Abstract
Metabolic engineering to increase the glucose uptake rate might be beneficial to improve microbial production of various fuels and chemicals. In this study, we enhanced the glucose uptake rate in Saccharomyces cerevisiae by overexpressing hexose transporters (HXTs). Among the 5 tested HXTs (Hxt1, Hxt2, Hxt3, Hxt4, and Hxt7), overexpression of high-affinity transporter Hxt7 was the most effective in increasing the glucose uptake rate, followed by moderate-affinity transporters Hxt2 and Hxt4. Deletion of STD1 and MTH1, encoding corepressors of HXT genes, exerted differential effects on the glucose uptake rate, depending on the culture conditions. In addition, improved cell growth and glucose uptake rates could be achieved by overexpression of GCR1, which led to increased transcription levels of HXT1 and ribosomal protein genes. All genetic modifications enhancing the glucose uptake rate also increased the ethanol production rate in wild-type S. cerevisiae. Furthermore, the growth-promoting effect of GCR1 overexpression was successfully applied to lactic acid production in an engineered lactic acid-producing strain, resulting in a significant improvement of productivity and titers of lactic acid production under acidic fermentation conditions.
INTRODUCTION
Saccharomyces cerevisiae has been used as an important cell factory for production of fuels and chemicals (1, 2). Although yeast can utilize a wide range of carbon sources, glucose is the most widely preferred carbon source for yeast fermentation (3). Glucose uptake in S. cerevisiae is elaborately regulated to adapt cellular metabolism in response to ever-changing environmental conditions (3, 4). However, to achieve high titers and productivity of target chemicals using metabolically engineered yeast cells, it may be beneficial to inactivate, circumvent, or modify some of such natural regulatory mechanisms.
The first regulatory step of glucose uptake is the facilitated diffusion of glucose through hexose transporters (HXTs) in the plasma membrane (4, 5). S. cerevisiae contains 18 HXT family members of hexose transporters, Hxt1 to Hxt17 and Gal2, among which Hxt1 to Hxt7 function as major glucose transporters (6). Expression of the HXTs, all of which exhibit different levels of glucose affinity, is differentially regulated depending on extracellular glucose concentrations (7–9). Hxt1 (Km = ∼100 mM) and Hxt3 (Km = ∼30 to 60 mM) are classified as low-affinity glucose transporters. HXT1 is highly induced in the presence of high (>1%) concentrations of glucose, whereas HXT3 is induced by both low and high levels of glucose. When the glucose concentrations are lowered to around 0.1%, HXT2 and HXT4, encoding moderate-affinity glucose transporters (Km = ∼5 to 10 mM), are expressed (10). HXT6 and HXT7, encoding highly homologous glucose transporters with very high glucose affinity (Km = ∼1 mM), are expressed just before the depletion of glucose in the medium (11). Expression of HXT5, encoding a moderate-affinity transporter (Km = ∼10 mM), is not regulated by extracellular glucose concentrations but by various environmental conditions affecting growth rates (12, 13).
The glucose concentration-dependent expression of HXT genes is achieved by a complex interconnection of both glucose induction and glucose repression mechanisms (14, 15). Induction of the HXT1, HXT2, HXT3, and HXT4 genes in the presence of glucose is mainly regulated by plasma membrane glucose sensors Snf3 and Rgt2 and by a transcriptional repressor, Rgt1 (16, 17). In the absence of glucose, HXT genes are repressed by Rgt1 in association with corepressors Mth1 and Std1 (18, 19). In the presence of glucose, binding of glucose to Snf3 and Rgt2 triggers a signal corresponding to the degradation of Mth1 and Std1, resulting in the release of Rgt1 from the promoters and subsequent derepression of HXT genes (20). On the other hand, HXT2, HXT3, HXT4, HXT6, and HXT7 are repressed by Mig1 in the presence of glucose (10, 21). Upon glucose starvation, activated Snf1 kinase phosphorylates Mig1, leading to its nuclear export and derepression of its target genes.
Previously, it was shown that overexpression of HXT1 or HXT7 in S. cerevisiae increases the rates of glucose uptake and ethanol production (22). Moreover, overexpression of HXT1 or HXT7 led to increased productivity and titers of lactic acid in S. cerevisiae cells engineered for lactic acid production, suggesting the possibility of manipulating the glucose uptake rate to improve fermentation performance for the production of various target chemicals under conditions in which optimal metabolic pathways toward the target product are provided (22). In addition to an increase in HXT protein levels in the plasma membrane, an increase in glycolytic flux might serve as a driving force to enhance the glucose uptake rate. However, so far, overexpression of glycolytic enzymes individually or in combination in experiments designed to significantly improve glycolytic flux has not been successful (23–27). In general, the highest yield of a desired chemical can be reached by maximizing the metabolic flux to the desired product while minimizing biomass synthesis. However, the drain of metabolites from essential pathways and/or product toxicity could inhibit cell growth, exerting a negative effect on the production of the target chemical by decreasing volumetric productivity. Therefore, a proper balance between growth rate and production flux is important to achieve both high yield and productivity (28).
In this study, we improved the glucose uptake rate in S. cerevisiae by overexpressing HXTs with various glucose affinities. In addition, improved cell growth and glucose uptake rates could be achieved by overexpressing a transcriptional activator, Gcr1, which led to increased expression levels of HXT1 and genes encoding ribosomal proteins (RPs). Overexpression of GCR1 was successfully applied to lactic acid production in an engineered strain, resulting in a significant improvement of lactic acid production under acidic fermentation conditions, where overcoming the growth-inhibitory effect is critical to improve lactic acid production.
MATERIALS AND METHODS
Yeast strains, media, and culture conditions.
The S. cerevisiae strains used in this study and the primers used for the construction of the strains are described in Table 1 and Table 2, respectively. All S. cerevisiae strains used in this study were derivatives of CEN.PK2-1C. JHY701 (mth1Δ), JHY702 (std1Δ), and JHY703 (mth1Δ std1Δ) strains were generated by deleting MTH1 and/or STD1 by PCR-mediated homologous recombination based on the Cre/loxP recombination system (31). The integration cassettes for the deletion of MTH1 and STD1 were obtained by PCR amplification from pUG72, using primer pair d_MTH1 F and d_ MTH1 R or primer pair d_STD1 F and d_ STD1 R. After confirmation of the correct integration of the cassette by PCR analysis using primer pairs consisting of ch_MTH1 F or ch_STD1 F with Ura-B and ch_MTH1 R or ch_STD1 R with Ura-C for each gene, the marker gene was removed by transformation with Cre recombinase expression vector pSH63. Strain JHY703 was generated by additional deletion of the STD1 gene in strain JHY701 by using the same method. Lactic acid-producing strains SP2006 and SP1130, possessing lactate dehydrogenase (LDH) genes originating from Pelodiscus sinensis subspecies japonicus (PsjLDH) or Bos taurus (BtLDH), were described previously (29). In CEN.PK2-1C, open reading frames (ORFs) of ADH1, PDC1, GPD1, and CYB2 genes were replaced with either the BtLDH gene or the PsjLDH gene controlled by the CCW12 promoter, resulting in strain SP2006 (Table 1) (29). To generate strain SP1130 (Table 1), PTDH3-controlled Escherichia coli mhpF and eutE genes, encoding acetylating acetaldehyde dehydrogenase (A-ALD), were introduced into the chromosome of SP2006, and ALD6 was additionally deleted (29). To generate strains SP2018 and SP1141 (overexpressing GCR1), the PTDH3-GCR1/HA-URA3-HA cassette was PCR amplified using primers i_GCR1 F and i_GCR1 R from plasmid pUC57-URA3-GCR1 and integrated into the chromosomes of SP2006 and SP1130, respectively, by replacing his3Δ1. After confirmation of the correct integration of the cassette by PCR analysis using primer pair ch_HIS3 F and ch_GCR1 R, the URA3 marker flanked with hemagglutinin (HA) tags was popped out (32). CEN.PK2-1C yeast cells were precultured in yeast extract-peptone-dextrose (YPD; 1% yeast extract, 2% Bacto peptone, and dextrose) medium or synthetic complete (SC; 0.67% yeast nitrogen base without amino acids and 0.2% amino acid dropout mixture lacking appropriate components for selection) medium containing 4% glucose and were inoculated to an A600 of 5 in YPD or SC medium containing 8% glucose. Cells in a 5-ml volume were cultivated in 50-ml screw-cap conical tubes at 30°C with shaking at 170 rpm. For lactic acid fermentation, lactic acid-producing strains were inoculated from an overnight seed culture at an A600 of 0.5 into 50 ml YPD medium containing 4% glucose with or without 5 g/liter CaCO3 in 250-ml flat-capped flasks and grown at 30°C under microaerobic conditions with shaking at 90 rpm.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| S. cerevisiae CEN.PK2-1C | MATa ura3-52 trp1-289 leu2-3_112 his3Δ1 MAL2-8c SUC2 | EUROSCARFa |
| S. cerevisiae JHY701 | CEN.PK2-1C mth1Δ | This study |
| S. cerevisiae JHY702 | CEN.PK2-1C std1Δ | This study |
| S. cerevisiae JHY703 | CEN.PK2-1C mth1Δ std1Δ | This study |
| S. cerevisiae SP2006 | CEN.PK2-1C pdc1Δ::Pccw12-BtLDH cyb2Δ::Pccw12-BtLDH gpd1Δ::Pccw12-PsjLDH adh1Δ::Pccw12-PsjLDH | 29 |
| S. cerevisiae SP2018 | SP2006 his3Δ::PTDH3-GCR1 | This study |
| S. cerevisiae SP1130 | SP2006 ald6Δ leu2Δ::PTDH3-mhpF pdc6Δ::PTDH3-eutE | 29 |
| S. cerevisiae SP1141 | SP1130 his3Δ::PTDH3-GCR1 | This study |
| Plasmids | ||
| pRS414GPD | CEN/ARS plasmid, PTDH3, TCYC1, TRP1 marker | 30 |
| pRS415GPD | CEN/ARS plasmid PTDH3, TCYC1, LEU2 marker | 30 |
| pRS416GPD | CEN/ARS plasmid, PTDH3, TCYC1, URA3 marker | 30 |
| pUG72 | Plasmid containing loxP-URA3-loxP deletion cassette | EUROSCARF |
| pSH63 | Plasmid containing a gene for Cre-recombinase, TRP1 marker | EUROSCARF |
| pRS414GPD-HXT1 | HXT1 ORF cloned between the BamHI and ClaI sites of pRS414GPD | This study |
| pRS414GPD-HXT2 | HXT2 ORF cloned between the BamHI and ClaI sites of pRS414GPD | This study |
| pRS414GPD-HXT3 | HXT3 ORF cloned between the BamHI and ClaI sites of pRS414GPD | This study |
| pRS414GPD-HXT4 | HXT4 ORF cloned between the BamHI and ClaI sites of pRS414GPD | This study |
| pRS414GPD-HXT7 | HXT7 ORF cloned between the BamHI and XhoI sites of pRS414GPD | This study |
| pRS416GPD-GCR1 | GCR1 ORF cloned between the XbaI and XhoI sites of pRS415GPD | This study |
| pRS415GPD-GCR2 | GCR2 ORF cloned between the XbaI and XhoI sites of pRS416GPD | This study |
| pUC57-URA3 | Plasmid containing HA-URA3-HA deletion cassette | 29 |
| pUC57-URA3–GCR1 | PTDH3-GCR1-TCYC1 cloned into the KpnI site of pUC57-URA3 | This study |
EUROSCARF, European Saccharomyces cerevisiae Archive for Functional Analysis.
TABLE 2.
Primers used in this study
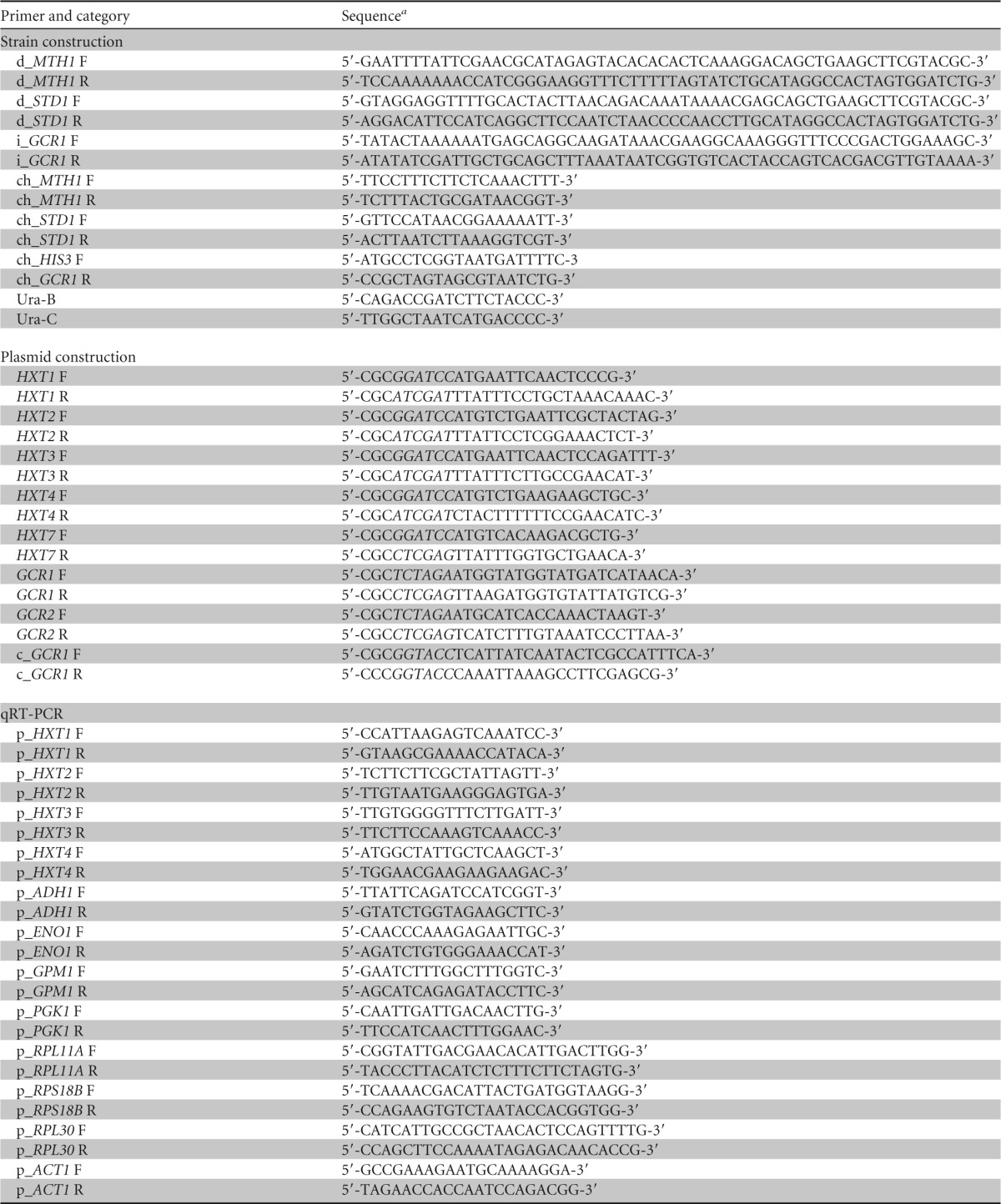
Utilized restriction sites are shown in italics.
Construction of plasmids.
Plasmids used in this study and primers used for the construction of the plasmids are described in Table 1 and Table 2, respectively. HXT1, HXT2, HXT3, and HXT4 ORFs were amplified by PCR using the primers shown in Table 2 and were inserted between the BamHI and ClaI sites of the pRS414GPD plasmid vector (33). The HXT7 ORF was inserted between the BamHI and XhoI sites of pRS414GPD, and GCR1 and GCR2 ORFs were inserted between the XbaI and XhoI sites of pRS416GPD and pRS415GPD, respectively. The PTDH3-GCR1-TCYC1 fragment was amplified from pRS416GPD-GCR1 by PCR using primers c_GCR1 F and c_GCR1 R and cloned into the KpnI site of pUC57-URA3 (29), generating pUC57-URA3-GCR1.
qRT-PCR analysis.
Total RNA was isolated from yeast cells, and the relative amounts of specific mRNA were determined by quantitative reverse transcription-PCR (qRT-PCR). Briefly, 1 mg of the total RNA was subjected to reverse transcription in a 20-μl reaction mixture containing 200 U of myeloblastosis virus reverse transcriptase (M-biotech, South Korea) and 0.1 mg of oligo(dT) at 42°C for 1 h. Quantitative PCR was performed with a LightCycler 480 II instrument (Roche Diagnostics, Germany) using SYBR green PCR master mix (Roche Diagnostics, Germany) and gene-specific primers (Table 2) under the following conditions: 95°C for 5 min, followed by 45 cycles of 95°C for 20 s, 60°C for 20 s, 72°C for 20 s, and cooling to 40°C for 30 s. All experiments were performed in triplicate, and the data were normalized using the ACT1 gene as a control.
Detection of metabolites.
To quantify the concentrations of glucose, ethanol, and lactic acid, 600 μl to approximately 1 ml of culture supernatants was collected and filtered through a 0.22-μm-pore-size syringe filter. High-performance liquid chromatography (HPLC) analysis was performed in an UltiMate 3000 HPLC system (Thermo Fisher Scientific) equipped with a Bio-Rad Aminex HPX-87H column (300 by 7.8 mm, 5 μm pore size) at 60°C with 5 mM H2SO4 and a flow rate of 0.6 ml/min and in a refractive index (RI) detector.
RESULTS
Effects of overexpressing HXTs with different glucose affinities on the glucose uptake rate.
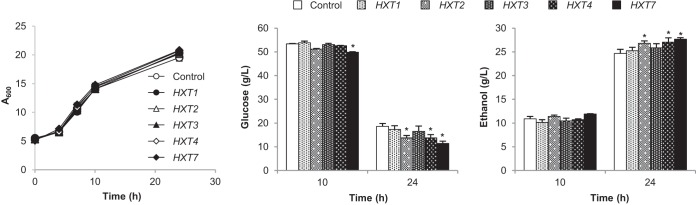
In a previous study, it was shown that overexpression of HXT1 and HXT7, encoding low- and high-affinity transporters, increases the glucose uptake rate with similar efficiencies (22). We reinvestigated the effects of HXT overexpression on the glucose uptake rate by individually overexpressing 5 HXT genes, HXT1, HXT2, HXT3, and HXT4 and HXT7, encoding glucose transporters with different affinities. The HXT genes were overexpressed under the control of a strong constitutive TDH3 promoter from a centromere (CEN)/autonomously replicating sequence (ARS)-based low-copy-number plasmid vector. In SC-Trp minimal medium containing 80 g/liter glucose, CEN.PK2-1C cells expressing each HXT gene showed higher glucose uptake and ethanol production rates than did cells of a control strain harboring an empty plasmid vector (Fig. 1). The amounts of glucose consumed in each strain after 10 and 24 h of cultivation were correlated with the amounts of ethanol produced, reflecting the fact that ethanol production is the major carbon flux during the yeast fermentation. Overexpression of high-affinity glucose transporter Hxt7 showed the greatest effect, followed by moderate-affinity glucose transporters Hxt2 and Hxt4. Overexpression of low-affinity transporters Hxt1 and Hxt3 showed marginal effects on glucose uptake and ethanol production (Fig. 1).
FIG 1.
Increased glucose uptake and ethanol production rates by overexpression of HXT genes. CEN.PK2-1C cells overexpressing each HXT gene (HXT1 to HXT4 and HXT7) were grown in SC-Trp medium with 80 g/liter glucose, and glucose and ethanol levels in the medium were detected after 10 and 24 h of cultivation. Cells harboring empty pRS414GPD vector were used as a control. Mean values of the results of triplicate experiments are shown with error bars indicating the relative standard deviations. *, P < 0.05 (compared to the wild type).
Effects of deleting Mth1 and Std1, corepressors of HXT genes, on the glucose uptake rate.
Mth1 and Std1 act as corepressors of the repressor Rgt1, whose target genes include HXT1 to HXT4 and HXT7 (16, 19). Although Std1 and Mth1 are mainly functional in the absence of glucose, deletion of STD1 and/or MTH1 has been shown to derepress HXT genes simultaneously to different extents depending on glucose concentrations (18). Therefore, we deleted STD1 and MTH1 individually or in combination to examine the effects of multiple upregulation of HXT genes on glucose uptake.
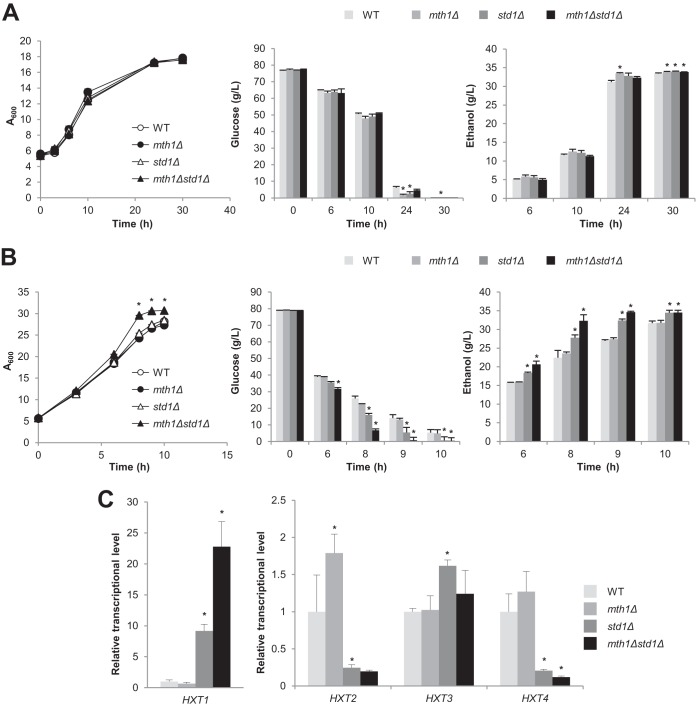
In SC medium, glucose uptake and ethanol production rates were slightly higher in the mth1Δ and std1Δ strains than in the wild type (Fig. 2A). The effect of double deletion was very subtle compared with those of single deletions (Fig. 2A). However, in rich YPD medium, the std1Δ and mth1Δ std1Δ strains showed a significant increase in glucose uptake and ethanol production rates compared with the wild type (Fig. 2B). The mth1Δ std1Δ strain, showing the highest glucose uptake rate, also showed a growth rate higher than those of the other strains. To elucidate the HXTs responsible for the differences in glucose uptake rates, we measured mRNA levels of HXT genes in each strain after 6 h of cultivation. HXT1 mRNA levels showed the most noticeable derepression in the std1Δ and mth1Δ std1Δ strains, suggesting that the increased expression of HXT1 might be the factor mainly responsible for the enhanced glucose uptake in these strains in YPD medium (Fig. 2C). HXT3 expression also slightly increased in the std1Δ strain but not in the mth1Δ std1Δ strain (Fig. 2C). In contrast, the expression levels of HXT2 and HXT4 decreased in the std1Δ strain and the mth1Δ std1Δ strain (Fig. 2C).
FIG 2.
Effects of deleting corepressor genes MTH1 and STD1 on glucose uptake and ethanol production rates. Wild-type (WT; CEN.PK2-1C) and mth1Δ, std1Δ, and mth1Δ std1Δ S. cerevisiae cells were grown in SC (A) or YPD (B) medium with 80 g/liter glucose, and glucose and ethanol levels were detected during growth. (C) Expression levels of HXT genes. The indicated strains were grown in YPD (80 g/liter) medium for 6 h, and the mRNA expression levels of each HXT gene were measured by qRT-PCR and normalized to that of the ACT1 gene. The mRNA levels relative to that of the wild-type control are indicated with standard deviations of the results of three independent experiments. *, P < 0.05 (compared to the wild type).
Taking the results together, the effects of deleting MTH1 and/or STD1 on the expression of HXT genes and glucose uptake appear to be variable, depending on culture conditions, which might be due to the condition-dependent expression levels of Mth1 and Std1 as well as the complex regulatory networks of the Mth1/Std1-Rgt1 transcriptional regulators, which regulate not only HXT genes but also other target genes.
Improvement of the glucose uptake rate by overexpressing GCR1.
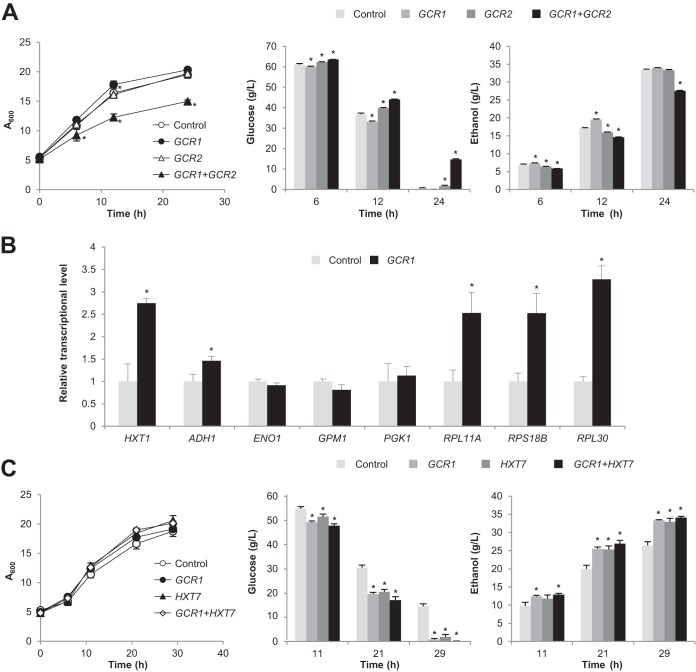
In previous studies, overexpression of genes encoding glycolytic enzymes was not effective in significantly improving glycolytic flux (23–27). Therefore, we tested whether overexpression of transcription factor Gcr1, which activates not only glycolytic genes but also other glucose-responsive target genes such as ribosomal protein (RP) genes, could improve the glucose uptake rate through facilitating glucose utilization and cell growth (34). Gcr1 binds to the CT (CTTCC) box in the promoters of glycolytic genes, and Gcr2 interacts with Gcr1 for the transcriptional activation. However, Gcr2 is dispensable for the activation of RP gene transcription by Gcr1 (34). CEN.PK2-1C cells overexpressing GCR1 showed increased glucose uptake and ethanol production rates (Fig. 3A). In contrast, overexpression of GCR2 decreased the glucose uptake rate somewhat, and overexpression of both GCR1 and GCR2 resulted in significant defects in growth and glucose uptake (Fig. 3A).
FIG 3.
Improvement of glucose uptake rate by overexpressing GCR1. (A) Effects of overexpressing GCR1 and/or GCR2 on the glucose uptake rate. CEN.PK2-1C cells overexpressing GCR1 and GCR2 alone or in combination were grown in SC-Leu-Ura medium with 80 g/liter glucose and monitored for glucose uptake and ethanol production. Cells expressing empty vectors pRS415GPD and pRS416GPD were used as a control. (B) Effects of overexpression of the GCR1 gene on the transcription levels of its target genes. CEN.PK2-1C cells overexpressing GCR1 or harboring pRS416GPD (control) were grown in SC-Ura medium with 80 g/liter glucose for 12 h, and mRNA expression levels of Gcr1 target genes were measured by qRT-PCR and normalized to the ACT1 level. The mRNA levels relative to those of the control are indicated with standard deviations of the results of three independent experiments. *, P < 0.05 (compared to control). (C) Effects of overexpressing GCR1 alone or in combination with HXT7. Cells overexpressing the indicated genes were grown in SC-Trp-Ura medium with 80 g/liter glucose and monitored for glucose uptake and ethanol production. Cells expressing empty vectors (pRS414GPD and pRS416GPD) were used as a control. Mean values of triplicate experiments are shown with error bars indicating standard deviations. *, P < 0.05 (compared to control).
To elucidate the effects of GCR1 overexpression, transcription levels of its potential target genes were investigated after 12 h of cultivation. In contrast to our initial expectation, transcription levels of glycolytic genes (ENO1, GPM1, and PGK1) were not elevated in cells overexpressing GCR1 (Fig. 3B). On the other hand, overexpression of GCR1 led to an increase in expression levels of RP genes (RPL11A, RPS18B, and RPL30), HXT1, and ADH1 (encoding alcohol dehydrogenase) (Fig. 3B). Expression levels of HXT2, HXT3, and HXT4 were not significantly changed by GCR1 overexpression (data not shown). Therefore, GCR1 overexpression might increase the glucose uptake rate mainly by enhancing ribosome biogenesis, which is tightly linked to cell growth, rather than by elevating the expression levels of glycolytic genes.
Overexpression of GCR1 was more efficient in enhancing the glucose uptake rate than overexpression of HXT7 (Fig. 3C). Slight additive effects were observed when the HXT7 gene was overexpressed together with GCR1 (Fig. 3C). Although the control cells harboring empty vector(s) showed glucose uptake rates in SC-Trp medium (Fig. 1) and SC-Trp-Ura medium (Fig. 3C) that were lower than that in SC-Leu-Ura medium (Fig. 3A), the effects of overexpression of HXT7 and GCR1 on the glucose uptake rate were reproducibly observed independently of the types of medium.
Improvement of lactic acid production by overexpressing GCR1.
Since overexpression of GCR1 was proven to be effective in increasing glucose uptake and ethanol production rates in wild-type S. cerevisiae, we next applied GCR1 overexpression to lactic acid production in engineered strains expressing lactate dehydrogenase. We used two lactic acid-producing strains, SP2006 and SP1130 (29). For high-yield lactic acid production, the pathways for glycerol and ethanol formation had been attenuated in SP2006 by deleting GPD1 (encoding glycerol-3-phosphate dehydrogenase), PDC1 (encoding pyruvate decarboxylase), and ADH1 (encoding alcohol dehydrogenase). In SP2006, insufficient production of acetyl-coenzyme A (CoA), a key precursor for the biosynthesis of various cellular components, could be one of the growth-limiting factors. Therefore, SP1130, an improved derivative of the construction of SP2006, was generated by increasing the cellular supply of acetyl-CoA via the introduction of bacterial genes for acetylating acetaldehyde dehydrogenase (A-ALD) enzymes MphF and EutE while deleting PDC6 encoding aldehyde dehydrogenase (29). In the present work, GCR1 was overexpressed in SP2006 and SP1130 by integrating the gene into the genome under the control of the TDH3 promoter, generating SP2018 and SP1141, respectively.
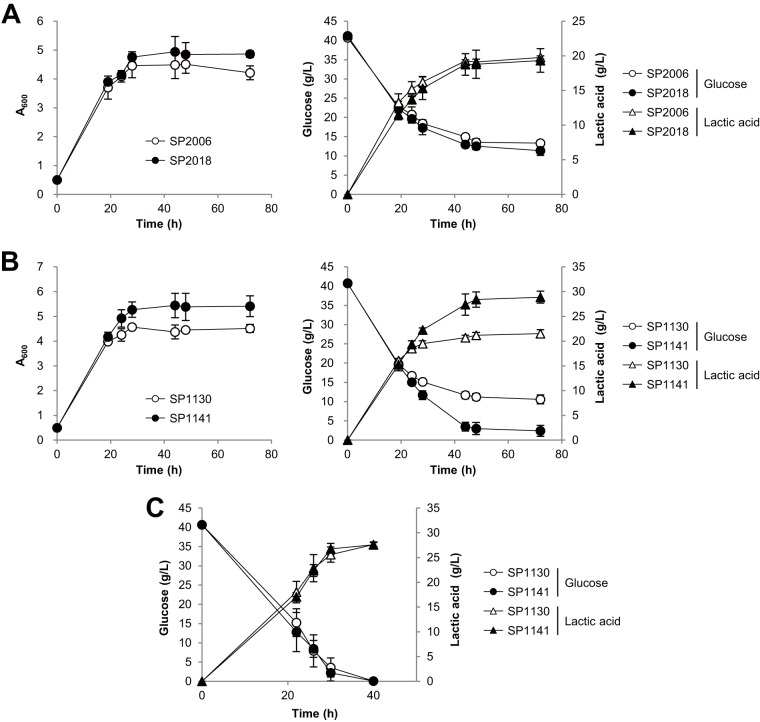
Interestingly, overexpression of GCR1 exhibited different effects depending on the lactic acid production ability of each strain. Note that the amounts of glucose uptake in the lactic acid-producing strains were limited to only 27.4 g/liter and 30.2 g/liter glucose, respectively, when SP2006 and SP1130 strains were grown in YPD medium containing 40 g/liter glucose (Fig. 4A and B). The volumetric productivity of lactic acid decreased over time in both SP2006 and SP1130, but SP1130 showed about 20% higher productivity than SP2006 at up to 28 h of cultivation (Fig. 4A and B). SP2006 produced 16.2 g/liter lactic acid after 28 h, whereas SP1130 produced 19.5 g/liter. After further cultivation to 72 h, SP2006 and SP1130 produced 19.8 g/liter and 21.5 g/liter lactic acid, respectively. Overexpression of GCR1 in SP2006 (strain SP2018) led to a slight increase in the glucose uptake level and final cell density (Fig. 4A). However, the increased amount of glucose transported into the SP2018 cells was not efficiently converted to lactic acid, with slightly decreased lactic acid production levels shown instead. On the other hand, GCR1 overexpression in the SP1130 background (strain SP1141) resulted in a significant increase in glucose consumption and lactic acid production levels (Fig. 4B). By overexpression of GCR1, glucose consumption increased from 30.2 g/liter to 38.4 g/liter and lactic acid production increased from 21.5 g/liter to 28.9 g/liter compared to the results seen with strain SP1130. The yield also increased from 0.71 g/g glucose to 0.75 g/g glucose.
FIG 4.
Improvement of lactic acid production by overexpressing GCR1. (A) Effects of GCR1 overexpression in strain SP2006 background. SP2006 and SP2018 (SP2006 overexpressing GCR1) strains were grown in YPD medium containing 40 g/liter glucose under microaerobic conditions. (B and C) Effects of GCR1 overexpression in strain SP1130 background. Glucose uptake and lactic acid production were measured in SP1130 and SP1141 (SP1130 overexpressing GCR1) strains during growth in YPD medium containing 40 g/liter glucose without CaCO3 (B) or with 5 g/liter CaCO3 (C) under microaerobic conditions. Mean values of the results of triplicate experiments are shown with error bars indicating standard deviations.
Next, we investigated whether the growth-promoting effect of GCR1 overexpression could also improve lactic acid production when the growth inhibitory acid stress was relieved by neutralization. When SP1130 cells were grown in YPD medium containing 40 g/liter glucose and 5 g/liter CaCO3 as a neutralizing reagent, the cells were found to have consumed all of the glucose in the medium after 40 h, producing 27.6 g/liter lactic acid. Under these fermentation conditions, SP1141 showed glucose uptake rates and lactic acid production levels similar to those shown by SP1130, suggesting that GCR1 overexpression might provide a selective advantage when cell growth is impaired.
Taking the data together, GCR1 overexpression might contribute to improving cell growth by facilitating glucose uptake and protein synthesis. However, the positive correlation between the growth-promoting effect and the production of a target chemical might be prominent only in the presence of efficient and strong metabolic flux toward the target chemical and when growth inhibition is one of the limiting factors for the production of the target chemical.
DISCUSSION
Glucose uptake is the first rate-limiting step to control cellular metabolism, depending on extracellular glucose availability and intracellular metabolic capacity. Very sophisticated regulatory mechanisms for glucose uptake have been evolved to ensure cell survival in natural environments with high fluctuation of glucose availability (6). However, engineering the natural regulatory networks to allow a higher glucose uptake rate might be beneficial for the production of various fuels and chemicals if the engineered strain has an efficient metabolic flux toward a target chemical.
As an effort to increase the glucose uptake rate, we tested various genetic modifications related to glucose uptake and cell growth. The most direct strategy was overexpressing HXTs with various affinities to glucose. Among the HXTs tested (Hxt1, Hxt2, Hxt3, Hxt4, and Hxt7), high-affinity transporter Hxt7 was most effective in increasing the glucose uptake rate, followed by moderate-affinity transporters Hxt2 and Hxt4. The effects of eliminating Mth1 and Std1 corepressors of HXT genes were more complicated due to the complex nature of the regulatory networks and the multiple targets regulated by Rgt1 (19). Mth1 and Std1 are homologous proteins acting as corepressors of Rgt1, but their cellular functions are distinguished mainly on the basis of their differential expression levels depending on glucose concentrations (35). Although expression levels of Std1 are not much affected by glucose concentrations, Mth1, whose expression is repressed by the Snf1-Mig1 glucose repression pathway, is abundant only under glucose starvation conditions (17, 35, 36). The effects of deletion of MTH1 and/or STD1 on glucose uptake were more prominent in YPD medium than in SC medium. In SC medium, deletion of MTH1 was most effective in increasing the glucose uptake rate, but in YPD medium, the std1Δ and std1Δ mth1Δ strains, but not the mth1Δ strain, showed an increased glucose uptake rate. Such culture condition-dependent effects of MTH1 and/or STD1 deletions on glucose uptake might reflect different levels of expression of Mth1 and Std1 as well as their slightly different regulatory mechanisms, which lead to different levels of contributions of each corepressor to the regulation of Rgt1 target genes under a given set of conditions. Different nutritional compositions and pH conditions of YPD and SC media and the faster pH drop in SC medium than in YPD medium might be responsible for the differential roles of Mth1 and Std1. In YPD medium, derepression of HXT1 seems to have been mainly responsible for the increase in glucose uptake rate in the std1Δ and std1Δ mth1Δ strains under our experimental conditions, although we cannot rule out the contributions of other Rgt1 target genes. Unexpectedly, the std1Δ and std1Δ mth1Δ strains exhibited reduced expression levels of HXT2 and HXT4, which might have been due to indirect effects of derepressing other Rgt1 target genes such as MIG2, which encodes a repressor for HXT2 and HXT4 (10, 35).
As another strategy to increase the glucose uptake rate, we overexpressed GCR1. Gcr1 plays an important role in glucose metabolism by activating glycolytic genes, HXT genes, and ADH1 (37). Furthermore, Gcr1 also promotes cell growth by activating the expression of translational components such as RP genes (34). Gcr2, a Gcr1-binding coactivator, is essential only for the expression of CT box-containing glycolytic genes, but not for RP genes, which do not have a CT box (34). Although overexpression of GCR1 was effective in increasing glucose uptake and ethanol production rates, overexpression of GCR2 alone or in combination with GCR1 showed a negative effect on glucose uptake. Overexpression of GCR1 led to increased expression levels of HXT1, ADH1, and RP genes but not glycolytic genes. These results might be in part related to the fact that transcriptional activation of glycolytic genes requires both Gcr1 and Gcr2 but Gcr1 alone can activate RP gene transcription. Therefore, the increase in the glucose uptake rate mediated by GCR overexpression might be caused by improving cell growth through promoting translational ability and by increased expression of HXT1 rather than by increasing glycolytic flux through activating the expression of glycolytic genes.
Overexpression of GCR1 was successfully applied to increase lactic acid production in engineered yeast strain SP1141. Inside lactic acid-producing cells, lactic acid molecules dissociate into acid anions and protons, inducing multiple stress conditions via cytosolic acidification and modifications of cellular components (38, 39). Furthermore, exporting the protons and acid anions through H+-ATPase and efflux pumps is a highly energy-consuming process (40). Because of such toxic effects of lactic acid, cell growth and glucose uptake halt when lactic acid production reaches a certain level. SP1130, the parental strain of SP1141, consumed only 30.2 g/liter glucose even in the presence of 40.0 g/liter glucose in the medium, producing 21.5 g/liter lactic acid. On the other hand, strain SP1141, overexpressing GCR1, showed increased glucose uptake up to 38.4 g/liter, and the imported glucose was successfully converted to lactic acid, reaching 28.9 g/liter. Therefore, the growth-promoting effect of GCR1 overexpression might increase the threshold level of the growth-inhibitory effects of lactic acid, leading to higher titers, yield, and efficiency of lactic acid production. However, GCR1 overexpression was not effective when applied to another lactic acid-producing strain, SP2006, which has a lower acetyl-CoA level and weaker lactic acid-producing capability than SP1130. Therefore, in SP2018, with inefficient metabolic flux to lactic acid, the increased amount of imported glucose might be mainly used for biomass synthesis rather than for lactic acid production.
Although GCR1 overexpression was effective in improving cell growth, it failed to exert a positive role for lactic acid production when the growth-inhibitory effect of the acid stress was alleviated by neutralizing the medium during the fermentation. Therefore, Gcr1 overexpression could be applied to improve lactic acid production during acidic fermentation. Acidic fermentation is preferred for lactic acid production to reduce the cost of neutralization during fermentation and recovery of lactic acid from the resulting lactate salt (33).
Taking the data together, increasing glucose uptake and cell growth rates might be a very useful strategy to improve the production of target metabolites if efficient metabolic pathways were to be provided to synthesize the desired molecules. Although overexpression of HXTs might affect only the glucose uptake rate, overexpression of GCR1 appears to exert an additional growth-facilitating effect by activating the transcription of RP genes. Although promoting cell growth and biomass synthesis might seem unfavorable in view of the goal of increasing the yield of a target chemical, improving cell growth could act in a positive way if impaired growth is a limiting factor for the production of a target chemical. Therefore, GCR1 overexpression might be a promising strategy for many other metabolic engineering applications where growth inhibition is one of the major bottlenecks impeding improved production.
ACKNOWLEDGMENT
This work was supported by a research grant funded by Samsung Electronics.
REFERENCES
- 1.Jensen MK, Keasling JD. 2015. Recent applications of synthetic biology tools for yeast metabolic engineering. FEMS Yeast Res 15:1–10. doi: 10.1111/1567-1364.12185. [DOI] [PubMed] [Google Scholar]
- 2.Krivoruchko A, Nielsen J. 2014. Production of natural products through metabolic engineering of Saccharomyces cerevisiae. Curr Opin Biotechnol 35:7–15. doi: 10.1016/j.copbio.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Broach JR. 2012. Nutritional control of growth and development in yeast. Genetics 192:73–105. doi: 10.1534/genetics.111.135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozcan S, Johnston M. 1999. Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev 63:554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boles E, Hollenberg CP. 1997. The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev 21:85–111. doi: 10.1111/j.1574-6976.1997.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 6.Busti S, Coccetti P, Alberghina L, Vanoni M. 2010. Glucose signaling-mediated coordination of cell growth and cell cycle in Saccharomyces cerevisiae. Sensors 10:6195–6240. doi: 10.3390/s100606195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reifenberger E, Boles E, Ciriacy M. 1997. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem 245:324–333. doi: 10.1111/j.1432-1033.1997.00324.x. [DOI] [PubMed] [Google Scholar]
- 8.Reifenberger E, Freidel K, Ciriacy M. 1995. Identification of novel Hxt genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol 16:157–167. doi: 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 9.Maier A, Volker B, Boles E, Fuhrmann GF. 2002. Characterisation of glucose transport in Saccharomyces cerevisiae with plasma membrane vesicles (countertransport) and intact cells (initial uptake) with single Hxt1, Hxt2, Hxt3, Hxt4, Hxt6, Hxt7 or Gal2 transporters. FEMS Yeast Res 2:539–550. doi: 10.1111/j.1567-1364.2002.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 10.Ozcan S, Johnston M. 1996. Two different repressors collaborate to restrict expression of the yeast glucose transporter genes HXT2 and HXT4 to low levels of glucose. Mol Cell Biol 16:5536–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye L, Berden JA, van Dam K, Kruckeberg AL. 2001. Expression and activity of the Hxt7 high-affinity hexose transporter of Saccharomyces cerevisiae. Yeast 18:1257–1267. doi: 10.1002/yea.771. [DOI] [PubMed] [Google Scholar]
- 12.Diderich JA, Schuurmans JM, Van Gaalen MC, Kruckeberg AL, Van Dam K. 2001. Functional analysis of the hexose transporter homologue HXT5 in Saccharomyces cerevisiae. Yeast 18:1515–1524. doi: 10.1002/yea.779. [DOI] [PubMed] [Google Scholar]
- 13.Verwaal R, Paalman JWG, Hogenkamp A, Verkleij AJ, Verrips CT, Boonstra J. 2002. HXT5 expression is determined by growth rates in Saccharomyces cerevisiae. Yeast 19:1029–1038. doi: 10.1002/yea.895. [DOI] [PubMed] [Google Scholar]
- 14.Horák J. 2013. Regulations of sugar transporters: insights from yeast. Curr Genet 59:1–31. doi: 10.1007/s00294-013-0388-8. [DOI] [PubMed] [Google Scholar]
- 15.Santangelo GM. 2006. Glucose signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 70:253–282. doi: 10.1128/MMBR.70.1.253-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulte F, Wieczorke R, Hollenberg CP, Boles E. 2000. The HTR1 gene is a dominant negative mutant allele of MTH1 and blocks Snf3- and Rgt2-dependent glucose signaling in yeast. J Bacteriol 182:540–542. doi: 10.1128/JB.182.2.540-542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flick KM, Spielewoy N, Kalashnikova TI, Guaderrama M, Zhu QZ, Chang HC, Wittenberg C. 2003. Grr1-dependent inactivation of Mth1 mediates glucose-induced dissociation of Rgt1 from HXT gene promoters. Mol Biol Cell 14:3230–3241. doi: 10.1091/mbc.E03-03-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt MC, McCartney RR, Zhang XD, Tillman TS, Solimeo H, Wolfl S, Almonte C, Watkins SC. 1999. Std1 and Mth1 proteins interact with the glucose sensors to control glucose-regulated gene expression in Saccharomyces cerevisiae. Mol Cell Biol 19:4561–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakshmanan J, Mosley AL, Ozcan S. 2003. Repression of transcription by Rgt1 in the absence of glucose requires Std1 and Mth1. Curr Genet 44:19–25. doi: 10.1007/s00294-003-0423-2. [DOI] [PubMed] [Google Scholar]
- 20.Mosley AL, Lakshmanan J, Aryal BK, Ozcan S. 2003. Glucose-mediated phosphorylation converts the transcription factor Rgt1 from a repressor to an activator. J Biol Chem 278:10322–10327. doi: 10.1074/jbc.M212802200. [DOI] [PubMed] [Google Scholar]
- 21.Johnston M, Kim JH. 2005. Glucose as a hormone: receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem Soc Trans 33:247–252. doi: 10.1042/BST0330247. [DOI] [PubMed] [Google Scholar]
- 22.Rossi G, Sauer M, Porro D, Branduardi P. 2010. Effect of HXT1 and HXT7 hexose transporter overexpression on wild-type and lactic acid producing Saccharomyces cerevisiae cells. Microb Cell Fact 9:15. doi: 10.1186/1475-2859-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinisch J. 1986. Isolation and characterization of the two structural genes coding for phosphofructokinase in yeast. Mol Gen Genet 202:75–82. doi: 10.1007/BF00330520. [DOI] [PubMed] [Google Scholar]
- 24.Schaaff I, Heinisch J, Zimmermann FK. 1989. Overproduction of glycolytic enzymes in yeast. Yeast 5:285–290. doi: 10.1002/yea.320050408. [DOI] [PubMed] [Google Scholar]
- 25.Davies SEC, Brindle KM. 1992. Effects of overexpression of phosphofructokinase on glycolysis in the yeast Saccharomyces cerevisiae. Biochemistry 31:4729–4735. doi: 10.1021/bi00134a028. [DOI] [PubMed] [Google Scholar]
- 26.Rosenzweig RF. 1992. Regulation of fitness in yeast overexpressing glycolytic enzymes: parameters of growth and viability. Genet Res 59:35–48. doi: 10.1017/S0016672300030159. [DOI] [PubMed] [Google Scholar]
- 27.Hauf J, Zimmermann FK, Muller S. 2000. Simultaneous genomic overexpression of seven glycolytic enzymes in the yeast Saccharomyces cerevisiae. Enzyme Microb Technol 26:688–698. doi: 10.1016/S0141-0229(00)00160-5. [DOI] [PubMed] [Google Scholar]
- 28.Venayak N, Anesiadis N, Cluett WR, Mahadevan R. 2015. Engineering metabolism through dynamic control. Curr Opin Biotechnol 34:142–152. doi: 10.1016/j.copbio.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Song JY, Park JS, Kang CD, Cho HY, Yang D, Lee S, Cho KM. 15 September 2015, posting date. Introduction of a bacterial acetyl-CoA synthesis pathway improves lactic acid production in Saccharomyces cerevisiae. Metab Eng doi: 10.1016/j.ymben.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Mumberg D, Muller R, Funk M. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 31.Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH. 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30:e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider BL, Seufert W, Steiner B, Yang QH, Futcher AB. 1995. Use of polymerase chain-reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya AN, Pandey RA, Mudliar S, Kumar MS, Chakrabarti T, Devotta S. 2005. Production and recovery of lactic acid for polylactide—an overview. Crit Rev Environ Sci Technol 35:429–467. doi: 10.1080/10643380590966181. [DOI] [Google Scholar]
- 34.Deminoff SJ, Santangelo GM. 2001. Rap1p requires Gcr1p and Gcr2p homodimers to activate ribosomal protein and glycolytic genes, respectively. Genetics 158:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabina J, Johnston M. 2009. Asymmetric signal transduction through paralogs that comprise a genetic switch for sugar sensing in Saccharomyces cerevisiae. J Biol Chem 284:29635–29643. doi: 10.1074/jbc.M109.032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lafuente MJ, Gancedo C, Jauniaux JC, Gancedo JM. 2000. Mth1 receives the signal given by the glucose sensors Snf3 and Rgt2 in Saccharomyces cerevisiae. Mol Microbiol 35:161–172. doi: 10.1046/j.1365-2958.2000.01688.x. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki H, Uemura H. 2005. Influence of low glycolytic activities in gcr1 and gcr2 mutants on the expression of other metabolic pathway genes in Saccharomyces cerevisiae. Yeast 22:111–127. doi: 10.1002/yea.1198. [DOI] [PubMed] [Google Scholar]
- 38.Valli M, Sauer M, Branduardi P, Borth N, Porro D, Mattanovich D. 2006. Improvement of lactic acid production in Saccharomyces cerevisiae by cell sorting for high intracellular pH. Appl Environ Microbiol 72:5492–5499. doi: 10.1128/AEM.00683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott DA, Suir E, van Maris AJ, Pronk JT. 2008. Physiological and transcriptional responses to high concentrations of lactic acid in anaerobic chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol 74:5759–5768. doi: 10.1128/AEM.01030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Maris AJA, Winkler AA, Porro D, van Dijken JP, Pronk JT. 2004. Homofermentative lactate production cannot sustain anaerobic growth of engineered Saccharomyces cerevisiae: possible consequence of energy-dependent lactate export. Appl Environ Microbiol 70:2898–2905. doi: 10.1128/AEM.70.5.2898-2905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]