Abstract
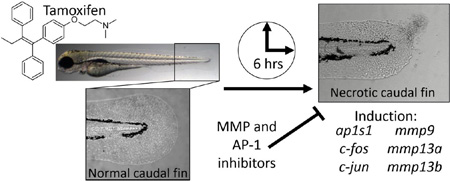
The zebrafish is a powerful alternative model used to link phenotypes with molecular effects to discover drug mode of action. Using a zebrafish embryo-larval toxicity bioassay, we evaluated the effects of tamoxifen - a widely used anti-estrogen chemotherapeutic. Zebrafish exposed to ≥10 µM tamoxifen exhibited a unique necrotic caudal fin phenotype that was rapidly induced regardless of developmental life-stage when treatment was applied. To define tamoxifen’s bioactivity resulting in this phenotype, targeted gene expression was used to evaluate 100 transcripts involved in tissue remodeling, calcium signaling, cell cycle and cell death, growth factors, angiogenesis and hypoxia. The most robustly misregulated transcripts in the tail were matrix metalloproteinases mmp9 and mmp13a, induced 127 and 1145 fold, respectively. Expression of c-fos, c-jun, and ap1s1 were also moderately elevated (3–7 fold), consistent with AP-1 activity - a transcription factor that regulates MMP expression. Immunohistochemistry confirmed high levels of induction for MMP13a in affected caudal fin skin epithelial tissue. The necrotic caudal fin phenotype was significantly attenuated or prevented by three functionally unique MMP inhibitors: EDTA (metal chelator), GM 6001 (broad MMP inhibitor), and SR 11302 (AP-1 transcription factor inhibitor), suggesting MMP-dependence. SR 11302 also inhibited induction of mmp9, mmp13a, and a putative MMP target, igfbp1a. Overall, our studies suggest that tamoxifen’s effect is the result of perturbation of the MMP system in skin leading to ectopic expression, cytotoxicity, and the necrotic caudal fin phenotype. These studies help advance our understanding of tamoxifen’s non-classical mode of action and implicates a possible role for MMPs in tissues such as skin.
Keywords: matrix metalloproteinases, mmp13a, skin epithelium, tamoxifen, zebrafish
Graphical abstract
1.0. Introduction
The chemotherapeutic drug tamoxifen is a prodrug commonly used for its anti-estrogenic effects to treat various reproductive disorders and diseases (Jordan, 2003; 2005). Tamoxifen and the CYP3A4/CYP2D6 derived metabolite 4-hydroxytamoxifen act as potent estrogen receptor α antagonists through competitive inhibition in breast and other tissues (Jin et al., 2005). These drugs also act as weak or partial agonists in endometrium and bone, and are thus classified as selective estrogen receptor modulators (SERMs). As a result of this activity, tamoxifen is primarily used for hormone therapy for prevention and treatment of ER-positive breast cancer for up to 10 years (Burstein et al., 2014). Tamoxifen is also used secondarily to treat a number of other reproductive health issues, such as anovulatory disorders, gynecomastia, and McCune-Albright syndrome (Eugster et al., 1998; Khan and Blamey, 2003; Steiner et al., 2005). As a multi-purpose hormone therapeutic, tamoxifen use is likely to remain high and become increasingly common.
Aside from tamoxifen’s clinical use for hormone therapy, its multifaceted bioactivity suggests potential for novel therapeutic applications. Many in vitro studies demonstrate tamoxifen to be highly cytotoxic and able to induce cellular transformation for a variety of cancerous cell types (Petinari et al., 2004). At low micromolar concentrations that typically exceed SERM levels, tamoxifen is pro-apoptotic to cancer cells in vitro, including breast (Salami and Karami-Tehrani, 2003), glioma (Kim et al., 2005), oral (Chu et al., 2007), and prostate (El Etreby et al., 2000). Tamoxifen modulates Ca2+ influx and triggers apoptosis in other cancer cell types such as bone (Lu et al., 2002), bladder (Chang et al., 2001), and liver (Kim et al., 1999). In malignant melanoma cells, tamoxifen induces cell death through an IGF-dependent pathway (Kanter-Lewensohn, 2000). These diverse effects on cell viability are under investigation for potential alternative applications.
Despite proven therapeutic value, a number of adverse side effects complicate and limit tamoxifen use. Reported side effects include increased risks for endometrial and uterine cancer, menopausal symptoms, stroke, deep vein thrombosis, pulmonary embolism, heart disease, osteoporosis, uterine cancer, sleep problems, weight gain, anxiety and depression, irritability and mood swings (Ganz, 2001; Lorizio et al., 2012). Gel based skin applications (e.g. afimoxifene) can be used to reduce systemic levels of tamoxifen to ameliorate side effects and provide localized transdermal treatment (Goyal et al., 2014). However, tamoxifen also induces skin-specific side effects including dermal fibrosis, epidermal atrophy, thinning of hair, thinning of skin, dryness, reduced vascularity, blistering, peeling, and loosening (Boström, 1999). For many drugs like tamoxifen and other emerging therapeutics, alternative in vivo approaches are needed to rapidly and comprehensively evaluate off-target effects.
The zebrafish model can be used to rapidly evaluate pharmaceuticals, such as tamoxifen, to identify novel effects and better understand drug side effects and toxicity (Barros et al., 2008; Strahle and Grabher, 2010; MacRae and Peterson, 2015). This increasingly popular and rapid throughput model can link unique adverse phenotypes with molecular events to elucidate mechanisms of action for toxicants in vivo (Hill et al., 2005; Bugel et al., 2014; Truong et al., 2014). Yet, many pharmaceuticals have not been thoroughly evaluated with this alternative model. Considering tamoxifen’s widespread use and therapeutic value, we sought to evaluate tamoxifen using the zebrafish embryo-larval toxicity bioassay with a goal to elucidate previously unappreciated bioactivity. Using a phenotypic anchoring approach, we demonstrated that tamoxifen rapidly induces widespread cell death resulting in a necrotic caudal fin phenotype that is both p53 and estrogen receptor independent. Targeted gene expression analysis and immunohistochemistry identified high levels of induction for matrix metalloproteinases (MMPs) in the skin, and several inhibitors for MMPs ameliorate the phenotype. Collectively these studies suggest that tamoxifen rapidly activated a robust MMP response in skin.
2.0. Materials and methods
2.1. Chemicals
Tamoxifen (≥99%, ICI146,474, CAS: 10540-29-1) was obtained from MP Biomedicals (Santa Ana, CA). Ethylenediaminetetraacetic acid (≥99%, EDTA, CAS: 60-00-4) and 17β-estradiol (≥98%, E2, CAS: 50-28-1) were obtained from Sigma-Aldrich (St. Louis, MO). Pifithrin α hydrobromide (≥98%, CAS: 63208-82-2), SR 11302 (≥98%, CAS: 160162-42-5), and G-15 (GPER antagonist, >99%, CAS: 1161002-05-6) were obtained from Tocris Bioscience (Minneapolis, MN). GM 6001 (≥98%, CAS: 142880-36-2) was obtained from Enzo Life Sciences (Farmingdale, NY). Dimethyl sulfoxide (≥99.9%, DMSO, CAS: 67-68-5) was obtained from Avantor Performance Materials (Center Valley, PA). All chemicals stocks were prepared in DMSO.
2.2. Zebrafish husbandry
Adult zebrafish (Danio rerio) were maintained on a 14:10 light:dark cycle at the Sinnhuber Aquatic Research Laboratory at Oregon State University (Corvallis, OR), in accordance with protocols approved by the Oregon State University Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Wild type tropical 5D were used for all studies except the apoptosis study, which required a secA5-YFP transgenic line to visualize apoptosis in vivo (van Ham et al., 2010). Prior to studies, chorions were enzymatically removed from embryos at 4-5 hours post fertilization (hpf) with pronase (Roche, Indianapolis, IN) following procedures by Usenko et al. (2007). Dechorionated embryos individually transferred into clear BD 353075 Falcon 96-well plates (Corning, Corning, NY) contained 100 µL embryo medium (15 mM NaCl, 0.5 mM KCl, 1 mM CaCl2, 0.15 mM KH2PO4, 0.05 mM Na2HPO4, 1 mM MgSO4, 0.05 mM NaHCO3).
2.3. Exposure protocols
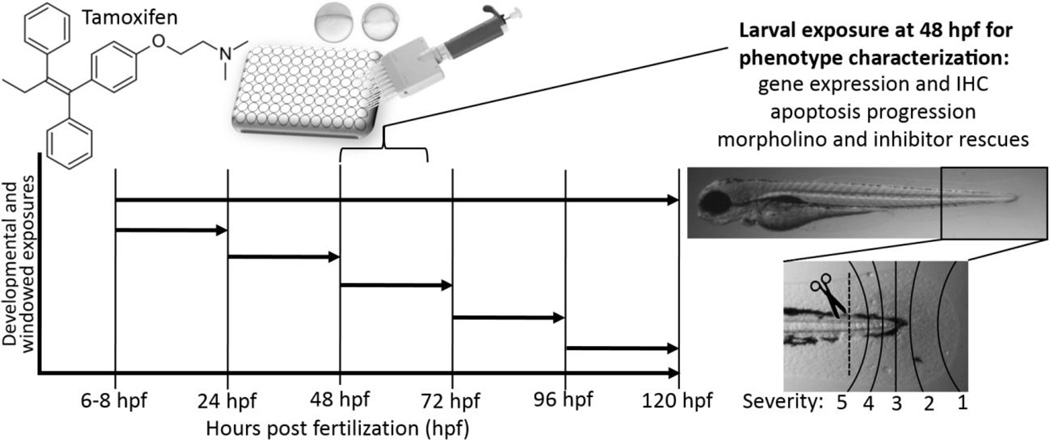
Exposures were either static throughout development, or staggered to determine developmental windows of sensitivity (Fig. 1). At the onset of exposure, treatments prepared in embryo medium were added to achieve the desired final concentration in 0.1% DMSO. Exposure plates were covered with parafilm and wrapped in aluminum foil to minimize chemical photo degradation, and incubated at 28.5 °C. Embryos were developmentally exposed from 6–120 hpf in an initial study to determine developmental toxicity, and observed daily for morphological defects (e.g. pericardial and yolk sac edemas, axial and craniofacial defects, circulatory effects, etc.). Concentrations tested were 1, 2.5, 5, 10, and 15 µM. Subsequent exposures to characterize the caudal fin phenotype were conducted at 48–72 hpf. For those studies, the necrotic caudal fin phenotype was the primary observation and scored as either present/absent or by severity for inhibitor rescue studies depending on how far the phenotype progressed (Fig. 1). For inhibitor rescue studies (EDTA, G-15, GM 6001, pifithrin α, SR 11302), inhibitors were co-added at previously determined maximum tolerable no observable adverse effect level (NOAEL) concentrations simultaneously with tamoxifen.
Figure 1.
Schematic of the exposure and study paradigm used to evaluate tamoxifen’s effects in the larval zebrafish model. Initially, embryos were developmentally exposed 6–120 hours post fertilization (hpf) and observed daily for toxicity. Embryo and larval animals were then exposed in 24 hour periods to determine the window of sensitivity. For most studies, larval 48 hpf animals were exposed to tamoxifen to characterize the phenotype and elucidate the mode of action using transgenic strains, gene expression, IHC and inhibitor rescue studies. Gene expression was evaluated in whole animal and isolated tails cut at the pigment gap (dashed line). For inhibitor rescue studies, phenotype progression was scored from 1 (least) to 5 (most severe).
2.4. Microinjection of morpholinos
Morpholino antisense oligonucleotides were used to investigate the potential involvement of tp53 and nuclear estrogen receptors (esr1, esr2a, esr2b) in the onset of the necrotic fin lesion caused by tamoxifen, using techniques adapted from Nasevicius and Ekker (2000). Morpholinos were designed and synthesized by Gene Tools, LLC (Philomath, OR) and were used to knock-down expression in an attempt to ameliorate the phenotype. Morpholinos were microinjected into 1–2 cell stage embryos (2–4 nL) directly into the yolk-stream and viable animals were selected and manually dechorionated at 24 hpf. The standard control morpholino (C-MO) used was 5′ -CCT CTT ACC TCA GTT ACA ATT TAT A-3′ and was injected at matching concentrations. The standard translation blocking tp53-MO: 5′ -GCG CCA TTG CTT TGC AAG AAT TG-3′ was injected at 1 mM, which has been widely used in zebrafish literature and our laboratory (Bill et al., 2009; Miller et al., 2012). The esr1-MO: 5′ -CAT GTA AAA CAG GCT GGT CAC CTT G-3′ is an E3I3 splice blocking morpholino injected at 1.5 mM, and was designed and validated by Griffin et al. (2013). Both esr2a-MO and esr2b-MO were newly validated. The esr2a-MO: 5′ -GTA CTT TCA GAG AGT CTT ACC TTG T-3′ is an E5I5 splice blocking morpholino and was injected at 1.5 mM. The esr2b-MO 5′ -CAC ACC TGT CGA AAC ACA CAA GAA C-3′ is an I4E5 splice blocking morpholino and was injected at 0.5 mM.
2.5. Analysis of mRNA expression by quantitative real-time polymerase chain reaction (qRT-PCR)
Messenger RNA expression for 100 transcripts of interest was evaluated using qRT-PCR methods adapted from Bugel et al. (2013). Transcripts selected for analysis were for genes involved in pathways identified in literature implicated or related to tamoxifen’s effects in various tissues, and were primarily involved in tissue remodeling, calcium signaling, cell cycle and cell death, growth factors, angiogenesis and hypoxia (Suppl. Data Table 1). Briefly, total RNA was isolated from whole animal larvae and isolated tail tissue using RNAzol® RT (Molecular Research Center, Inc., Cincinnati, OH). For whole animal expression analysis, four pools of 12 larval animals were sampled at 3, 6, and 12 hours post-exposure (hpe). For tail specific expression analysis, four pools of 80 tails were sampled 6 hpe. Tails were cut using a glass microdissection blade at the ventral pigment gap (Fig. 1). Isolated total RNA was converted to complementary DNA using the Applied Biosystems High-Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA). qRT-PCR was performed using a StepOnePlus™ Real-Time PCR System with Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA). Primers used are listed in Suppl. Data Table 1, and were purchased from Integrated DNA Technologies (Coralville, IA). Primers were designed to amplify 75–400 bp fragments of each transcript and were verified for specificity using the NCBI BLAST. OligoAnalyzer (IDT) was used to evaluate primer sets for hetero/homo-dimers (ΔG > -9), formation of hairpins (Tm of hairpin ≪ Tm of oligo) and Tm of primers (60 ± 2 °C). Melt curves were generated for each run to ensure a single product, and product size was verified by gel electrophoresis. β-actin was used as a housekeeping transcript for normalization, and relative expression was quantified using the ΔΔCt method (Pfaffl, 2001).
2.6. Whole mount immunohistochemistry (IHC) for MMP13a
For in situ whole mount IHC visualization of MMP13a protein expression in the caudal fin in response to tamoxifen, an IgG rabbit polyclonal anti-MMP-13a (hinge) antibody for detecting both proenzyme and cleaved active MMP was used (Anaspec, Inc., Fremont, CA, Cat. No. 55114). The manufacturer’s protocol was adapted with minor changes. Briefly, animals were fixed in 4% paraformaldehyde in PBS at 4 °C overnight, then rinsed 3 times in PBSTx for 10 minutes each, then blocked in blocking buffer 1 (PBSTx with 5% BSA, 5% NGS, 0.1% DMSO) for 6 hours at 4 °C. Primary antibody was applied overnight in blocking buffer 2 (PBSTx with 0.5% BSA, 0.1% DMSO) with a 1:500 dilution at 4 °C. Primary was rinsed off with PBSTx 2 × 5 minutes, then 4 × 30 minutes. AlexaFluor 555 goat anti-rabbit IgG secondary antibody (1:5000 dilution, Molecular Probes, Eugene, OR) was applied in blocking buffer 2 at room temperature for 2 hours. Secondary was washed off with PBSTx 2 × 5 minutes, then 4 × 30 minutes. Stained embryos were then visualized on an inverted Zeiss Axiovert 200M epifluorescence microscope using a Zeiss Axiocam HR camera (Carl Zeiss, Oberkochen, Germany).
2.7. Data and statistical analyses
Statistical tests were performed using SigmaPlot™ (v. 11.0) or R (v. 3.2.2), and a p-value ≤ 0.05 was regarded as significantly different for all studies. For discrete presence/absence data, a Fisher’s exact test was used to compare control versus treatment. Gene expression data was evaluated using either one-way ANOVA (Student-Newman-Keuls), or Student’s t-test (Wilcoxon when normality/variance failed). For gene expression, GENE-E (v. 3.0.204) was used to generate heat maps only for significantly altered transcripts with a ≥1.5 fold change for whole animal samples and ≥2 fold change for isolated tail samples.
3.0. Results
3.1. Phenotypic characterization of tamoxifen toxicity in zebrafish
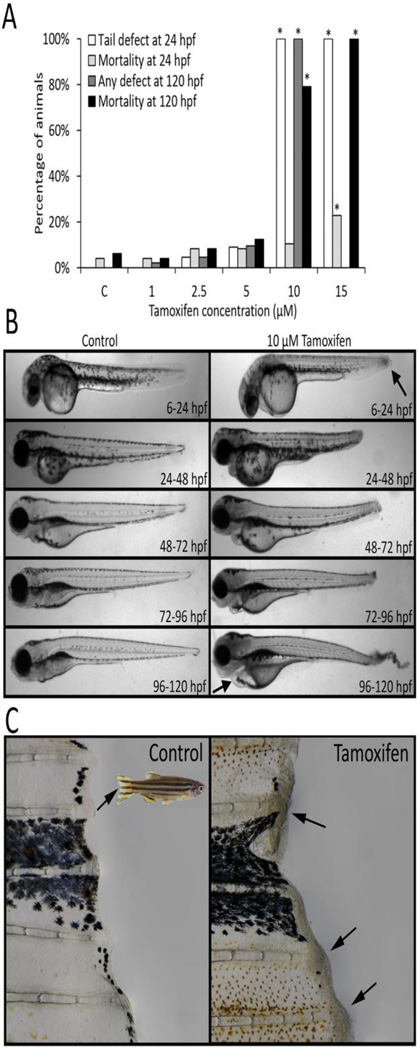
A series of developmental exposure studies were conducted to evaluate the effects of tamoxifen using the zebrafish embryo-larval toxicity bioassay (Fig. 1). Initially, embryos were exposed to tamoxifen (1–15 µM, static non-renewal) throughout development, from 6–120 hpf, and observed daily for morphological defects and mortality (N = 24 per treatment). There was no significant developmental toxicity (morphology or mortality) observed for 1, 2.5, and 5 µM tamoxifen at any time point (Fig. 2A). However, 10 µM and 15 µM significantly induced a highly specific necrosis-like caudal fin defect in 100% of animals by 24 hpf, with some mortality at 15 µM. With continued exposure, >75% of animals died in the 10 µM group, while all animals perished with 15 µM by 120 hpf (Fig. 2A).
Figure 2.
Exposure to tamoxifen induced a necrotic tail lesion in zebrafish that was not dependent on life-stage or development. (A) Zebrafish developmentally exposed to 10 µM tamoxifen or greater from 6–120 hours post fertilization (hpf) showed the presence of a necrotic tail phenotype at 24 hpf, and high levels of mortality at 120 hpf with continued exposure. *Indicates significantly elevated percentage relative to controls (Fisher’s exact test, p ≤ 0.05, N = 48 per treatment). (B) Exposure to 10 µM tamoxifen for 24 hours beginning at any time point throughout embryonic development significantly induced the tail lesion in 100% of animals (Fisher’s exact test, p ≤ 0.05, N = 24 per group). (C) Adult zebrafish exposed to 10 µM tamoxifen for 1.5 hours exhibited similar necrotic lesions widespread on the caudal fin (shown), pectoral fins, and skin of the body. All control and treatment groups contained 0.1% DMSO.
Acute exposures within discrete developmental windows were conducted to determine whether the fin defect was due to perturbation of tail formation during embryogenesis (Fig. 1). Embryonic and larval zebrafish were exposed to 10 µM tamoxifen for 24 hours starting at 6, 24, 48, 72, and 96 hpf (N = 24 per group). Within all exposure windows, tamoxifen significantly induced the necrotic caudal fin phenotype in 100% of treated animals, indicating developmental stage-independence (Fig. 2B). The necrotic phenotype was also recapitulated in adult animals acutely exposed to 10 µM tamoxifen (Fig. 2C). Fin necrosis and skin lesions were more widespread in the adult and observed in pectoral fins and skin universally.
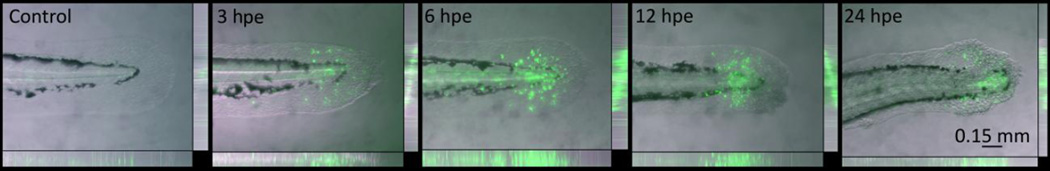
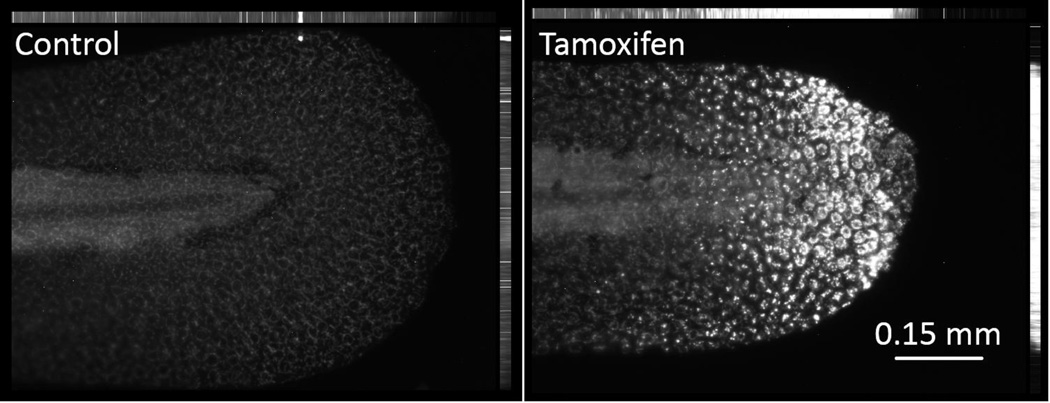
To characterize the onset and progression of the caudal fin phenotype, a study was conducted using 48 hpf larvae exposed to 10 µM tamoxifen. Larvae at 48 hpf were chosen for subsequent studies because tail tissue is differentiated, and enabled the use of morpholinos for later functional studies. Transgenic secA5-YFP zebrafish allowed for non-invasive in vivo visualization of apoptosis within the necrotic fin phenotype (van Ham et al., 2010). Tamoxifen rapidly induced robust changes in cell morphology at the apex of the caudal fin by 3 hpe, which progressively worsened by 6, 12 and 24 hpe (Fig. 3). Apoptosis was observed as early as 3 hpe, and co-localized to the lesion as it advanced. Apoptotic cells were typically adjacent proximally to the necrotic tissue, in normal appearing tissue that later became necrotic-like. A time-lapse video of the development of the phenotype demonstrated that tamoxifen rapidly induced the phenotype starting with a necrotic-like lesion and propagating into adjacent phenotypically normal tissue (Suppl. Data Video 1). Initially, changes in cell morphology rapidly occurred by 3 hpe followed by swelling and lysis of apical cells by 6 and 12 hpe. Leukocytes were also observed migrating towards the distal part of the damaged tail tissue. Proximal to the caudal fin, skin was observed to behave increasingly fluid-like as the phenotype progressed.
Figure 3.
Tamoxifen rapidly induced the necrotic caudal fin phenotype in 48 hour post fertilization (hpf) larvae exposed to 10 µM tamoxifen, and apoptosis co-localized temporally and spatially with the lesion. The secA5-YFP transgenic strain was used to visualize apoptosis in vivo. Representative z-stack photomicrographs at 3, 6, 12 and 24 hours post exposure (hpe) are shown.
3.2. Evaluation of gene expression
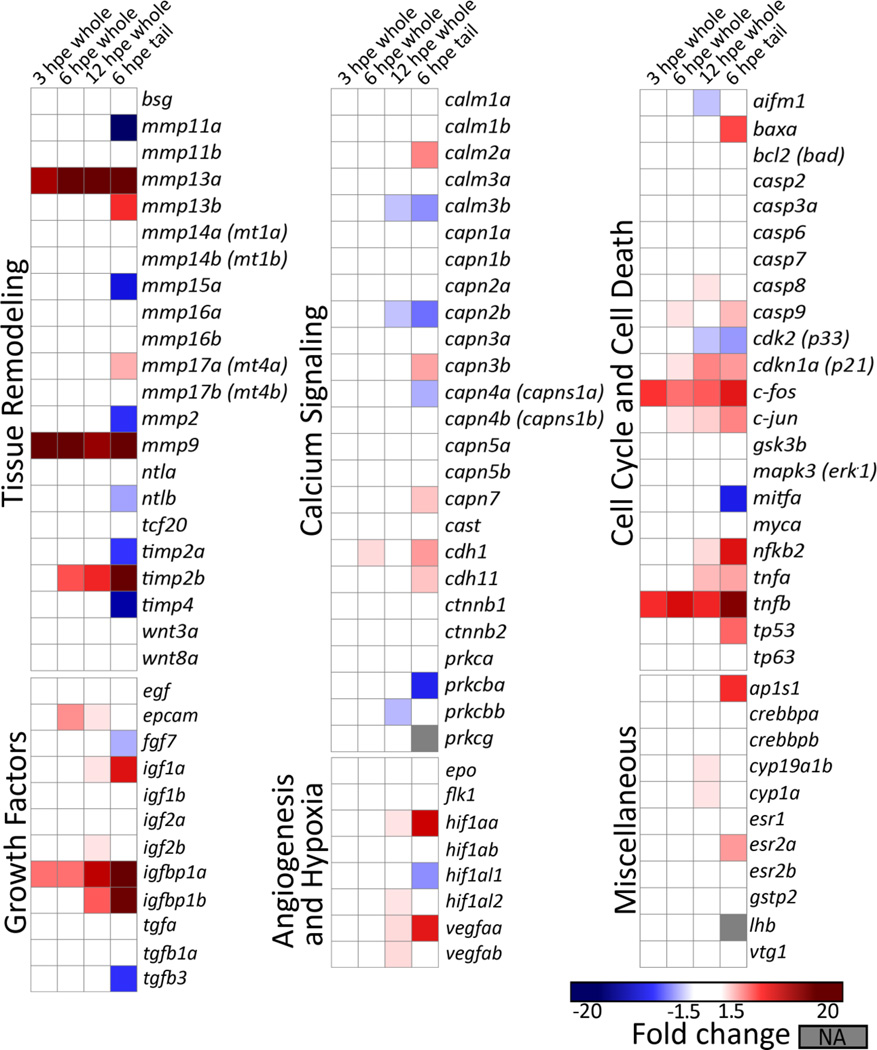
Targeted gene expression was evaluated for 100 transcripts in 48 hpf larval animals acutely exposed to 10 µM tamoxifen. Whole animal expression was evaluated at 3, 6, and 12 hpe, whereas tail specific expression was evaluated only at 6 hpe. Tamoxifen treatment rapidly induced significant and robust changes in expression for a number of transcripts in both the whole animal and isolated tails (Fig. 4 and Suppl. Data Table 2). Generally, whole animal gene expression was concordant between time points and with the 6 hpe tail dataset. In the whole animal, the number of transcripts and the magnitude fold change generally increased with exposure time. At 3 hpe, expression of five transcripts were significantly elevated in the whole animal (mmp9 > mmp13a > tnfb > c-fos > igfbp1a), all of which were also significantly elevated in the 6 hpe tail dataset. At 6 hpe, expression of eleven transcripts were significantly elevated in whole animal samples, ten of which were similarly elevated in the tail dataset (top 6: mmp9 > mmp13a > tnfb > timb2b > igfbp1a > c-fos), and one that was not (epcam). At 12 hpe, 26 genes were elevated in whole animal samples, 17 of which were also significantly increased in the tail specific dataset (top 10: mmp13a, mmp9, igfbp1a, timp2b, tnfb, igfbp1b, c-fos, cdkn1a, tnfa, c-jun).
Figure 4.
Heat map for targeted mRNA gene expression analysis revealed significant effects on transcripts and pathways involved in tissue remodeling, growth factors, and cell cycle and death. Gene expression was evaluated in larval animals treated with 10 µM tamoxifen at 48 hours post fertilization (hpf). Whole animal expression was evaluated at 3, 6 and 12 hours post exposure (hpe), and tail specific expression only at 6 hpe in isolated tails (cut at pigment gap). Data represents fold changes (treatment to control ratio) calculated using the ΔΔCt method. Only significantly altered transcripts with a fold change greater than the cutoff are shown (Student’s t-test or Wilcoxon when normality/variance failed, p ≤ 0.05, N = 4 replicates with either 12 whole animals each or 75 tails each). The fold change cutoff for whole animal and isolated tail samples was ≥1.5 and ≥2, respectively. Gray shaded cells (NA) indicate expression below detection.
Overall, the highest number of transcripts with significantly altered expression levels was observed in the isolated tail at 6 hpe (41 out of 100 tested transcripts). Focusing on the 6 hpe dataset, transcripts for tissue remodeling and growth factor pathway genes were the most robustly altered. These involved several matrix metalloproteinases and other genes involved in MMP regulation or function, such as tissue specific inhibitors, and insulin-like growth factors. The top 10 transcripts most robustly altered (by fold change) in the 6 hpe tail specific dataset were: mmp13a (1146 fold) > igfbp1a (150 fold) > mmp9 (127 fold) > timp2b (40 fold), mmp11a (-19 fold) > igfbp1b (16 fold) > tnfb (14 fold) > timp4 (-11 fold) > hif1aa (8 fold) > nfkb2 (7 fold). Similarly, the top six transcripts most altered in the 6 hpe whole animal dataset were: mmp9 (54 fold) > mmp13a (42 fold) > tnfb (7 fold) > timp2b (4 fold) > igfbp1a (3 fold) = c-fos (3 fold). In the 6 hpe tail dataset, a number of transcripts for genes involved in cell cycle/cell death were significantly altered with modest fold changes (± 3–6 fold), notably c-fos, c-jun, tp63, tnfa, tnfb. A number of transcripts for genes involved in calcium signaling were also significantly different with low fold-changes (± 2–3 fold), specifically the calmodulins, calpains, and cadherins.
3.3. Investigation of the potential role for p53 in the necrotic caudal fin phenotype
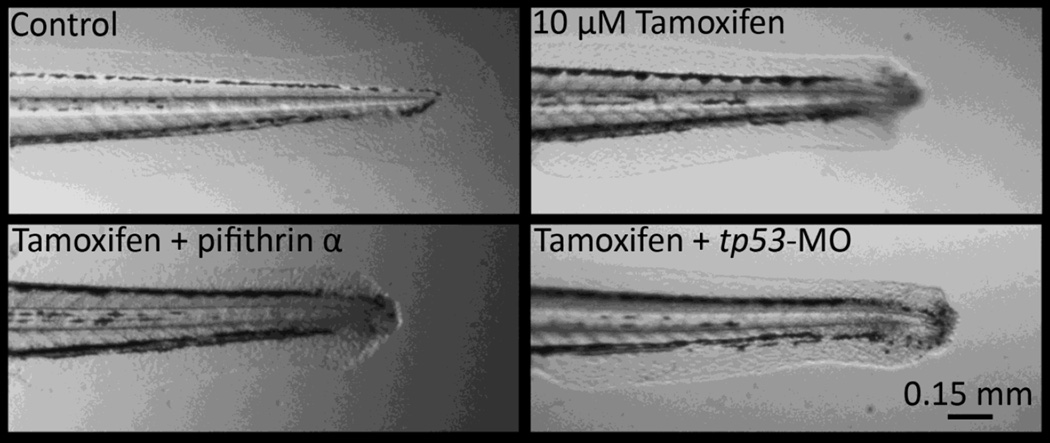
To explore the possibility for a tp53 role in mediating the tamoxifen induced caudal fin lesion, anti-sense morpholinos and chemical inhibition was used to suppress p53 signaling. For these studies, 48 hpf larval animals were exposed to 10 µM tamoxifen with and without morpholino or inhibitor, and observed at 3, 6, 12, and 24 hpe (N = 24). The tp53 translation blocking morpholino is widely used in zebrafish literature to knock-down mRNA expression and concentrations used were similar to those effectively used previously (Bill et al., 2009; Miller et al., 2012). The inhibitor, pifithrin α, directly binds the p53 DNA-binding domain, though there may be off-target effects on related tp63 and tp73 because of sequence similarity (Levrero et al., 2000). The concentration of pifithrin α used (5 µM) was similar to effective concentrations previously used and was a maximum tolerable NOAEL concentration (Davidson et al., 2014; Duffy and Wickstrom, 2014). In the present study, neither method of p53 suppression, morpholino antisense knockdown nor pifithrin α inhibition provided any beneficial effect against the tamoxifen induced caudal fin lesion at any observed time point (Fig. 5). The tamoxifen treatments, with or without morpholino/inhibitor, produced caudal fin necrosis in 100% of animals. Severity between all treatment groups treated or co-treated with tamoxifen were qualitatively similar at all evaluated time points.
Figure 5.
Suppression of tumor protein 53 with either a specific inhibitor (5 µM pifithrin α) or morpholino gene knockdown (tp53-MO) did not prevent nor ameliorate the necrotic caudal fin phenotype induced by 10 µM tamoxifen in 48 hours post fertilization (hpf) larval animals. Photomicrographs are 12 hours post exposure (hpe), and similarly, no amelioration of severity was observed at 3, 6, or 24 hpe. A control morpholino group was included. 100% of animals exposed to tamoxifen exhibited the phenotype with or without inhibitor/morpholino (N = 24 per group).
3.4. Demonstration of an MMP-dependent role for tamoxifen toxicity in the necrotic caudal fin epithelium
The role of matrix metalloproteinases as key mediators of the necrotic caudal fin phenotype was investigated using the 48 hpf larval exposure paradigm. First, whole mount in situ immunohistochemistry was used to visualize MMP13a protein expression in the caudal fin in response to tamoxifen. Similar to the mRNA study, MMP13a protein expression was robustly induced by 10 µM tamoxifen at 6 hpe in the caudal fin lesion of all treated animals (Fig. 6). Low level, MMP13a expression was widely observed in a ring like pattern resembling a secreted protein specifically localized to large skin cells across the entire body of control animals. The antibody used detects both pro-enzyme and active cleaved MMP13a.
Figure 6.
Whole mount in situ immunohistochemistry analysis revealed robust and widespread induction of MMP13a protein expression in skin epithelial ionocytes in the area surrounding the necrotic caudal fin lesion. Photomicrographs are shown for 48 hours post fertilization (hpf) larval animals treated with 10 µM tamoxifen at 6 hours post exposure (hpe). MMP13a expression was localized primarily to skin epithelial ionocytes in controls at low level, and high levels in treated animals. Both proenzyme and cleaved active MMP13a are co-measured.
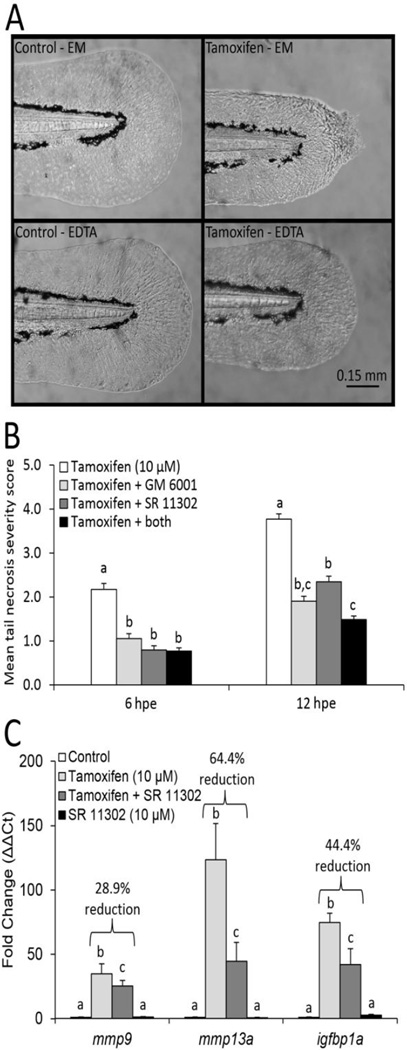
Next, the MMP-dependence of the tamoxifen-induced caudal fin phenotype was evaluated using three functionally unique MMP inhibitors: EDTA, GM 6001 and SR 113302. EDTA is a divalent metal cation chelator commonly used to broadly inhibit MMP activity, and the NOAEL concentration (1 mM) used in our studies was consistent with those used previously (Hillegass et al., 2007). GM 6001 is a broad competitive inhibitor that complexes with the zinc binding site of MMPs to inhibit enzymatic activity. The maximum tolerable NOAEL for GM 6001 (100 µM) has been previously used (Hillegass et al., 2007). SR 11302 is an inhibitor to AP-1, a transcription factor responsible for regulating MMP gene expression. SR 11302 was used at 10 µM, the maximum tolerable NOAEL. For these studies, severity of the lesion was evaluated on a scale from 1–5 (Fig. 1). Only EDTA completely prevented the development of the tamoxifen-induced fin lesion at 3, 6, 12 and 24 hpe. Co-treatment of tamoxifen with EDTA in embryo medium (EM) resulted in 0% of animals exhibiting the lesion, compared to 100% of animals when treated with tamoxifen in EM without EDTA (Fig. 7A). GM 6001 and SR 113302 only partially ameliorated the severity of the lesion, but did not block the prevalence of the lesion (Fig. 7B). Co-treatment of tamoxifen with both GM 6001 and SR 113302 moderately reduced the severity of the necrotic caudal fin phenotype at all evaluated time points, though only slightly better than each inhibitor tested alone when co-treated with tamoxifen (Fig. 7B, only 6 and 12 hpe are shown). A gene expression study confirmed the ability of SR 11302 to inhibit the robust induction of the two primary MMP transcripts of interest, mmp9 and mmp13a, and a putative downstream MMP target, igfbp1a. For this study, tail tissue was isolated at 3 hpe, and co-treatment of tamoxifen with SR 11302 significantly inhibited induction of mmp9, mmp13a and igfbp1a by 28.9%, 64.4% and 44.4%, respectively (Fig. 7C).
Figure 7.
MMP inhibitors broadly prevented or ameliorated the severity of the necrotic caudal fin phenotype induced by 10 µM tamoxifen in 48 hour post fertilization (hpf) larval animals. (A) 1 mM EDTA completely blocked the phenotype at 3, 6, 12 and 24 hours post exposure (hpe). Example photomicrographs show 6 hpe. Tamoxifen treatment in EDTA did not appear qualitatively different than control groups in embryo medium (EM) or EDTA, while tamoxifen treatments in embryo medium induced the phenotype in 100% of animals (Fisher’s exact test, p ≤ 0.05, N = 36 animals per group). (B) Co-treatment of 10 µM tamoxifen with 100 µM GM 6001 or 10 µM SR 11302, reduced the severity of the necrotic caudal fin phenotype. Data are reported as mean ± stdev. Bars not labelled with the same letter are significantly different (ANOVA on ranks, Student-Newman-Keuls, p ≤ 0.05, N = 36 per group). (C) SR 11302, an AP-1 transcription factor inhibitor, significantly reduced the robust tail specific induction of mmp9, mmp13a and igfbp1a by 10 µM tamoxifen at 3 hpe. Fold changes (ΔΔCT) are relative to the 0.1% DMSO control group. Data are reported as mean ± stdev. Bars not labelled with the same letter are significantly different (ANOVA on ranks, Student-Newman-Keuls, p ≤ 0.05, N = 4 replicates with 75 tails each). Percent inhibition was calculated using fold change of the co-treatment group relative to the tamoxifen group.
3.5. Evaluation of tamoxifen’s anti-estrogenic potency and potential role of estrogen receptors
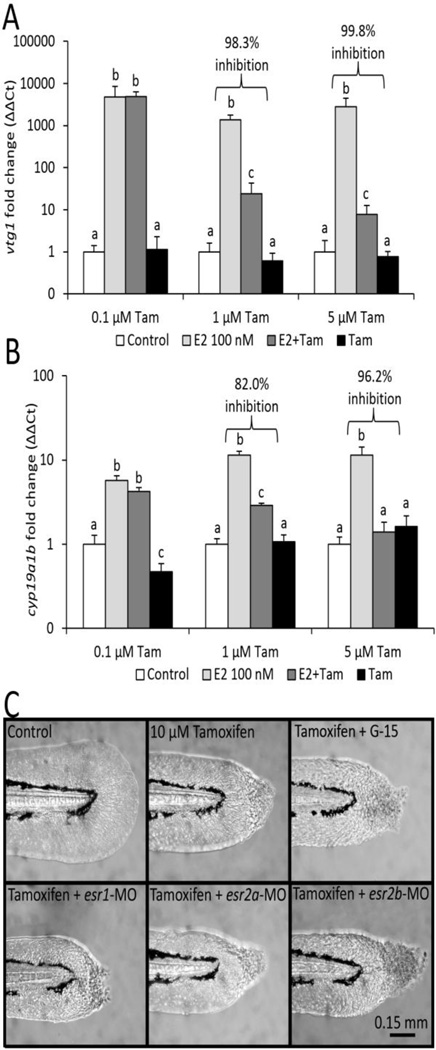
To determine the anti-estrogenic potency of tamoxifen in larval zebrafish, a co-exposure study was conducted from 6–120 hpf with either 0.1% DMSO (control), E2 (100 nM 17β-estradiol), tamoxifen, or a combination of E2 + tamoxifen (Fig. 8A–B). NOAEL concentrations of tamoxifen tested were 0.1, 1, and 5 µM. Gene expression was measured in whole animal samples for estrogen responsive transcripts (A) vtg1, a highly specific hepatic biomarker, and (B) cyp19a1b, the highly specific brain aromatase. Robust inductions of both estrogen biomarkers were significantly inhibited by 1 and 5 µM, but not 0.1 µM tamoxifen co-treatment. Hepatic vtg1 expression was inhibited by greater than 98% at 1 µM tamoxifen and above (Fig. 8A). Induction of brain aromatase was inhibited by 82% at 1 µM tamoxifen, and 96% at 5 µM tamoxifen (Fig. 8B). Together, this demonstrated that the necrotic caudal fin phenotype, which developed at 10 µM tamoxifen and higher, exceeded concentrations that elicited highly potent antiestrogenic effects on gene expression.
Figure 8.
Tamoxifen was highly anti-estrogenic at 1 µM or greater, though the necrotic caudal fin lesion was not regulated by estrogen receptors. Developmental exposure to 100 nM 17β-estradiol (E2) from 6–120 hours post fertilization (hpf) robustly induced estrogen responsive genes (A) vtg1 and (B) cyp19a1b at 120 hpf, which were significantly inhibited by co-treatment with 1 and 5 µM, but not 0.1 µM tamoxifen. Gene expression was evaluated in whole animals and fold changes (ΔΔCT) are relative to the 0.1% DMSO control group. Data are reported as mean ± stdev. Bars not labelled with the same letter were significantly different (ANOVA on ranks, Student-Newman-Keuls, p ≤ 0.05, N = 4 replicates with 12 animals each). Percent inhibition was calculated using the fold inductions for the co-treatment group relative to the E2 group. (C) Co-treatment with G-protein estrogen receptor antagonist G-15 (25 µM) or morpholino gene knockdown of esr1, esr2a, or esr2b did not prevent nor ameliorate the necrotic caudal fin phenotype induced by 10 µM tamoxifen in 48 hpf larval animals. Photomicrographs are 6 hours post exposure (hpe), and similarly, no amelioration of severity was observed at 3, 12, or 24 hpe. Control groups included morpholino and inhibitor alone. 100% of animals exposed to tamoxifen exhibited the phenotype with or without morpholino or inhibitor (N = 24 per group).
To explore the potential role of estrogen receptors in the tamoxifen induced caudal fin lesion, either the chemical inhibitor G-15 was used to antagonize the g-protein estrogen receptor (GPER), or splice blocking morpholinos were used to knock-down expression of nuclear estrogen receptors (Fig. 8C). For these studies, 48 hpf larval animals were exposed to 10 µM tamoxifen with and without inhibitor or individual morpholinos, and observed at 3, 6, 12, and 24 hpe (N = 24). The concentration of G-15 (25 µM) was previously determined to effectively inhibit 5 dpf developmental toxicity of the GPER agonist G-1 in zebrafish in our lab (data not shown), and others (Jayasinghe and Volz, 2012). The esr1 morpholino was developed and validated by Griffin et al. (2013) and used at similar concentrations, and those for esr2a and esr2b were newly developed and validated prior to this study. In the present study, inhibition of the membrane g-protein estrogen receptor with G-15 or knockdown of nuclear estrogen receptors using morpholinos did not ameliorate nor provide a beneficial effect against the tamoxifen induced caudal fin lesion at any observed time point. The tamoxifen treatments, with or without morpholino or inhibitor, produced the caudal fin defect in 100% of animals and were qualitatively the same at all time points.
4.0. Discussion
In the present studies, we used the zebrafish embryo-larval developmental toxicity bioassay as an alternative model to define the effects of tamoxifen, a highly prescribed pharmaceutical with diverse bioactivity in many tissue and cell types. A comprehensive evaluation of tamoxifen’s toxicity in vivo may provide a better understanding of secondary effects and alternative modes of action (Ganz, 2001; Lorizio et al., 2012). To our knowledge, tamoxifen has not been fully evaluated in zebrafish, with the exception of its specific effects on the cardiovascular, hepatic, and reproductive systems (Burns et al., 2005; van der Ven et al., 2007; Bopp and Lettieri, 2008). Our studies demonstrated that acute exposure to tamoxifen rapidly induced a unique tail lesion regardless of developmental life-stage when tamoxifen was applied (Fig. 2). In a high-throughput developmental toxicity screen of 1060 compounds, relatively few chemicals (79) induced any type of adverse effects on the caudal fin in zebrafish, suggesting similar effects to be uncommon (Truong et al., 2014). It was therefore the focus of these studies to investigate the causative mode of action for this unique phenotype to further elucidate tamoxifen’s bioactivity.
Tamoxifen induced the caudal fin phenotype as rapidly as 3 hpe, as observed with both secA5-YFP transgenic zebrafish and time-lapse video (Fig. 3, Suppl. Data Video 1). This finding paralleled previous studies, which found tamoxifen to be cytotoxic and pro-apoptotic in vitro to multiple cell types at low micromolar concentrations (Petinari et al., 2004). Apoptotic cells were typically adjacent to the necrotic fin tissue, suggesting apoptosis to be a secondary effect. The misregulation of transcripts involved in cell cycle and cell death pathways reflected the widespread tissue damage (Fig. 4 and Suppl. Data Table 2). Altered expression of regulatory molecules associated with cell death suggested potential for a tp53 pathway mediated effect, including c-fos, c-jun, cdkn1a (p21), nfkb2, tnfa, tnfb, and tp53. Tumor protein p53 is a pleiotropic transcription factor that drives a variety of cellular responses and programmed cell death, including both apoptosis and necrosis (Chipuk and Green, 2006; Baumann, 2012). The aforementioned transcripts identified in our study are similarly involved in cellular responses and interact with tp53 through a variety of mechanisms (Elkeles et al., 1999; Ryan et al., 2000; Lohr et al., 2003; Pastor et al., 2010). We therefore attempted to mitigate the necrotic caudal fin phenotype using two methods of p53 suppression. However, neither morpholino knockdown of tp53 nor chemical inhibition by pifithrin α ameliorated the phenotype, suggesting the effect to be tp53-independent (Fig. 5). In addition, significantly altered transcript levels were observed for several calmodulins, caspases and calpains, which are involved with programmed cell death signaling cascades (Schuler et al., 2000; Harwood et al., 2005). Calmodulins and calpains are a family of calcium-dependent genes involved in cell cycle progression, gene expression, and cell death (Goll et al., 2003). For many cell types, tamoxifen’s in vitro cytotoxicity involved calcium signaling (Kim et al., 1999; Chang et al., 2001; Lu et al., 2002). However, changes in expression for calm, casp and capn genes were generally not robust or compelling (Fig. 4). Taken together, our data suggested the apoptosis to be secondary to the widespread tissue damage, and not likely directly involved with initiation of the phenotype.
Overall, a number of genes in other related pathways were significantly misregulated in the tail, providing valuable molecular insight into the onset and progression of the phenotype. The most robustly misregulated transcripts were the MMPs (Fig. 4 and Suppl. Data Table 2). This family of calcium-dependent zinc-containing endopeptidases is responsible for extracellular matrix degradation and tissue remodeling critical for regulation of skin physiology, function, and diseases (Page-McCaw et al., 2007). They play important roles in tumor progression for various cancers (e.g. melanoma), through alteration of tumor microenvironment thereby affecting proliferation, migration, invasion and metastasis (Hofmann et al., 2000; Kessenbrock et al., 2010; Lu et al., 2012). The zebrafish has great potential as a versatile tool for studying chemical effects on MMP function in vivo (Crawford and Pilgrim, 2005). Our studies identified mmp9 and mmp13a as the primary MMPs involved in the necrotic caudal fin phenotype, based on robust induction and inhibition studies (Figs. 6–7). Like many MMPs, these two respond to toxicants and drugs and are potential therapeutic targets for treating various diseases (Overall and Kleifeld, 2006; Vartak and Gemeinhart, 2007). In the present studies, robust induction of MMPs offered an explanation for cytotoxicity in the necrotic caudal fin phenotype considering their function as endopeptidases and their roles in extracellular gelatin and collagen matrix degradation. Further support for a role of MMPs in the tamoxifen caudal fin phenotype came from the complete or partial amelioration of the phenotype by MMP inhibitors (Fig. 7). Three MMP inhibitors chosen for their differential mechanism of action ameliorated the necrotic caudal fin phenotype and were: EDTA, a divalent metal cation chelator; GM 6001, a broad-spectrum MMP competitive inhibitor, and SR 11302, an inhibitor of AP-1 transcription factor signaling. EDTA and GM 6001 provided direct evidence that the MMPs were involved in the development of the necrotic caudal fin phenotype, and SR 11302 suggested that the ectopic induction of mmp9 and mmp13a in skin by tamoxifen was mediated through an AP-1 regulated pathway. AP-1 is an inducible transcriptional complex composed of c-fos and c-jun proteins that broadly modulate MMP gene expression, and as a family, nearly all have AP-1 binding sites in their promoters (Benbow and Brinckerhoff, 1997). In our studies, expression of c-fos, c-jun, and the ap1s1 subunit were all moderately elevated (3–7 fold), consistent with elevated AP-1 activity (Fig. 4 and Suppl. Data Table 2). Numerous estrogen receptor ligands, including tamoxifen, transactivate AP-1 through ERβ binding (Paech, 1997). Our dataset found that tamoxifen modestly decreased isolated tail tissue expression of esr2a (−2.7 fold), the zebrafish ERβ ortholog (Fig. 4 and Suppl. Data Table 2). Additionally, tamoxifen is a ligand for the membrane g-protein estrogen receptor (GPER), which can subsequently activate c-jun/c-fos (Maggiolini et al., 2004; Prossnitz and Barton, 2011). However, the failure of esr1, esr2a and esr2b morpholinos and the GPER specific antagonist (G-15) to provide a beneficial effect suggested the caudal fin phenotype to be ER-independent (Fig. 8C). Furthermore, robust changes in expression were observed for multiple IGF transcripts (igf1a, igfbp1a and igfbp1b), which are known to be cleaved and activated by MMPs and play roles in physiological and pathological conditions (McCawley and Matrisian, 2001; Samani et al., 2007). These results relate to findings by Kanter-Lewensohn (2000) where tamoxifen induced apoptosis in malignant melanoma cells in vitro by an IGF-dependent pathway. Taken together, these studies suggest a critical role for MMPs, though they were not able to determine whether the caudal fin phenotype was due to direct MMP action (i.e. extracellular matrix breakdown), or a secondary MMP-mediated downstream response (e.g. IGF signaling). Future studies may address this and use tamoxifen to further understand how MMPs and IGFs are ectopically regulated in skin in vivo.
The skin epithelium is a relatively simple structure in the zebrafish, particularly in the larval animal (Le Guellec et al., 2004; Chang and Hwang, 2011). Embryonic and larval zebrafish have only two epidermal layers: the surface enveloping layer and epidermal basal layer. Cell types include keratinocytes, ionocytes and mucous cells. Skin in adult zebrafish has an additional intermediate layer and club cells. These differences provided an advantage in using the simpler larval model to characterize the necrotic caudal fin phenotype and elucidate the mode of action. The simple larval caudal fin is a non-muscular limb limited to actinotrichia (bony ray precursor), neurons, and skin, while lacking vasculature in the region where the necrotic phenotype originated. Immunohistochemistry for MMP13a demonstrated a specific and widespread pattern of expression across the entire body of the larval animal in very large epithelial cells (Fig. 6). The distribution, morphology, and size of the stained epithelial cells were consistent with ionocytes based on studies by Kwong et al. (2013). Ionocytes are widespread in zebrafish skin and have several sub-types, generally defined by their dominantly expressed ion pump (Janicke et al., 2007; Chang and Hwang, 2011). Specific ionocytes sub-types can be regiospecific in larvae, possibly offering an explanation for the caudal fin specificity at this life-stage. Unlike the larval animal, the effect was not restricted to a particular region of the adult skin and was observed broadly. Furthermore, the response in the ionocytes may be a secondary effect, due to signals from adjacent cells, such as keratinocytes, which are known to have a highly reactive MMP responsive to certain chemicals (Murphy et al., 2004). Further studies using cell specific stains would be necessary to positively identify other skin cell types involved.
The aqueous concentrations used in our study to produce the necrotic caudal fin phenotype are relatable to blood plasma levels reported in patients using low- and high-dose tamoxifen therapy, and also in vitro studies demonstrating cytotoxic effects of tamoxifen. Patients using low-dose tamoxifen hormone therapy typically have sub-micromolar blood plasma levels, ranging from 0.1 to 0.3 µM (MacCallum et al., 2000; Gallicchio et al., 2004; Langenegger et al., 2006), whereas low-micromolar levels of 1–10 µM and greater can be achieved with high dose therapy (Ducharme et al., 2003). A relatively high concentration of tamoxifen (10 µM) was required to elicit the caudal fin phenotype. This concentration exceeded SERM concentrations that are typically in the low to sub-micromolar range. In our studies, the anti-estrogenic effects of tamoxifen on the induction of E2 responsive genes was only observed at ≥1 µM (Fig. 8A–B). In contrast, van der Ven et al. (2007) showed in a partial life-cycle study with zebrafish that exposure to tamoxifen at ~0.1 µM resulted in reproductive toxicity in adults, suggesting larvae to be less sensitive to tamoxifen than later stages. Furthermore, the concentrations used to produce cytotoxicity in vitro with a broad range of cell types typically exceeded 10 µM (Kanter-Lewensohn, 2000; Kim et al., 2005; Chu et al., 2007). Therefore, concentrations used to elicit effects on the MMP system and the resulting necrotic caudal fin phenotype in our studies may be pharmacologically relevant.
Taken together, our studies use a phenotype anchoring approach to provide evidence to suggest a role for MMPs in mediating tamoxifen’s effects in skin epithelium. In summary, we discovered a unique necrotic caudal fin phenotype that was rapidly induced after exposure to tamoxifen. The onset of this phenotype correlated with robust induction of several MMP (mmp9 and mmp13b) genes as well as genes involved with the AP-1 transcriptional factor complex (ap1s1, c-fos, c-jun) that regulates MMP expression. The necrotic caudal fin phenotype was attenuated by three unique MMP inhibitors, one of which was an AP-1 inhibitor that also inhibited the robust induction of MMP genes. We demonstrated that perturbation of the MMP system in skin results in ectopic MMP expression, cytotoxicity and ultimately a unique necrotic caudal fin phenotype, implicating MMP involvement as the mode of action. This action is likely due to either direct MMP action through extracellular matrix breakdown, or an MMP-mediated downstream secondary effect. This may help to understand tamoxifen’s effects for other cell types and tissues, and perhaps implicates a role for MMPs in skin-specific side effects (Boström, 1999). Overall, our study highlights the potential for zebrafish to be used to evaluate unique effects for new, old, and emerging pharmaceuticals for mode of action discovery.
Supplementary Material
List of transcripts selected for targeted qRT-PCR and PCR primer sequences used.
Summary data for gene expression dataset used to create heat map in Fig 4. Mean ± stdev for fold changes (treatment to control ratio) and p-values are provided. N = 4 for each time point and gene.
Time-lapse video for the first 12 hours progression of the necrotic caudal fin lesion induced by 10 µM tamoxifen. Approximately 15 minutes real time is shown each second (12 hours in 50 seconds). The video begins immediately after application of treatment in a 48 hours post fertilization (hpf) larval animal.
Highlights.
Tamoxifen rapidly induced a unique necrotic caudal fin phenotype in zebrafish
Apoptosis co-localized temporally and spatially in the necrotic tail
The necrotic fin phenotype was p53, GPER and ER independent
The necrotic fin phenotype was dependent on ectopic MMP induction and activity in skin
The necrotic fin phenotype occurred at concentrations exceeding anti-estrogenic effects
Acknowledgments
We would like to thank the staff at the Oregon State University Sinnhuber Aquatic Research Laboratory for animal and husbandry support. We would also like to thank Randall Peterson (Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts, USA) for providing the secA5-YFP transgenic zebrafish. This work was conducted at Oregon State University with support by U.S. National Institute of Environmental Health Sciences (NIEHS) Environmental Health Sciences Core Center grant P30 ES000210, NIEHS Training grant T32 ES007060, and an NIEHS Superfund Basic Research Program grant P42 ES016465.
Abbreviations
- E2
17β-estradiol
- hpe
hours post exposure
- hpf
hours post fertilization
- MMP
matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflicts of interest:
The authors have no potential conflicts of interest to declare.
Author contributions:
SMB: Supervised project, designed and performed experiments, analyzed data, generated figures and tables and prepared manuscript
LCW: Designed and performed experiments, analyzed data and prepared manuscript
JKL: Assisted with experiments and prepared manuscript
RLT: Supervised overall performance of project and prepared manuscript
Contributor Information
Sean M. Bugel, Email: Sean.Bugel@oregonstate.edu.
Leah C. Wehmas, Email: wehmasl@onid.oregonstate.edu.
Jane K. La Du, Email: Jane.LaDu@oregonstate.edu.
Robert L. Tanguay, Email: Robert.Tanguay@oregonstate.edu.
References
- Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S. Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br. J. Pharmacol. 2008;154:1400–1413. doi: 10.1038/bjp.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K. Cell death: multitasking p53 promotes necrosis. Nat. Rev. Mol. Cell Biol. 2012;13:480–481. doi: 10.1038/nrm3401. [DOI] [PubMed] [Google Scholar]
- Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp SK, Lettieri T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008;8:8. doi: 10.1186/1471-2210-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström Å. Radiation Recall: Another Call With Tamoxifen. Acta Oncologica. 1999;38:955–960. doi: 10.1080/028418699432653. [DOI] [PubMed] [Google Scholar]
- Bugel SM, Tanguay RL, Planchart A. Zebrafish: A marvel of high-throughput biology for 21 century toxicology. Current environmental health reports. 2014;1:341–352. doi: 10.1007/s40572-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel SM, White LA, Cooper KR. Inhibition of vitellogenin gene induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin is mediated by aryl hydrocarbon receptor 2 (AHR2) in zebrafish (Danio rerio) Aquat. Toxicol. 2013;126:1–8. doi: 10.1016/j.aquatox.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat. Chem. Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H-T, Huang J-K, Wang J-L, Cheng J-S, Lee K-C, Lo Y-K, Lin M-C, Tang K-Y, Jan C-R. Tamoxifen-induced Ca 2+ mobilization in bladder female transitional carcinoma cells. Arch. Toxicol. 2001;75:184–188. doi: 10.1007/s002040100212. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Hwang PP. Development of zebrafish epidermis. Birth defects research. Part C, Embryo today : reviews. 2011;93:205–214. doi: 10.1002/bdrc.20215. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- Chu ST, Huang CC, Huang CJ, Cheng JS, Chai KL, Cheng HH, Fang YC, Chi CC, Su HH, Chou CT, Jan CR. Tamoxifen-induced [Ca2+]i rises and Ca2+-independent cell death in human oral cancer cells. Journal of receptor and signal transduction research. 2007;27:353–367. doi: 10.1080/10799890701699660. [DOI] [PubMed] [Google Scholar]
- Crawford BD, Pilgrim DB. Ontogeny and regulation of matrix metalloproteinase activity in the zebrafish embryo by in vitro and in vivo zymography. Dev. Biol. 2005;286:405–414. doi: 10.1016/j.ydbio.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Davidson W, Ren Q, Kari G, Kashi O, Dicker AP, Rodeck U. Inhibition of p73 function by Pifithrin-α as revealed by studies in zebrafish embryos. Cell Cycle. 2014;7:1224–1230. doi: 10.4161/cc.7.9.5786. [DOI] [PubMed] [Google Scholar]
- Ducharme J, Fried K, Shenouda G, Leyland-Jones B, Wainer IW. Tamoxifen metabolic patterns within a glioma patient population treated with high-dose tamoxifen. Br. J. Clin. Pharmacol. 2003;43:189–193. doi: 10.1046/j.1365-2125.1997.05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KT, Wickstrom E. Zebrafish tp53 knockdown extends the survival of irradiated zebrafish embryos more effectively than the p53 inhibitor pifithrin. Cancer Biology & Therapy. 2014;6:675–678. doi: 10.4161/cbt.6.5.3956. [DOI] [PubMed] [Google Scholar]
- EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- El Etreby MF, Liang Y, Lewis RW. Induction of apoptosis by mifepristone and tamoxifen in human LNCaP prostate cancer cells in culture. The Prostate. 2000;43:31–42. doi: 10.1002/(sici)1097-0045(20000401)43:1<31::aid-pros5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Elkeles A, Juven-Gershon T, Israeli D, Wilder S, Zalcenstein A, Oren M. The c-fos Proto-Oncogene Is a Target for Transactivation by the p53 Tumor Suppressor. Mol. Cell. Biol. 1999;19:2594–2600. doi: 10.1128/mcb.19.4.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster EA, Shankar R, Feezle LK, Pescovitz OH. Tamoxifen Treatment of Progressive Precocious Puberty in a Patient With McCune-Albright Syndrome † 420. Pediatric Research. 1998;43:74–74. doi: 10.1515/jpem.1999.12.5.681. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Lord G, Tkaczuk K, Danton M, Lewis LM, Lim CK, Flaws JA. Association of tamoxifen (TAM) and TAM metabolite concentrations with self-reported side effects of TAM in women with breast cancer. Breast Cancer Res Treat. 2004;85:89–97. doi: 10.1023/B:BREA.0000021050.92539.b0. [DOI] [PubMed] [Google Scholar]
- Ganz PA. Impact of Tamoxifen Adjuvant Therapy on Symptoms, Functioning, and Quality of Life. JNCI Monographs. 2001;2001:130–134. doi: 10.1093/oxfordjournals.jncimonographs.a003450. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol. Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Goyal A, Mansel R, Le Nestour E, Masini-Etévé V. Topical tamoxifen gel (afimoxifene) is associated with low plasma levels of 4-OHT while achieving therapeutic local antiestrogenic effect in premenopausal women with cyclical mastalgia. Cancer Res. 2014;69:2154. [Google Scholar]
- Griffin LB, January KE, Ho KW, Cotter KA, Callard GV. Morpholino-mediated knockdown of ERalpha, ERbetaa, and ERbetab mRNAs in zebrafish (Danio rerio) embryos reveals differential regulation of estrogen-inducible genes. Endocrinology. 2013;154:4158–4169. doi: 10.1210/en.2013-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood SM, Yaqoob MM, Allen DA. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann. Clin. Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hillegass JM, Villano CM, Cooper KR, White LA. Matrix metalloproteinase-13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol. Sci. 2007;100:168–179. doi: 10.1093/toxsci/kfm192. [DOI] [PubMed] [Google Scholar]
- Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. The Journal of investigative dermatology. 2000;115:337–344. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- Janicke M, Carney TJ, Hammerschmidt M. Foxi3 transcription factors and Notch signaling control the formation of skin ionocytes from epidermal precursors of the zebrafish embryo. Dev. Biol. 2007;307:258–271. doi: 10.1016/j.ydbio.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Jayasinghe BS, Volz DC. Aberrant ligand-induced activation of G protein-coupled estrogen receptor 1 (GPER) results in developmental malformations during vertebrate embryogenesis. Toxicol. Sci. 2012;125:262–273. doi: 10.1093/toxsci/kfr269. [DOI] [PubMed] [Google Scholar]
- Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- Kanter-Lewensohn L. Tamoxifen-induced cell death in malignant melanoma cells: possible involvement of the insulin-like growth factor-1 (IGF-1) pathway. Mol. Cell. Endocrinol. 2000;165:131–137. doi: 10.1016/s0303-7207(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HN, Blamey RW. Endocrine treatment of physiological gynaecomastia. BMJ. 2003;327:301–302. doi: 10.1136/bmj.327.7410.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-A, Kang YS, Jung M-W, Lee SH, Lee YS. Involvement of Ca2+ influx in the mechanism of tamoxifen-induced apoptosis in HepG2 human hepatoblastoma cells. Cancer Lett. 1999;147:115–123. doi: 10.1016/s0304-3835(99)00284-0. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Lee CJ, Lee U, Yoo YM. Tamoxifen-induced cell death and expression of neurotrophic factors in cultured C6 glioma cells. Journal of neuro-oncology. 2005;71:121–125. doi: 10.1007/s11060-004-0984-z. [DOI] [PubMed] [Google Scholar]
- Kwong RW, Kumai Y, Perry SF. The role of aquaporin and tight junction proteins in the regulation of water movement in larval zebrafish (Danio rerio) PloS one. 2013;8:e70764. doi: 10.1371/journal.pone.0070764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenegger T, Wahl P, Schiesser D, Thurlimann B. Plasma levels of tamoxifen, N-desmethyl tamoxifen and anastrozole in a patient with metastatic breast cancer and chronic hemodialysis. Breast Cancer Res Treat. 2006;100:177–181. doi: 10.1007/s10549-006-9243-7. [DOI] [PubMed] [Google Scholar]
- Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio) Int. J. Dev. Biol. 2004;48:217–231. doi: 10.1387/ijdb.15272388. [DOI] [PubMed] [Google Scholar]
- Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J. Cell Sci. 2000;113(Pt 10):1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]
- Lohr K, Moritz C, Contente A, Dobbelstein M. p21/CDKN1A mediates negative regulation of transcription by p53. J. Biol. Chem. 2003;278:32507–32516. doi: 10.1074/jbc.M212517200. [DOI] [PubMed] [Google Scholar]
- Lorizio W, Wu AH, Beattie MS, Rugo H, Tchu S, Kerlikowske K, Ziv E. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat. 2012;132:1107–1118. doi: 10.1007/s10549-011-1893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-C, Jiann B-P, Chang H-T, Huang J-K, Chen W-C, Su W, Jan C-R. Effect of the Anti-Breast Cancer Drug Tamoxifen on Ca2+ Movement in Human Osteosarcoma Cells. Pharmacology and Toxicology. 2002;91:34–39. doi: 10.1034/j.1600-0773.2002.910106.x. [DOI] [PubMed] [Google Scholar]
- MacCallum J, Cummings J, Dixon JM, Miller WR. Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br. J. Cancer. 2000;82:1629–1635. doi: 10.1054/bjoc.2000.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015;14:721–731. doi: 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J. Biol. Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Miller GW, Labut EM, Lebold KM, Floeter A, Tanguay RL, Traber MG. Zebrafish (Danio rerio) fed vitamin E-deficient diets produce embryos with increased morphologic abnormalities and mortality. The Journal of nutritional biochemistry. 2012;23:478–486. doi: 10.1016/j.jnutbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KA, Villano CM, Dorn R, White LA. Interaction between the aryl hydrocarbon receptor and retinoic acid pathways increases matrix metalloproteinase-1 expression in keratinocytes. J. Biol. Chem. 2004;279:25284–25293. doi: 10.1074/jbc.M402168200. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- Paech K. Differential Ligand Activation of Estrogen Receptors ER and ER at AP1 Sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor DM, Irby RB, Poritz LS. Tumor necrosis factor alpha induces p53 up-regulated modulator of apoptosis expression in colorectal cancer cell lines. Diseases of the colon and rectum. 2010;53:257–263. doi: 10.1007/DCR.0b013e3181c522c7. [DOI] [PubMed] [Google Scholar]
- Petinari L, Kohn LK, de Carvalho JE, Genari SC. Cytotoxicity of tamoxifen in normal and tumoral cell lines and its ability to induce cellular transformation in vitro. Cell Biol. Int. 2004;28:531–539. doi: 10.1016/j.cellbi.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nature reviews. Endocrinology. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- Salami S, Karami-Tehrani F. Biochemical studies of apoptosis induced by tamoxifen in estrogen receptor positive and negative breast cancer cell lines. Clin. Biochem. 2003;36:247–253. doi: 10.1016/s0009-9120(03)00007-9. [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 Induces Apoptosis by Caspase Activation through Mitochondrial Cytochrome c Release. J. Biol. Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Terplan M, Paulson RJ. Comparison of tamoxifen and clomiphene citrate for ovulation induction: a meta-analysis. Human reproduction. 2005;20:1511–1515. doi: 10.1093/humrep/deh840. [DOI] [PubMed] [Google Scholar]
- Strahle U, Grabher C. The zebrafish embryo as a model for assessing off-target drug effects. Disease models & mechanisms. 2010;3:689–692. doi: 10.1242/dmm.006312. [DOI] [PubMed] [Google Scholar]
- Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014;137:212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven LTM, van den Brandhof E-J, Vos JH, Wester PW. Effects of the Estrogen Agonist 17β-Estradiol and Antagonist Tamoxifen in a Partial Life-Cycle Assay with Zebrafish (Danio Rerio) Environ. Toxicol. Chem. 2007;26:92. doi: 10.1897/06-092r1.1. [DOI] [PubMed] [Google Scholar]
- van Ham TJ, Mapes J, Kokel D, Peterson RT. Live imaging of apoptotic cells in zebrafish. FASEB J. 2010;24:4336–4342. doi: 10.1096/fj.10-161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartak DG, Gemeinhart RA. Matrix metalloproteases: underutilized targets for drug delivery. J Drug Target. 2007;15:1–20. doi: 10.1080/10611860600968967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of transcripts selected for targeted qRT-PCR and PCR primer sequences used.
Summary data for gene expression dataset used to create heat map in Fig 4. Mean ± stdev for fold changes (treatment to control ratio) and p-values are provided. N = 4 for each time point and gene.
Time-lapse video for the first 12 hours progression of the necrotic caudal fin lesion induced by 10 µM tamoxifen. Approximately 15 minutes real time is shown each second (12 hours in 50 seconds). The video begins immediately after application of treatment in a 48 hours post fertilization (hpf) larval animal.