---|---|
| Gender | Male- 100% (n=15) |
| Age at death | 75 ± 10 years |
| PMD | 4.5 ± 2.4 hours |
| pH | 6.56 ± 0.36 |
| Age of onset of illness | 22 ± 7 years |
| Duration of illness | 53 ± 10 years |
| D-A interval1 | 17 ± 12 months |
| Daily AP dose2 | 521.30 ± 498.27 mg/day |
| Clinical Scales | |
| PANSS Positive | 25.53 ± 8.13 |
| PANSS Negative | 32.53 ± 10.26 |
| PANSS General | 53.60 ± 15.35 |
| CGI-SCH Positive | 4.40 ± 1.64 |
| CGI-SCH Negative | 5.53 ± 1.06 |
| CGI-SCH Depressive | 2.20 ± 1.26 |
| CGI-SCH Cognitive | 4.60 ± 1.59 |
Mean ± standard deviation or relative frequency are shown for each variable; PMD, postmortem delay; RIN, RNA integrity number; D-A, Death to clinical assessment interval; AP, antipsychotic; PANSS, Positive and Negative Syndrome Scale; CGI-SCH, Clinical Global Impression-Schizophrenia Scale; N/A, not applicable. All deaths were due to natural causes.
D-A Interval is defined as the number of months between clinical assessments and the time of death.
Last chlorpromazine equivalent dose was calculated based on the electronic records of drug prescriptions of the patients.
Specimens, extending from the pial surface to white matter and only including gray matter, were dissected and stored at −80°C. The study was approved by the Institutional Ethics Committee of Parc Sanitari Sant Joan de Déu.
2.2. Cell culture
Cerebellar granule neurons were obtained from P6 rats and cultured in 25mM KCl as previously described (Bilimoria and Bonni, 2008). All protocols regarding the use of animals were approved by the Committee for the Humane Use of Animals at Tufts University School of Medicine. Neurons were transfected with GFP and artificial N-terminal FLAG tagged truncated Sp4 derivatives by nucleofection at DIV0 (Kang et al., 2009; Ramos et al., 2007). S770 point mutants were generated in FLAG-Sp4 full-length and (610-784) deletion as described (Saia et al., 2014). At DIV 4, cells were treated with culture medium containing serum, 5mM KCl and 100μg/mL cycloheximide for the indicated times and protein was analyzed by Western blot.
2.3. Western blotting
Cells were lysed in RIPA buffer supplemented with protease inhibitors (Roche) and phosphatase inhibitors (Santa Cruz), and 10-20 μg of protein lysates were analyzed. Human brain samples were homogenized on ice in a Dounce homogenizer with NP40 lysis buffer as described (Pinacho et al., 2011) and 50 μg of human protein lysates were analyzed in a blinded manner. Equal amounts of protein were analyzed by SDS/PAGE and immunoblotted with the indicated primary antibodies: FLAG-M2 (Sigma); green fluorescent protein (GFP) (Life technologies); Sp4 (Santa Cruz); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Millipore); pSp4 (Saia et al., 2014); or pSp4 with 0.8 mg/ml of the phosphopeptide [CKKAAISQDpSNPATPN]. Linear range exposures were quantified by densitometry using Quantity One software (BioRad) in duplicate samples for human samples or Fiji software for Sp4 S770 point mutant experiments. The values from human samples were normalized to GAPDH and referred to an internal control standard sample to standardize the data between different sets of samples loaded in two independent gels and between repetitions. Cell culture lysates were normalized to GFP as both loading and transfection efficiency control. For each human sample, phospho-SP4/total SP4 ratio was calculated by referring phospho-SP4 normalized values to total SP4 normalized values.
2.4. Statistical analysis
For the analysis of postmortem brain tissue from control and patients, D'Agostino & Pearson omnibus normality test was carried out to test whether the variables followed a normal distribution. Outliers were detected by Peirce method. Non-parametric Mann-Whitney test or unpaired t test were used to compare pSP4/SP4 ratio or SP4 protein levels between control and bipolar disorder groups, respectively, according to the distribution of each variable. Spearman correlations were used for association with negative symptoms, since the scales did not follow a normal distribution, and for association between pSP4 and total SP4 levels. The effect size was calculated using Cohen's method (d) for parametric variables or the rank-biserial correlation (r) for Mann-Whitney (Wentd's method) for non-parametric variables. Bivariate analyses were carried out to detect association of our variables with potential confounding factors (age, gender, postmortem delay, toxicology and pH) (Table 3 and Table 4), using Mann–Whitney test for two groups, Kruskal-Wallis for more than two groups and Spearman correlation for quantitative variables. For studies of Sp4 S770 point mutants in rat cerebellar granule neurons, all results represent the mean ± SEM from at least 3 independent experiments. ANOVA followed by Bonferonni's post hoc test for multiple comparisons were performed where indicated. Statistical analysis was performed with GraphPad Prism version 5.00. The significance level was set to 0.05.
Table 3.
Association analysis of other variables in the study.
|
BD-C cohort (n=20) pSP4/SP4 ratio |
||
|---|---|---|
| Cerebellum | Prefrontal cortex | |
| Age | ||
| Spearman's r | −0.063 | 0.456 |
| 95% CI | (−0.5026, 0.4022) | (0.0027, 0.7538) |
| p value | 0.7912 | 0.0433 |
| Gender | ||
| U | 30.00 | 17.00 |
| p value | 0.8873 | 0.1707 |
| PMD | ||
| Spearman's r | 0.296 | 0.165 |
| 95% CI | (−0.1820, 0.6612) | (−0.3124, 0.5756) |
| p value | 0.2046 | 0.4875 |
| Toxicology | ||
| K-W | 1.692 | 4.381 |
| p value | 0.6386 | 0.2232 |
| pH | ||
| Spearman's r | −0.423 | −0.180 |
| 95% CI | (−0.7358, 0.03785) | (−0.5861, 0.2981) |
| p value | 0.0629 | 0.4473 |
r, Spearman's r correlation; CI, confidence interval; U, Mann-Whitney U; PMD, postmortem delay; K-W, Kruskal-Wallis statistic; BD, Bipolar Disorder; C, Control; ns, not significant. Significant p values are indicated in bold.
Table 4.
Association analysis of other variables in the pSP4/SP4 correlation analysis with negative symptoms in the cerebellum of schizophrenia subjects
|
SZ cohort (n=15) pSP4/SP4 ratio |
|
|---|---|
| Age | |
| Spearman's r | −0.131 |
| 95% CI | (−0.6132, 0.4230) |
| p value | 0.6427 |
| PMD | |
| Spearman's r | 0.134 |
| 95% CI | (−0.4201, 0.6154) |
| p value | 0.6339 |
| pH | |
| Spearman's r | 0.320 |
| 95% CI | (−0.2459, 0.7232) |
| p value | 0.2450 |
| CPZd | |
| Spearman's r | −0.034 |
| 95% CI | (−0.5488, 0.4995) |
| p value | 0.9043 |
| DI | |
| Spearman's r | 0.075 |
| 95% CI | (−0.4679, 0.5770) |
| p value | 0.7901 |
r, Spearman's r correlation; CI, confidence interval; PMD, postmortem delay; CPZd, chlorpromazine dose: last mean daily chlorpromazine equivalent dose; DI, duration of illness.
3. Results
3.1. Increased phospho-SP4 S770 in postmortem BD cerebellum
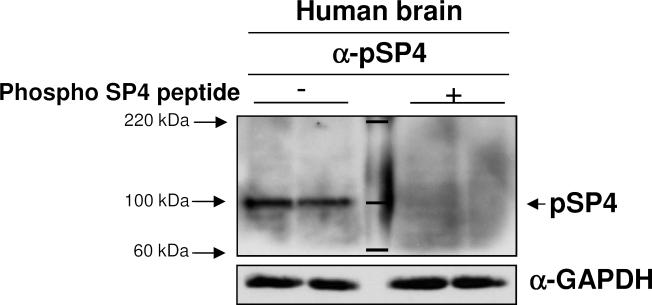
We investigated if phosphorylation at SP4 serine 770 is altered in BD subjects. We first determined whether this modification was present in human brain using a phospho-S770 SP4 specific antisera (Saia et al., 2014). The specificity of the phosphoSP4 (pSP4) signal detected in human postmortem brain by the pSP4 antibody was confirmed by blocking the signal with the specific phospho-peptide antigen (Figure 1).
Figure 1. Expression of phosphorylated SP4 in human postmortem brain tissue.
Specificity of phospho-SP4 S770 antibody was determined by competition assay with peptide antigen in protein extracts from human postmortem cerebellum. Full images of immunoblots for phospho-SP4 S770 (left), phospho-SP4 S770 antibody co-incubated with peptide antigen (right) and GAPDH in postmortem cerebellum samples are shown from a representative bipolar disorder sample in duplicate. Total SP4 levels for this sample are shown in Figure 2A, since it corresponds to the fourth BD sample. Molecular marker weights are labeled and shown in kDa.
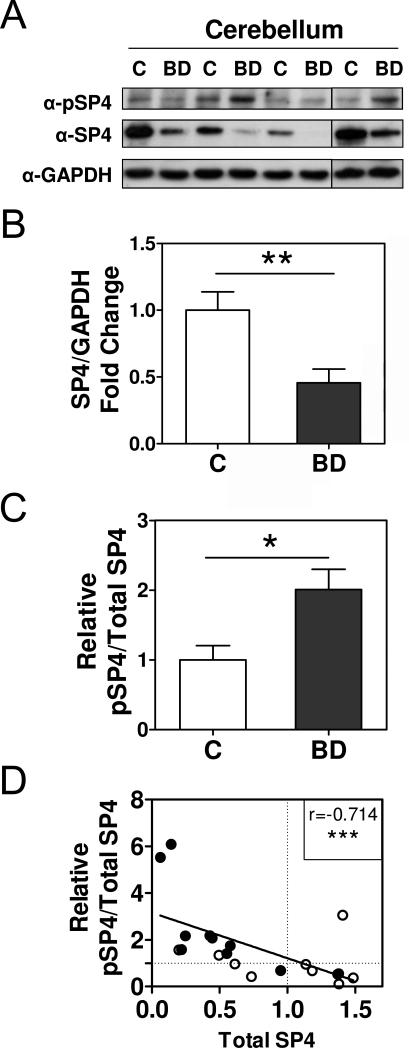
We determined SP4 phosphorylation levels and SP4 protein levels in protein lysates from the postmortem cerebellum of bipolar disorder and control subjects. We first confirmed that SP4 protein levels were reduced in the postmortem cerebellum in BD (t=3.160; df=18; 95% CI (0.1824, 0.9059); p=0.0027; mean±S.E.M: control=1.00±0.14, BD=0.46±0.10) (Figure 2A, 2B and S1), as we reported previously (Pinacho et al., 2011). The effect size was −1.25 (Cohen's d). We referred SP4 phosphorylation levels to total SP4 protein levels (ratio pSP4/SP4) in these samples. The ratio of pSP4/SP4 was significantly increased in BD (U=18.00; p=0.0147; median±S.E.M: control=0.81±0.27, BD=1.9±0.60) (Figure 2A, 2C and S1) and the effect size was 0.64 (Wentd's). Moreover, the relative abundance of phosphorylated SP4 compared to total SP4 levels also inversely and significantly associated with the total amount of SP4 protein, irrespective of disease status (Spearman's r=−0.714; 95%CI (−0.882, −0.385), p=0.0004) (Figure 2D), and remained significant after two outlier samples were excluded from the analysis (Spearman's r= −0.610; 95%CI (−0.8426, −0.1855), p=0.0072, n=18).
Figure 2. Increased phospho-SP4 S770 in the postmortem cerebellum of bipolar disorder patients.
Levels of phospho-SP4 S770 (pSP4), total SP4, and GAPDH were determined in protein extracts from cerebellar postmortem tissue of control (C) and bipolar disorder (BD) individuals. A. Images show representative pSP4, SP4, and GAPDH immunoblots from four control individuals (C) and four BD subjects (BD). B. The graph shows the mean and standard error of the mean of SP4 normalized to GAPDH for control and BD individuals from two independent analyses. Statistical analysis was performed using unpaired t test. C. The graph shows the mean and standard error of the mean of the ratio of pSP4 and total SP4 for control and BD individuals from two independent analyses. Statistical analysis was performed using the Mann-Whitney test. D. The graph shows the correlation between the ratio of pSP4 and total SP4 of control (open symbols) and bipolar disorder subjects (closed symbols). Both variables were normalized to the mean of the control group which is labeled with a dotted line for both axes. The solid line is adjusted to a linear regression of the values. Each value represents the mean of two independent analyses. The uncorrected Spearman coefficient r is shown in the graph. Corrected Spearman coefficient after exclusion of two outliers detected by Peirce's criterion was: Spearman's r= −0.610; 95%CI (−0.8426, −0.1855), p=0.0072, n=18. (*p<0.05, **p<0.01, *** p<0.001).
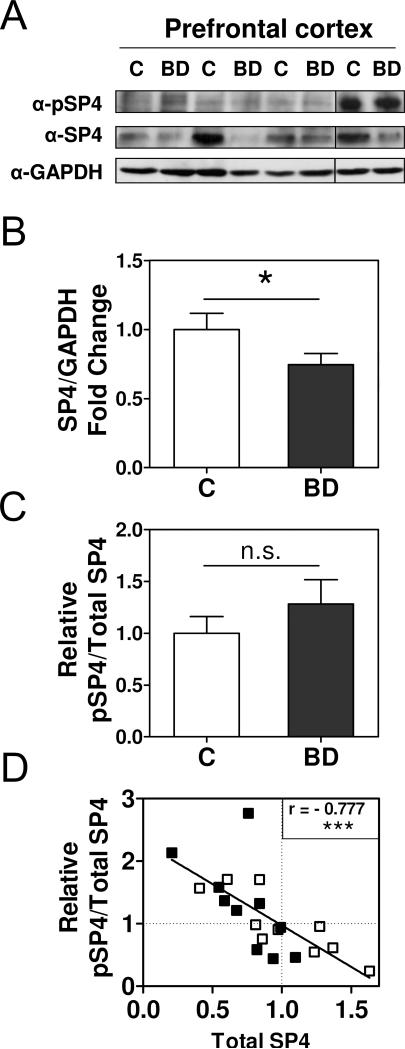
We further determined SP4 phosphorylation levels compared to SP4 protein levels in the postmortem prefrontal cortex of the same BD and control subjects. We confirmed that SP4 protein levels were reduced in the postmortem prefrontal cortex in BD (t=1.772; df=18; 95% CI (−0.0471, 0.5555); p=0.0466; mean±S.E.M: control=1.00±0.12, BD=0.75±0.08) (Figure 3A, 3B and S2) as reported in our previous study (Pinacho et al., 2011). The effect size was −0.68 (Cohen's d). We found that the ratio of phosphorylated SP4 to total SP4 levels in the prefrontal cortex was not statistically significant between groups (U=42.00; p=0.5781; median±S.E.M: control=0.93±0.16, BD=1.27±0.24) (Figure 3A, 3C and S2). However, the relative abundance of phosphorylated SP4 compared to total SP4 levels in this brain region also inversely and significantly associated with the total amount of SP4 protein, irrespective of disease status (Spearman's r=−0.777; 95%CI (−0.910, −0.500), p<0.0001) (Figure 3D).
Figure 3. Total SP4 protein levels are inversely correlated with pSP4/SP4 ratio in the postmortem prefrontal cortex of control and bipolar disorder subjects.
Levels of phospho-SP4 S770 (pSP4), total SP4, and GAPDH were determined in protein extracts from postmortem prefrontal cortex tissue of control (C) and bipolar disorder (BD) individuals. A. Images show representative pSP4, SP4, and GAPDH immunoblots from four control individuals (C) and four BD subjects (BD). B. The graph shows the mean and standard error of the mean of SP4 normalized to GAPDH for control and BD individuals from two independent analyses. Statistical analysis was performed using unpaired t test. C. The graph shows the mean and standard error of the mean of the ratio of pSP4 and total SP4 for control and BD individuals from two independent analyses. Statistical analysis was performed using the Mann-Whitney test. D. The graph shows the correlation between the ratio of pSP4 and total SP4 of control (open symbols) and bipolar disorder subjects (closed symbols). Both variables were normalized to the mean of the control group which is labeled with a dotted line for both axes. The solid line is adjusted to a linear regression of the values. Each value represents the mean of two independent analyses. The Spearman coefficient r is shown. (ns, not significant; *p<0.05; *** p<0.001).
We have also analyzed the influence of other demographic and tissue-derived variables such as age, postmortem delay or pH on the ratio of pSP4/SP4 in the BD cohort. This analysis did not show any significant associations for cerebellum, indicating that pSP4/SP4 ratio was not influenced by any of the potential confounding factors (Table 3). However, this analysis in the prefrontal cortex showed that older subjects have a higher pSP4/SP4 ratio in the prefrontal cortex (Table 3), in line with the significant inverse association of SP4 levels with age we described in our previous SP4 study in bipolar disorder (Pinacho et al., 2011). These results indicate that phospho-SP4 S770 levels are significantly increased in the cerebellum of patients with BD as compared to control subjects and that the levels of S770 phosphorylated SP4 are inversely correlated with total SP4 levels both in the human cerebellum and prefrontal cortex independently of disease status.
3.2. The relative abundance of phospho-SP4 S770 to total SP4 protein levels associates with negative symptom severity in postmortem cerebellum of schizophrenia subjects
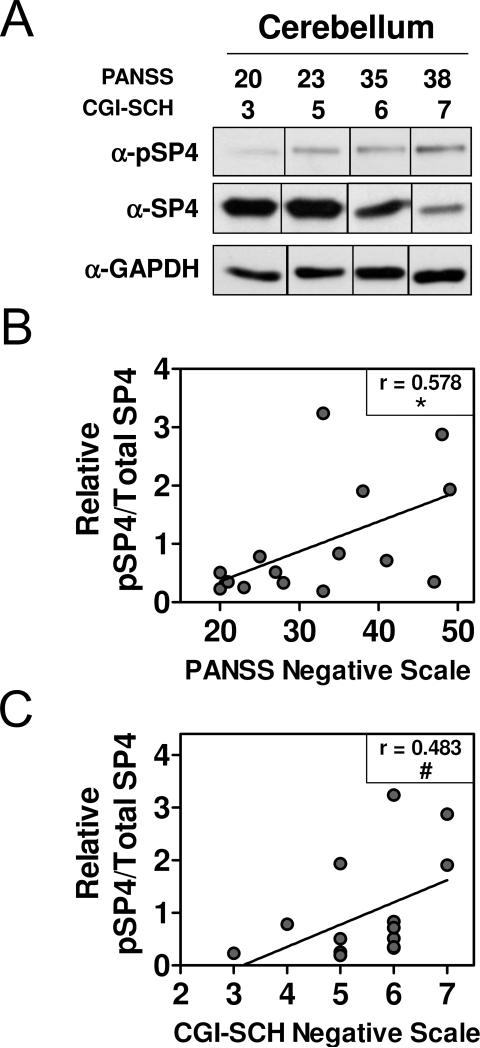
We determined SP4 S770 phosphorylation levels in protein lysates from the postmortem cerebellum of 15 subjects with chronic schizophrenia (Figure 4A and S3). We calculated pSP4/SP4 ratio in these samples by referring SP4 phosphorylation levels to total SP4 protein levels reported in our previous study (Pinacho et al., 2013). The ratio of pSP4/SP4 was significantly associated with negative symptoms in schizophrenia measured by the PANSS negative scale (Spearman's r= 0.578; 95%CI (0.076, 0.846), p= 0.0241) (Figure 4B), and showed a trend to associate with SZ negative symptom severity measured by the GCI-SCH scale (Spearman's r= 0.483; 95%CI (−0.055, 0.804), p= 0.0679) (Figure 4C).
Figure 4. pSP4/SP4 ratio in the cerebellum associates with negative symptom severity in schizophrenia.
Protein extracts from cerebellum of subjects with schizophrenia (n=15) were analyzed by immunoblotting for pSP4 and GAPDH and pSP4/SP4 ratio was computed by referring pSP4 to previously reported SP4 protein levels from the same samples (Pinacho et al., 2013). (A) Images show representative pSP4, SP4 and GAPDH immunoblots of four representative schizophrenia subjects and their score for negative symptoms measured by the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression-Schizophrenia scale (CGI-SCH). Full-length blots are shown in Figure S3. The graphs represent pSP4/SP4 ratio and negative symptom severity measured with the PANSS (B) and the CGI-SCH (C) scales. Data were adjusted to a linear regression. Statistical analysis was performed using Spearman correlation; Spearman's r is indicated for each analysis (*p<0.05; #p=0.0679).
We have also analyzed the influence of other demographic and tissue-derived variables such as age, postmortem delay, pH, last mean daily chlorpromazine equivalent dose and duration of illness on the ratio of pSP4/SP4 in the SZ cohort. This analysis did not show any significant associations, indicating that the association of pSP4/SP4 ratio in cerebellum with SZ negative symptom severity was not influenced by any of the potential confounding factors (Table 4).
3.3. Sp4 S770 point mutants contribute to changes in protein stability
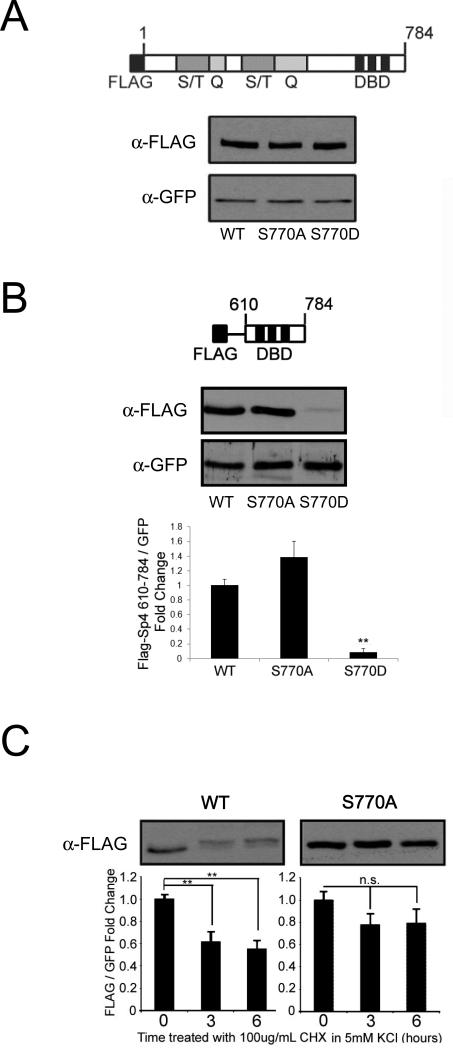
Since we observed a correlation between reduced SP4 levels and increased SP4 phosphorylation in human postmortem brain (Figure 2D and 3D), in line with our previous observations in cultured rat cerebellar granule neurons (Saia et al., 2014), we considered the possibility that phosphorylation may promote Sp4 degradation. We used non-phosphorylatable (S770A) and phospho-mimetic (S770D) FLAG-Sp4 point mutants to examine the role of phosphorylation in protein degradation. Consistent with previous findings, mutation of S770 did not alter steady state levels of full-length Sp4 in cerebellar granule neurons (Figure 5A) (Saia et al., 2014). In addition, full-length Sp4 S770D mutant did not show a statistically significant change in stability up to 8 hours in the presence of the protein synthesis inhibitor cycloheximide (Figure S4). These findings suggest that phosphorylation at S770 is not sufficient to determine the accumulation or stability of Sp4. We did, however, observe a trend towards decreased stability of the full-length S770D mutant Sp4, and we reasoned that in an artificial truncated protein, not found endogenously, S770 mutants may have a more pronounced effect on stability. We therefore analyzed S770A and S770D point mutants in the context of an Sp4 deletion mutant (610-784) that we previously showed to be phosphorylated at S770 in response to reduced membrane potential in rat cerebellar granule neurons (Saia et al., 2014).
Figure 5. Mutation of S770 alters levels and stability of truncated Sp4.
A. Cerebellar granule neurons were transfected at DIV0 with full-length (1-784) FLAG-Sp4 wild-type (WT), S770A, or S770D point mutants together with GFP and protein expression was analyzed by Western blot at DIV4. Top: diagram of FLAG-Sp4 construct; S/T, serine-threonine rich region; Q, glutamine-rich region; DBD, DNA binding domain. Bottom: representative immunoblots from an experiment performed at least 3 times. B. Cerebellar granule neurons were transfected at DIV0 with truncated FLAG-Sp4(610-784) wild-type (WT), S770A, or S770D point mutants together with GFP and protein extraction was performed at DIV4. Protein expression was analyzed by Western blot. Top: diagram of FLAG-Sp4 construct analyzed; DBD, DNA binding domain. Middle: a representative immunoblot from an experiment performed at least 3 times. Bottom: FLAG-Sp4 was quantified relative to co-transfected GFP in three independent experiments. **p <0.01 compared to WT. C. Cerebellar granule neurons transfected at DIV0 with truncated FLAG-Sp4(610-784) wild-type or S770A were treated at DIV4 with 5mM KCl and 100ug/mL cycloheximide for the indicated times and analyzed by Western blot. FLAG-Sp4 was quantified relative to co-transfected GFP in at least 3 independent experiments. **p<0.01 compared to time point zero.
In the Sp4 deletion mutant, the phospho-mimetic S770D was very poorly expressed, consistent with the hypothesis that this protein is constitutively degraded (Figure 5B) (F=22.04; df=2,6; p=0.002). We also examined the stability of truncated Sp4 (610-784) in non-depolarizing conditions in the presence of cycloheximide. Although the wild-type FLAG-Sp4 deletion derivative was rapidly degraded (F=11.27; df=2,9; p=0.0035), as previously observed for endogenous Sp4 (Pinacho et al., 2011), the non-phosphorylatable S770A mutant exhibited increased stability (F=1.48; df=2,8; p=0.3001) (Figure 5C). Taken together, these data suggest that phosphorylation at S770 may be part of a pathway regulating degradation of the Sp4 protein, although there are likely to be additional mechanisms acting on full-length Sp4.
4. Discussion
Our rationale for undertaking this study was based on observations that reduced levels of SP4 protein were observed in the BD cerebellum and in association with negative symptom severity in the SZ cerebellum, and that conditions in rat cerebellar granule neuron culture that lead to reduced Sp4 also lead to increased phosphorylation (Pinacho et al., 2011; Pinacho et al., 2013; Saia et al., 2014). Here we report a significant increase in SP4 phosphorylation at S770 in the postmortem cerebellum of BD subjects that mainly committed suicide. Moreover, we have found that increased SP4 phosphorylation at S770 associates with reduced SP4 protein levels both in human cerebellum and prefrontal cortex independently of disease status. We have also confirmed that increased cerebellar pSP4/SP4 ratio associates with more severe negative symptoms in schizophrenia. Also, we show that in the context of a truncated Sp4, a phospho-mimetic mutant was poorly expressed, while the non-phosphorylatable mutant exhibited increased stability. Our findings suggest a pathway by which changes in Sp4 phosphorylation may contribute to the physiopathology of bipolar disorder and/or suicide and of negative symptoms in schizophrenia via impacts on Sp4 activity and abundance.
Our data strongly supports altered post-translational regulation of transcription factor SP4 in the cerebellum in BD and SZ, indicated by increased phosphorylation of SP4 at S770. Phosphorylation of many proteins alters their ubiquitination status and, in fact, some ubiquitin E3 ligases preferentially target phosphorylated substrates, priming them for subsequent degradation by the proteasome (Ravid and Hochstrasser, 2008). Using point mutations we show here that a phospho-mimetic in a truncated Sp4 was poorly expressed, while the non-phosphorylatable mutant exhibited increased Sp4 stability. Mutation of S770 was not sufficient, however, to significantly alter the abundance or stability of full-length Sp4 protein (Figure 5, S4 and (Saia et al., 2014)). Consistent with the involvement of additional mechanisms, we observed that the contribution of pSP4/SP4 ratio to the variance of total SP4 varied from 50 to 60% (cerebellum, R2=0.510; PFC, R2=0.604). Taken together, we hypothesize that there are likely to be additional sites of regulation governing the stability of Sp4, and that phosphorylation at S770 represents part of a signaling pathway controlling Sp4 levels in cerebellar granule neurons.
We previously reported that SP4 mRNA and protein levels are decreased in the prefrontal cortex in bipolar disorder, indicating that SP4 decrease in this particular area may be due to transcriptional regulation (Pinacho et al., 2011). In line with this, we have observed here that although pSP4/SP4 ratio correlates with total SP4, there are no significant differences in pSP4/SP4 ratio between control and patient groups in this region. This suggests that while phosphorylation-dependent mechanisms may affect SP4 protein levels in the prefrontal cortex, these mechanisms seem to be independent of the disease status. The decrease in SP4 protein levels in the prefrontal cortex in BD is more likely to be due to transcriptional regulation mechanisms than to a change in SP4 protein stability. This is in contrast to the disease-specific post-transcriptional regulation we observe for SP4 in the cerebellum in our bipolar disorder cohort.
While classically the cerebellum has been considered a motor function coordinator, an increasing body of evidence indicates that the cerebellum also contributes to affective, psychological and cognitive deficits is psychiatric disorders (Hoppenbrouwers et al., 2008; Konarski et al., 2005; Schmahmann et al., 2007). Recent molecular and imaging studies (Adler et al., 2007; Kim et al., 2013) have shown that the cerebellum contributes to the neurological basis of psychiatric disorders, i.e. altered expression of gamma-aminobutyric acid (GABA) receptors has been reported in cerebellum of BD subjects (Fatemi et al., 2013). Moreover, cerebellar impairments in schizophrenia have been shown to correlate with cerebellar neurologic defects and negative symptoms (Ho et al., 2004; Thomann et al., 2009), suggesting that the cerebellum may contribute to negative symptoms. These evidences, combined with our results here and in our previous reports of reduced SP4 in the cerebellum of BD subjects and in SZ subjects with severe negative symptoms (Pinacho et al., 2011; Pinacho et al., 2013), provide further evidence for a role of the cerebellum in the pathophysiology of BD and SZ.
Our findings provide insights into the pathophysiological mechanisms that may be contributing to BD. An increase in the relative levels of SP4 phosphorylation in BD implicates alterations in the upstream signaling pathways regulating SP4 in the disorder and/or in suicide. We have previously reported that the NMDA receptor dependent activation of a PP1/PP2A protein phosphatase reduces the levels of Sp4 phosphorylation in primary neurons (Saia et al., 2014). Although this signaling pathway, by itself, was not sufficient to influence the total levels of Sp4, it may act to prime Sp4 for degradation. Dysfunction of the glutamatergic system has been implicated in the pathogenesis of several neuropsychiatric disorders, including BD, major depression and schizophrenia (Fountoulakis, 2012; Javitt, 2007; Marsden, 2011). Some reports also have provided evidence of deregulation of NMDA signaling pathways in suicide (Choudary et al., 2005; Kekesi et al., 2012; Nagy et al., 2014; Nowak et al., 1995; Sequeira et al., 2009). Postmortem brain studies have shown changes in transcripts and expression of glutamate receptors and associated signaling components in bipolar disorder and/or suicide (Beneyto et al., 2007; Beneyto and Meador-Woodruff, 2008; Clinton and Meador-Woodruff, 2004; Choudary et al., 2005; Eastwood and Harrison, 2010; Kekesi et al., 2012; McCullumsmith et al., 2007; Nowak et al., 1995; Sequeira et al., 2009). Thus, our finding of increased pSP4 could implicate NMDA receptor hypofunction and/or the reduced activity of the PP1/PP2A phosphatase in the pathophysiology of BD and/or suicide.
Glutamate hypofunction is also proposed as one of the main contributors to symptom emergence in schizophrenia (Jentsch and Roth, 1999; Kantrowitz and Javitt, 2010; Krystal et al., 1994). Moreover, an increasing body of evidence has suggested that Sp4 transcription factor has a role in complex psychotic disorders through its regulation of a large network of genes, including components of NMDAR signaling pathways (Fuste et al., 2013; Ji et al., 2013; Pinacho et al., 2011; Pinacho et al., 2013; Sun et al., 2015; Zhou et al., 2005). Notably, we also observed reduced SP4 protein abundance without changes in its mRNA expression in the cerebellum of schizophrenia subjects and in peripheral blood mononuclear cells from first-episode psychosis patients (Fuste et al., 2013; Pinacho et al., 2013). These findings support the view that post-translational regulation of SP4 is likely to play a role in the modulation of SP4 steady-state protein levels in many psychiatric disorders. Thus, our findings of increased SP4 S770 phosphorylation and reduced SP4 levels in BD and severe SZ subjects not only provide further evidence on SP4 post-translational regulation in BD that committed suicide and SZ subjects with severe negative symptoms but also open the possibility that altered phosphorylation could also appear in peripheral blood mononuclear cells from first-episode psychosis. Further studies are needed to confirm this hypothesis and remain an important ongoing effort.
Sp4 has been shown to be important for a variety of physiological processes in neurons, including dendrite outgrowth, the regulation of NMDA receptors, and long-term potentiation (Ji et al., 2013; Liu et al., 2003; Priya et al., 2013; Ramos et al., 2007; Ramos et al., 2009; Zhou et al., 2005; Zhou et al., 2010). It is unclear if changes in SP4 phosphorylation and levels represent an adaptive response to an upstream deficit, or if these changes contribute directly to the pathology of BD and SZ. Connectivity defects and aberrant neuronal dendritic arbours have been reported in subjects with psychotic and mood disorders (Friston and Frith, 1995; McGuire and Frith, 1996; Miguel-Hidalgo and Rajkowska, 2002; Rosoklija et al., 2000; Savitz and Drevets, 2009). Sp4 is essential for the regulation of dendritic morphogenesis in developing cerebellar granule neurons, by limiting dendrite branching and promoting the elimination of excess primary dendrites (Ramos et al., 2007; Ramos et al., 2009). We have recently shown that Sp4 S770 phosphorylation impairs Sp4-dependent maturation of cerebellar granule neuron primary dendrites (Saia et al., 2014). Here, we report an increase of S770 phosphorylation in the cerebellum of bipolar disorder patients that committed suicide, and in schizophrenia patients with more severe negative symptoms. This opens the possibility that an increase in Sp4 S770 phosphorylation may also be impairing Sp4-dependent dendrite maturation, leading to altered connectivity in these disorders. Regardless, our results reveal new insights and directions of study into molecular pathways associated with BD and/or suicide and negative symptomatology in SZ.
The use of human postmortem brain entails certain limitations, but it is a key tool for the study of molecular mechanisms disrupted in psychiatric disorders. For example, potential confounding factors, including age, gender, postmortem delay, antipsychotic treatments and pH, have to be carefully considered. We have controlled for the influence of these variables in the analysis of pSP4 in postmortem samples, ruling out their influence in the findings of increased SP4 S770 phosphorylation in the cerebellum in BD and in the association of pSP4/SP4 ratio with negative symptoms in SZ. Unfortunately, the possible effect of long term treatments in our sample cannot be ruled out, since this data was not available. However, the analysis of drugs present in blood at the time of death in BD subjects and the daily antipsychotic dose in SZ subjects suggests the last treatment did not influence our findings. Also, the prevalence of suicide in the bipolar cohort (eight out of ten BD subjects) examined here may suggest a possible confounder effect of this mechanism of death on our molecular findings. Further studies will be needed in order to explore this possibility in non bipolar disorder subjects that committed suicide or in a cohort of BD with a different mechanism of death. Moreover, these findings should be extended to larger and independent cohort of patients to confirm whether the increased SP4 S770 phosphorylation in cerebellum is a consistent molecular alteration among BD and SZ subjects. Despite these limitations, this study may contribute towards a better understanding of the regulatory mechanisms modulating SP4 protein post-translational modification and abundance, and their contribution to the pathophysiology of psychotic disorders.
Supplementary Material
Acknowledgements
We thank X. Sun, N. Villalmanzo, and staff members of the Basque Institute of Legal Medicine, Bilbao, Spain for technical assistance.
Role of funding sources
This work was supported by MCINN-Spain: Plan Nacional de I+D BFU2008-01103 to B.R., and, ISCIII and Fondo de Investigación Sanitaria: PFIS to R.P; and grants from the National Institutes of Health: RO1 HD043364 to G.G. and support for G.S. from the T32 NS061764 training grant. The funding bodies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author R.P. performed the processing, expression measurements and statistical analysis of human postmortem tissue; and co-wrote the first draft of the manuscript. Author G.S. performed the experiments, expression measurements and the statistical analysis of the cerebellar granule neuron experiments; and co-wrote the first draft of the manuscript. Author J.J.M. contributed to clinical protocol and statistical analysis. Author G.G. co-designed the study, assisted with the CGN experiment analysis, and co-wrote the first draft of the manuscript. Author B.R. co-designed the study, assisted with the human protein expression analysis, and co-wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
Conflict of interest
All authors declare that they have no financial conflicts of interest.
References
- Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Molecular pharmacology. 2005;68:317–329. doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry. 2007;61:776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Belmaker RH. Bipolar disorder. The New England journal of medicine. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Bilimoria PM, Bonni A. Cultures of cerebellar granule neurons. CSH protocols 2008. 2008 doi: 10.1101/pdb.prot5107. pdb prot5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL. Prepulse inhibition of the startle reflex: a window on the brain in schizophrenia. Curr Top Behav Neurosci. 2011;4:349–371. doi: 10.1007/7854_2010_61. [DOI] [PubMed] [Google Scholar]
- Braff DL, Freedman R, Schork NJ, Gottesman Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA Receptor and Associated Intracellular Molecules in the Thalamus in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:1353–1362. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr., Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Markers of glutamate synaptic transmission and plasticity are increased in the anterior cingulate cortex in bipolar disorder. Biological psychiatry. 2010;67:1010–1016. doi: 10.1016/j.biopsych.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. Expression of GABAA alpha2-, beta1- and epsilon-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl Psychiatry. 2013;3:e303. doi: 10.1038/tp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN. The possible involvement of NMDA glutamate receptor in the etiopathogenesis of bipolar disorder. Curr Pharm Des. 2012;18:1605–1608. doi: 10.2174/138161212799958585. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Fuste M, Pinacho R, Melendez-Perez I, Villalmanzo N, Villalta-Gil V, Haro JM, Ramos B. Reduced expression of SP1 and SP4 transcription factors in peripheral blood mononuclear cells in first-episode psychosis. J Psychiatr Res. 2013;47:1608–1614. doi: 10.1016/j.jpsychires.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Hajek T, Carrey N, Alda M. Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar Disord. 2005;7:393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- Haro JM, Kamath SA, Ochoa S, Novick D, Rele K, Fargas A, Rodriguez MJ, Rele R, Orta J, Kharbeng A, Araya S, Gervin M, Alonso J, Mavreas V, Lavrentzou E, Liontos N, Gregor K, Jones PB. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003:16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–1153. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Schutter DJ, Fitzgerald PB, Chen R, Daskalakis ZJ. The role of the cerebellum in the pathophysiology and treatment of neuropsychiatric disorders: a review. Brain Res Rev. 2008;59:185–200. doi: 10.1016/j.brainresrev.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Ji B, Wang X, Pinto-Duarte A, Kim M, Caldwell S, Young JW, Behrens MM, Sejnowski TJ, Geyer MA, Zhou X. Prolonged Ketamine Effects in Hypomorphic Mice: Mimicking Phenotypes of Schizophrenia. PLoS One. 2013;8:e66327. doi: 10.1371/journal.pone.0066327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Ramu S, Lee S, Aguilar B, Ganesan SK, Yoo J, Kalra VK, Koh CJ, Hong YK. Phosphate-buffered saline-based nucleofection of primary endothelial cells. Analytical biochemistry. 2009;386:251–255. doi: 10.1016/j.ab.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain research bulletin. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kekesi KA, Juhasz G, Simor A, Gulyassy P, Szego EM, Hunyadi-Gulyas E, Darula Z, Medzihradszky KF, Palkovits M, Penke B, Czurko A. Altered functional protein networks in the prefrontal cortex and amygdala of victims of suicide. PLoS One. 2012;7:e50532. doi: 10.1371/journal.pone.0050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cho HB, Dager SR, Yurgelun-Todd DA, Yoon S, Lee JH, Lee SH, Lee S, Renshaw PF, Lyoo IK. Posterior cerebellar vermal deficits in bipolar disorder. J Affect Disord. 2013;150:499–506. doi: 10.1016/j.jad.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. J Psychiatr Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–186. [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Liu A, Zhuang Z, Hoffman PW, Bai G. Functional analysis of the rat N-methyl-D-aspartate receptor 2A promoter: multiple transcription starts points, positive regulation by Sp factors, and translational regulation. J Biol Chem. 2003;278:26423–26434. doi: 10.1074/jbc.M211165200. [DOI] [PubMed] [Google Scholar]
- Mahon K, Wu J, Malhotra AK, Burdick KE, DeRosse P, Ardekani BA, Szeszko PR. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34:1590–1600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Yang SH, Simpkins JW, Barger SW. Glutamate receptor activation evokes calpain-mediated degradation of Sp3 and Sp4, the prominent Sp-family transcription factors in neurons. J Neurochem. 2007;100:1300–1314. doi: 10.1111/j.1471-4159.2006.04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden WN. Stressor-induced NMDAR dysfunction as a unifying hypothesis for the aetiology, pathogenesis and comorbidity of clinical depression. Med Hypotheses. 2011;77:508–528. doi: 10.1016/j.mehy.2011.06.021. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain research. 2007;1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychol Med. 1996;26:663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Morphological brain changes in depression: can antidepressants reverse them? CNS Drugs. 2002;16:361–372. doi: 10.2165/00023210-200216060-00001. [DOI] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.21. 10.1038/mp.2014.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- Pinacho R, Villalmanzo N, Lalonde J, Haro JM, Meana JJ, Gill G, Ramos B. The transcription factor SP4 is reduced in postmortem cerebellum of bipolar disorder subjects: control by depolarization and lithium. Bipolar disorders. 2011;13:474–485. doi: 10.1111/j.1399-5618.2011.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinacho R, Villalmanzo N, Roca M, Iniesta R, Monje A, Haro JM, Meana JJ, Ferrer I, Gill G, Ramos B. Analysis of Sp transcription factors in the postmortem brain of chronic schizophrenia: a pilot study of relationship to negative symptoms. J Psychiatr Res. 2013;47:926–934. doi: 10.1016/j.jpsychires.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Priya A, Johar K, Wong-Riley MT. Specificity protein 4 functionally regulates the transcription of NMDA receptor subunits GluN1, GluN2A, and GluN2B. Biochim Biophys Acta. 2013;1833:2745–2756. doi: 10.1016/j.bbamcr.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos B, Gaudilliere B, Bonni A, Gill G. Transcription factor Sp4 regulates dendritic patterning during cerebellar maturation. Proc Natl Acad Sci U S A. 2007;104:9882–9887. doi: 10.1073/pnas.0701946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos B, Valin A, Sun X, Gill G. Sp4-dependent repression of neurotrophin-3 limits dendritic branching. Mol Cell Neurosci. 2009;42:152–159. doi: 10.1016/j.mcn.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Nature reviews. Molecular cell biology. 2008;9:679–690. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M, Escanilla A, Monje A, Baño V, Planchat L, Costa J, Haro J. Banco de tejidos neurológicos de Sant Joan de Déu-Serveis de Salut Mental para la investigación de las enfermendades mentales. La importancia de un programa de donación en vida. Psiquiatría Biológica. 2008;15:73–79. [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Saia G, Lalonde J, Sun X, Ramos B, Gill G. Phosphorylation of the transcription factor Sp4 is reduced by NMDA receptor signaling. J Neurochem. 2014;129:743–752. doi: 10.1111/jnc.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Pinacho R, Saia G, Punko D, Meana JJ, Ramos B, Gill G. Transcription factor Sp4 regulates expression of nervous wreck 2 to control NMDAR1 levels and dendrite patterning. Dev Neurobiol. 2015;75:93–108. doi: 10.1002/dneu.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- Thomann PA, Roebel M, Dos Santos V, Bachmann S, Essig M, Schroder J. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2009;173:83–87. doi: 10.1016/j.pscychresns.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Wadehra S, Pruitt P, Murphy ER, Diwadkar VA. Network dysfunction during associative learning in schizophrenia: Increased activation, but decreased connectivity: an fMRI study. Schizophr Res. 2013;148:38–49. doi: 10.1016/j.schres.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Zhou X, Long JM, Geyer MA, Masliah E, Kelsoe JR, Wynshaw-Boris A, Chien KR. Reduced expression of the Sp4 gene in mice causes deficits in sensorimotor gating and memory associated with hippocampal vacuolization. Mol Psychiatry. 2005;10:393–406. doi: 10.1038/sj.mp.4001621. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nie Z, Roberts A, Zhang D, Sebat J, Malhotra D, Kelsoe JR, Geyer MA. Reduced NMDAR1 expression in the Sp4 hypomorphic mouse may contribute to endophenotypes of human psychiatric disorders. Hum Mol Genet. 2010;19:3797–3805. doi: 10.1093/hmg/ddq298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Qyang Y, Kelsoe JR, Masliah E, Geyer MA. Impaired postnatal development of hippocampal dentate gyrus in Sp4 null mutant mice. Genes, brain, and behavior. 2007;6:269–276. doi: 10.1111/j.1601-183X.2006.00256.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Tang W, Greenwood TA, Guo S, He L, Geyer MA, Kelsoe JR. Transcription factor SP4 is a susceptibility gene for bipolar disorder. PloS one. 2009;4:e5196. doi: 10.1371/journal.pone.0005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.