Abstract
Background
The improper invasion of trophoblast cells (TC) can cause various diseases. BRCT-repeat inhibitor of hTERT expression (BRIT1) is involved in the invasion of tumors. Here, we analyzed the effects of BRIT1 on the invasion of TC.
Material/Methods
The expression of BRIT1 in JEG-3, B6Tert, and HTR8/SVneo cells was evaluated by transcription-quantitative polymerase chain reaction (RT-qPCR) and Western blotting. The viability, invasion, and migration of HTR8/SVneo cells were measured using cell counting kit-8 (CCK-8) and Transwell assays. The activities of pro-matrix metalloproteinase-2 (pro-MMP-2) and pro-MMP-9 were tested by gelatin zymography assay. The levels of invasion- and Wnt/β-catenin pathway-related factors were assessed by RT-qPCR and Western blotting.
Results
Levels of BRIT1 in HTR8/SVneo cells were higher than that of JEG-3 and B6Tert cells. The transfection efficiency of BRIT1 siRNA-2 was better than BRIT1 siRNA-1 in HTR8/SVneo cells. BRIT1 siRNA-2 did not change cell viability, whereas it promoted cell invasion and migration. BRIT1 siRNA-2 enhanced the activities of pro-MMP-2 and pro-MMP-9, as well MMP-2 and MMP-9 levels, and reduced tissue inhibitor of metalloproteinases-1 (TIMP-1) and TIMP-2 expression. Moreover, BRIT1 siRNA-2 significantly increased the levels of Wnt2, Wnt3, and β-catenin.
Conclusions
BRIT1 silencing accelerated the invasion and migration of TC and activated the Wnt/β-catenin pathway. Our results may provide new insights for finding new molecular targets to cure disease caused by insufficient invasion of TC.
MeSH Keywords: Neoplasm Invasiveness, Trophoblastic Neoplasms, Wnt Signaling Pathway
Background
Trophoblast cells (TC) are the cell population derived from the development of the nourishing ectoderm outside the blastocyst and are covered with the surface of the villi [1]. TC have biological characteristics similar to the morphology and infiltration of tumor cells. TC on the maternal decidua and maternal spiral arteries moderate invasion, which is the key to establish maternal fetal blood circulation and successful pregnancy [2,3]. During the development of human placenta, 3 cell types are involved: cytotrophoblasts (CTBs), syncytiotrophoblasts (STBs), and extrovillous trophoblasts (EVTs). Each cell type plays an important role in regulating the invasion of TC [4–6]. Many complex events are related to each other in the process of invasion of TC, which is subject to stringent temporal regulation. Regulation disorder can lead to disease, specifically, invasive deficiencies can cause spontaneous abortion, intrauterine growth retardation, preeclampsia, and other diseases [7–9], but excessive invasion may cause hydatidiform mole and choriocarcinoma [10,11]. Based on these understandings, the academic community has launched a study on the invasion and regulation of TC [12–14].
BRCT-repeat inhibitor of hTERT (human telomerase reverse transcriptase) expression (BRIT1), also called microcephalin 1 (MCPH1) BRIT1 gene mutation, is one of the main causes of the primary head deformity. As early as 1998, Jackson et al. studied the gene sequence of small head deformities to determine the location of the mutation [15]. The BRIT1 gene is located at the human chromosome at the position of 8p23.1, which is widely expressed in various tissues, such as the brain, kidney, and liver [16–18]. BRIT1 has been proved to be involved in DNA damage, cell cycle regulation, chromosome agglutination, and cell death, and is associated with many diseases [19–22]. BRIT1 also plays a role in the development of tumors, including cell invasion, migration, and apoptosis [23,24]. Nevertheless, the influences of BRIT1 on the invasion and migration of TC are unclear.
The Wnt signaling pathway plays a pivotal regulatory role in the processes of cell proliferation, differentiation, migration, polarity, adhesion, and stem cell renewal [25,26]. It is believed that there are 3 different activation pathways in the Wnt signaling pathway: the classical pathway (Wnt/β-catenin pathway) and the 2 non-classical pathways (PCP pathway and Wnt/Ca2+ pathway). Activation of the classical pathway is particularly closely related to a variety of important physiological functions, such as cell differentiation, embryo development, and tissue and organ regeneration [27]. β-catenin cell nuclear transfer is used as an indicator of the classical pathway, and activation of this pathway can enhance the transcriptional activity of the target genes (MMPs and c-Myc), thereby promoting the developments of TC and tumor cells [28–31].
In this study, we explored the expression of BRIT1 in the human choriocarcinoma cell and normal TC. Moreover, the effects of BRIT1 on the viability, invasion, and migration of TC were detected by cell counting kit-8 and Transwell assays. Subsequently, the potential pathway of BRIT1 in TC was measured by Western blotting.
Material and Methods
Cell culture and transfection
The human choriocarcinoma cell line (JEG-3 cells) and normal trophoblast cell lines (B6Tert and HTR8/SVneo cells) were provided by Shanghai Junrui Biotechnology Co., Ltd. All cells were maintained in high-glucose Dulbecco’s modified Eagle medium (DMEM) (Solarbio, Beijing, China) supplemented with 10% fetal bovine serum (10% FBS, Solarbio, Beijing, China). Then, the cells were transferred into a 37°C incubator with 5% CO2 (SHH01, Jianheng, Shanghai, China). BRIT1 siRNA-1, BRIT2 siRNA-2, and unspecific scrambled siRNA plasmids were designed by GenePharma (Suzhou, Jiangsu, China), and plasmids were transfected into HTR8/SVneo cells using Hieff Trans™ Liposomal Transfection Reagent (Yeasen, Shanghai, China). Transfection efficiency was detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Western blotting.
Cell viability analysis
The viability of HTR8/SVneo cells was analyzed by CCK-8 (MSK, Wuhan, Hubei, China). Firstly, cells were cultured in 96-well plates at the density of 3×103 cells/well in incubator for 24 h. Secondly, cells were treated with PBS (Control), unspecific scrambled siRNA plasmid (Control siRNA), or BRIT1 siRNA-2 plasmid for 12, 24, and 48 h. Thirdly, CCK-8 reagent was dripped to the cells, and cells were transferred into incubator for 4 h. Finally, the absorbance at 450 nm was examined using an HBS-1096B microplate reader (Detie, Nanjing, Jiangsu, China).
Cell migration and invasion analysis
The migration and invasion of HTR8/SVneo cells were assessed by Transwell assay. Cell invasion detection required the application of BD matrigel (Qcbio, Shanghai, China), but the detection of cell migration does not need it. In brief, BD matrigel was spread out in the upper Transwell chamber at room temperature until solidification. DMEM containing 15% FBS was poured into the lower Transwell chamber. Cells were plated to the upper chamber (containing DMEM without FBS) and maintained in the 37°C incubator. After 24 h, the cells in the lower chamber were collected and fixed with 4% paraformaldehyde (Nanjing Reagent, Nanjing, Jiangsu, China) for 10 min at 4°C. Subsequently, cells were stained using 0.1% Crystal violet (Solarbio, Beijing, China) for 20 min at room temperature. Finally, cells were observed and photographed under an inverted microscope (Olympus, Japan). The numbers of invaded and migrated cell was counted from 5 randomly selected fields.
Matrix metalloproteinase activity analysis
The activities of pro-matrix metalloproteinase-2 (pro-MMP-2) and pro-MMP-9 in HTR8/SVneo cells were determined by gelatin zymography assay. The details of the experimental steps have been presented in a previous paper [32]. Briefly, the proteins of cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel was incubated, dyed, and decolorized at room temperature. The gel was observed and photographed using a gel imaging instrument (UVCI-1000, MajorScience, Shanghai, China). The gray value was calculated with Quantity One 4.6.2 software (BioRad, USA). The gray value in the control group was normalized to “1”.
RT-qPCR analysis
The total RNA of B6Tert, HTR8/SVneo, and JEG-3 cells were collected and cracked using Trizol reagent (Leagene, Beijing, China). The TIANscript RT Kit (TianGen, Beijing, China) was used to synthesize cDNA according to the manufacturer’s instructions. Then, SYBR Premix Ex Taq™ II (Takara, Japan) was used to amplify cDNA following the manufacturer’s instruction. All primers were obtained from Genewiz (Suzhou, Jiangsu China) and are listed in Table 1. GAPDH was used as an internal control. The formula 2−ΔΔCT was used to analyze the gene expression according to the method described previously [33].
Table 1.
The sequences of primers.
| Primer name | Sequence (5′-3′) | Product size (bp) |
|---|---|---|
| BRIT1-forward | AAACCATCTCCAGTCCTCGG | 248 |
| BRIT1-reverse | TGTGCTTTTCAGACCAACCG | |
| MMP-2-forward | CAGCCCTGCAAGTTTCCATT | 210 |
| MMP-2-reverse | GTTGCCCAGGAAAGTGAAGG | |
| MMP-9-forward | GAGACTCTACACCCAGGACG | 238 |
| MMP-9-reverse | GAAAGTGAAGGGGAAGACGC | |
| TIMP-1-forward | AGACCACCTTATACCAGCGT | 217 |
| TIMP-1-reverse | GCCACAAAACTGCAGGTAGT | |
| TIMP-2-forward | AAGCGGTCAGTGAGAAGGAA | 250 |
| TIMP-2-reverse | ACGATGAAGTCACAGAGGGT | |
| GAPDH-forward | CCATCTTCCAGGAGCGAGAT | 222 |
| GAPDH-reverse | TGCTGATGATCTTGAGGCTG |
Western blotting
The total proteins of B6Tert, HTR8/SVneo, and JEG-3 cells were collected and cracked by high RIPA buffer (Solarbio, Beijing, China). The concentration of proteins was detected using a BCA kit (Yeasen, Shanghai, China). Then, the SDS-PAGE was used to separate the proteins, and the proteins were transferred to a nitrocellulose (NC) membrane (Haoran, Shanghai, China). The NC membrane was blocked by 5% non-fat milk at room temperature for 1.5 h, and then incubated with the primary antibodies (anti-BRIT1, Abcam, ab121277, dilution: 1: 900; anti-matrix metalloproteinase-2 (MMP-2), R&D, IC903G, dilution: 1: 700; anti-MMP-9, Abcam, ab38898, dilution: 1: 1000; anti-tissue inhibitor of metalloproteinases-1 (TIMP-1), R&D, IC970G, dilution: 1: 700; anti-TIMP-2, R&D, MAB971, dilution: 1: 500; anti-Wnt2, Abcam, ab27794, dilution: 1: 600; anti-Wnt3, Abcam, ab32249, dilution: 1: 600; anti-β-catenin, Abcam, ab16051, dilution: 1: 600; anti-GAPDH, R&D, MAB5718, dilution: 1: 800) at 4°C overnight. Subsequently, the NC membrane was incubated with the secondary antibodies at room temperature for 1.5 h (goat anti-mouse IgG, Abcam, ab6785, 1: 8000; donkey anti-rabbit IgG, R&D, NL004, 1: 5000; mouse anti-rabbit IgG, Invitrogen, BA1034, 1: 7000). Chemiluminescence detection was carried out using ECL reagent (Huiying, Shanghai, China).
Statistical analysis
All experimental data are presented as mean ±SD. Statistical analysis used SPSS 20 statistics software. One-way analysis of variance (ANOVA) was carried out to evaluate differences between the experimental groups. Statistical significant was set at P<0.05.
Results
BRIT1 was highly expressed in HTR8/SVneo cells
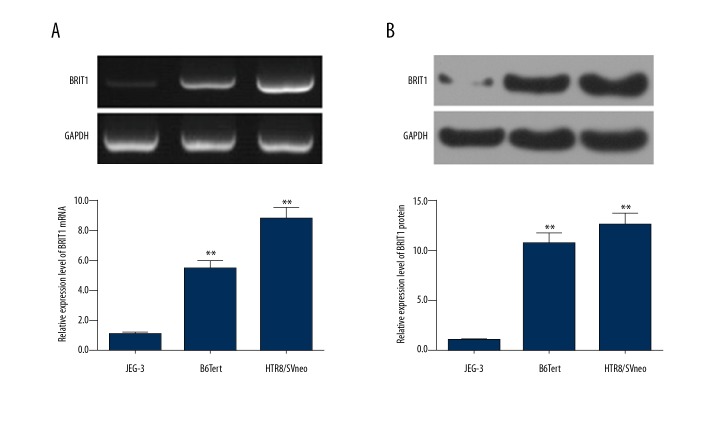
The expression of BRIT1 in B6Tert, HTR8/SVn, and JEG cells were analyzed by RT-qPCR and Western blotting. The mRNA level of BRIT1 in human normal trophoblast cell lines (B6Tert and HTR8/SVneo cells) was higher than in choriocarcinoma cell line (JEG-3 cells). BRIT1 mRNA level was highest in HTR8/SVneo cells (Figure 1A). The protein level of BRIT1 in HTR8/SVneo cells was the highest (Figure 1B).
Figure 1.
Expression of BRIT1 in different trophoblast cells. (A) The mRNA level of BRIT1 in human choriocarcinoma cell line (JEG-3 cells) and normal trophoblast cell lines (B6Tert and HTR8/SVneo cells) was detected by RT-qPCR. (B) The proteins levels of BRIT1 were measured by Western blotting. * P<0.05, ** P<0.01, versus JEG-3.
BRIT1 siRNA-2 did not affect the viability of HTR8/SVneo cells
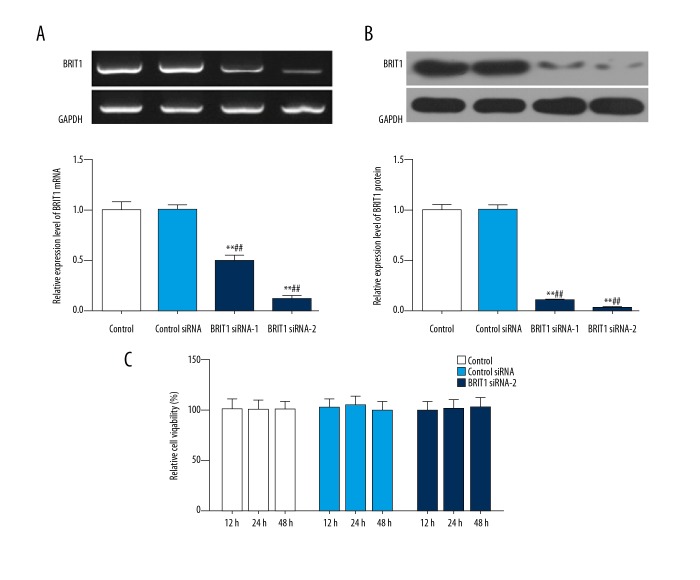
To explore the transfection efficiency of BRIT1 siRNA-1 and BRIT1 siRNA-2 in HTR8/SVneo cells, we used RT-qPCR and Western blotting. As the RT-qPCR and Western blotting data shown, when cells were transfected with BRIT1 siRNA-1 and BRIT1 siRNA-2, the mRNA and protein levels of BRIT1 were down-regulated relative to control siRNA. Moreover, the levels of BRIT1 in BRIT1 siRNA-2 were lower than that of BRIT1 siRNA-1 (Figure 2A, 2B). Therefore, BRIT1 siRNA-2 was selected to transfect into HTR8/SVneo cells. The viability of cells was examined by CCK-8. The CCK-8 results showed that there was no significant change in relative cell viability in each group (Figure 2C).
Figure 2.
Effect of BRIT1 siRNA-2 on the viability of HTR8/SVneo cells. HTR8/SVneo cells were transfected with PBS (control), unspecific scrambled siRNA (control siRNA), BRIT1 siRNA-1, and BRIT1 siRNA-2 plasmids. (A) RT-qPCR was performed to test the mRNA level of BRIT1. (B) Western blotting was used to assess the protein level of BRIT1. (C) Cell viability was analyzed by CCK-8 analysis. * P<0.05, ** P<0.01, versus control; # P<0.05, ## P<0.01, versus control si-RNA.
BRIT1 siRNA-2 accelerated the invasion and migration of HTR8/SVneo cells
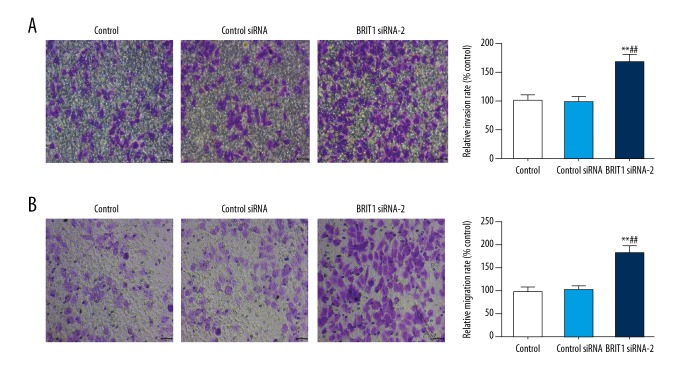
The abilities of invasion and migration in HTR8/SVneo cells exposed to BRIT1 siRNA-2 were detected using the Transwell assay. Our results revealed that the relative invasion rate in BRIT1 siRNA-2 was higher than that of control siRNA (Figure 3A). Migration was markedly increased in BRIT1 siRNA-2 compared to control siRNA (Figure 3B).
Figure 3.
Effect of BRIT1 siRNA-2 on the invasion and migration of HTR8/SVneo cells. (A, B) Transwell assay was carried out to examine the invasion (A) and migration (B) of HTR8/SVneo cells. * P<0.05, ** P<0.01, versus control; # P<0.05, ## P<0.01, versus control siRNA. Bar=50 μm.
BRIT1 siRNA-2 modulated the invasion-associated factors in HTR8/SVneo cells
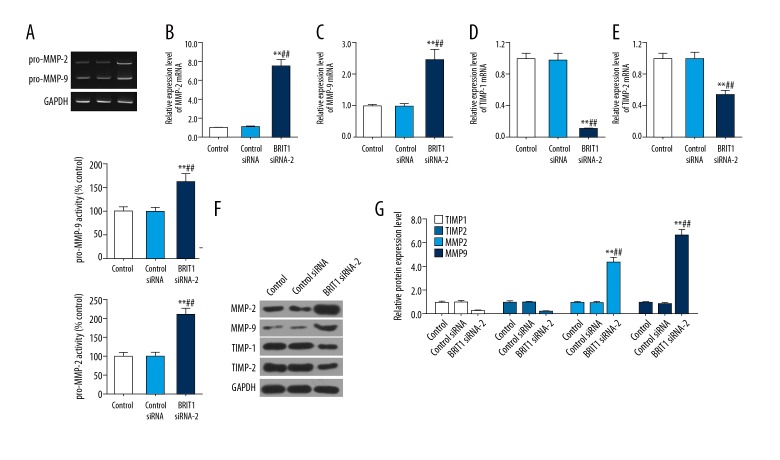
To explore the invasion-associated factors in HTR8/SVneo cells treated with BRIT1 siRNA-2, the activities of pro-MMP-2 and pro-MMP-9 were evaluated by gelatin zymography assay and the expression levels of MMP-2. MMP-9, TIMP-1, and TIMP-2 were assessed by RT-qPCR and Western blotting. The gelatin zymography data showed that BRIT1 siRNA-2 obviously enhanced pro-MMP-2 and pro-MMP-9 activities (Figure 4A). Similarly, BRIT1 siRNA-2 dramatically increased the mRNA and proteins levels of MMP-2 and MMP-9. However, expression of TIMP-1 and TIMP-2 in the mRNA and protein was inhibited in BRIT1 siRNA-2 (Figure 4B–4G).
Figure 4.
Effect of BRIT1 siRNA-2 on invasion-related factors in HTR8/SVneo cells. (A) The activities of pro-MMP-2 and pro-MMP-9 were investigated by gelatin zymography assay. (B–E) The mRNA levels of MMP-2 (B), MMP-9 (C), TIMP-1 (D), and TIMP-2 (E) were surveyed using RT-qPCR. (F, G) The proteins levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 were identified by Western blotting. * P<0.05, ** P<0.01, versus control; # P<0.05, ## P<0.01, versus control siRNA.
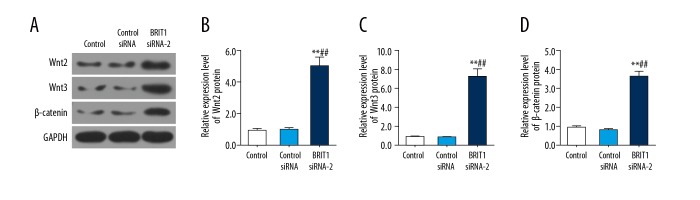
BRIT1 siRNA-2 up-regulated the Wnt/β-catenin pathway in HTR8/SVneo cells
To study the potential mechanism of BRIT1 in HTR8/SVneo cells, the Wnt/β-catenin pathway-related factors (Wnt2, Wnt3, and β-catenin) were evaluated using Western blotting. The results revealed that BRIT1 siRNA-2 conspicuously boosted the relative expression levels of Wnt2, Wnt3, and β-catenin compared to control siRNA (Figure 5A–5D).
Figure 5.
Effect of BRIT1 siRNA-2 on Wnt/β-catenin pathway in HTR8/SVneo cells. (A–D) Western blotting was performed to test the protein levels of Wnt2 (B), Wnt3 (C), and β-catenin (D). * P<0.05, ** P<0.01, versus control; # P<0.05, ## P<0.01, versus control siRNA.
Discussion
Recent studies have shown that BRIT1 expression is reduced in many human cancers [22,24,34,35]. Zhang et al. found that the expression of MCPH1 gene was depressed in lung cancer tissues compared to normal lung tissues [35]. Mai et al. proved that in comparison with normal renal tissues, MCPH1 levels were markedly attenuated in cancer tissues [34]. Consistent with previous research results, we found that BRIT1 expression in JEG-3 cells was lower than in B6Tert and HTR8/SVneo cells. Mai et al. has also indicated that knockdown of BRIT1 gene can obviously repress the invasion and migration of cervical cancer cells [34]. Other studies have proved that over-expression of BRIT1 reduces growth, migration, and invasion of renal carcinoma oral squamous cell carcinoma [24,36]. Hence, we thought that BRIT1 might have a certain effect on TC. Our results show that BRIT1 siRNA-2 had no significant effect on the viability of HTR8/SVneo cells, but increased the capacities of invasion and migration, suggesting that silencing BRIT1 can expedite the invasion and migration of TC.
It is well known that the invasion of TC is regulated by a series of factors of the trophoblast and the autocrine or paracrine uterine cells [37, 38]. For example, villous trophoblast cells secrete MMPs to infiltrate the maternal uterus and mediate cells-to-cell adhesion and migration. Among members of the MMP family, MMP-2 and MMP-9 are the most studied and play the most important roles in trophoblast cell invasion [39–41]. Its inhibitory factor, TIMPs, inhibits invasion [42,43]. To test our hypothesis, we studied the impact of si-BRIT1 on the activities of MMP-2, MMP-9, TIMP-1, and TIMP-2 in TC. Our data show that BRIT1 siRNA-2 apparently boosted pro-MMP-2 and pro-MMP-9 activities and increased the expression of MMP-2 and MMP-9, but depressed TIMP-1 and TIMP-2 expression in HTR8/SVneo cells. These findings show that knockdown of BRIT1 accelerates cell invasion via the up-regulation of MMPs and the down-regulation of TIMPs.
Mounting evidence has validated that the Wnt/β-catenin pathway participates in the regulation of the trophoblast cells invasion process [44,45]. In HTR8/SVneo cells, Zuo Q et al. showed that SPRY4-IT1 over-expression inhibits the capacities of invasion and migration through down-regulating Wnt/β-catenin signaling [45], and Rao et al. have confirmed that SATB1 silencing attenuates invasion and migration via the suppression of Wnt/β-catenin signaling [44]. Here, we hypothesized that the mechanism by which BRIT1 affects HTR8/SVneo cells was Wnt/β-catenin signaling. Our Western blotting data revealed BRIT1 siRNA-2 dramatically up-regulated the levels of Wnt2, Wnt3, and β-catenin in HTR8/SVneo cells. This result suggests that silencing of BRIT1 facilitated the invasion and migration of TC by stimulating Wnt/β-catenin signaling.
Conclusions
Our results show that BRIT1 had higher expression in human normal TC compared to choriocarcinoma cells. Moreover, silencing of BRIT1 strengthened the abilities of invasiveness and migration in TC, accompanied by down-regulating TIMPs expression, but up-regulating MMPs expression and Wnt/β-catenin signaling. These findings may provide a new insight for discovery of a new molecular target to cure disease caused by insufficient invasion of TC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Cross JC, Hemberger M, Lu Y, et al. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol. 2002;187(1–2):207–12. doi: 10.1016/s0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 2.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2s):S745–s61. doi: 10.1016/j.ajog.2017.11.577. [DOI] [PubMed] [Google Scholar]
- 3.Paulesu L, Rao CV, Ietta F, et al. hCG and its disruption by environmental contaminants during human pregnancy. Int J Mol Sci. 2018;19(3) doi: 10.3390/ijms19030914. pii: E914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion – a review. Placenta. 2000;21(Suppl A):S55–60. doi: 10.1053/plac.2000.0521. [DOI] [PubMed] [Google Scholar]
- 5.Chiarello DI, Salsoso R, Toledo F, et al. Foetoplacental communication via extracellular vesicles in normal pregnancy and preeclampsia. Mol Aspects Med. 2018;60:69–80. doi: 10.1016/j.mam.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Fierro ML, Hernandez-Delgadillo GP, Flores-Morales V, et al. Current model systems for the study of preeclampsia. Exp Biol Med. 2018;243(6):576–85. doi: 10.1177/1535370218755690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu L, Zeng H, Liu N. [Expression of H19 long non-coding RNA and ZEB1 in the trophoblast of women with spontaneous abortion]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):179–83. doi: 10.11817/j.issn.1672-7347.2018.02.013. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 8.James-Allan LB, Whitley GS, Leslie K, et al. Decidual cell regulation of trophoblast is altered in pregnancies at risk of pre-eclampsia. J Mol Endocrinol. 2018;60(3):239–46. doi: 10.1530/JME-17-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohli S, Hoffmann J, Lochmann F, et al. p45 NF-E2 regulates syncytiotrophoblast differentiation by post-translational GCM1 modifications in human intrauterine growth restriction. Cell Death Dis. 2017;8(4):e2730. doi: 10.1038/cddis.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voloshchuk IN, Barinova IV, Kondrikov NI, Barto RA. [Twin pregnancy with complete hydatidiform mole]. Arkh Patol. 2017;79(5):43–48. doi: 10.17116/patol201779543-48. [in Russian] [DOI] [PubMed] [Google Scholar]
- 11.Zheng W, Liu T, Sun R, et al. Daidzein induces choriocarcinoma cell apoptosis in a dose-dependent manner via the mitochondrial apoptotic pathway. Mol Med Rep. 2018;17(4):6093–99. doi: 10.3892/mmr.2018.8604. [DOI] [PubMed] [Google Scholar]
- 12.Kim RH, Ryu BJ, Lee KM, et al. Vitamin D facilitates trophoblast invasion through induction of epithelial-mesenchymal transition. Am J Reprod Immunol. 2018;79(2) doi: 10.1111/aji.12796. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Zhai J, Liu J, et al. BMAL1 facilitates trophoblast migration and invasion via SP1-DNMT1/DAB2IP pathway in recurrent spontaneous abortion. Oncotarget. 2017;8(52):89451–64. doi: 10.18632/oncotarget.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Multhaup A, Huppertz B, Gohner C, et al. N-cadherin knockdown leads to disruption of trophoblastic and endothelial cell interaction in a 3D cell culture model – New insights in trophoblast invasion failure. Cell Adh Migr. 2017 doi: 10.1080/19336918.2017.1386822. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson AP, McHale DP, Campbell DA, et al. Primary autosomal recessive microcephaly (MCPH1) maps to chromosome 8p22-pter. Am J Hum TGenet. 1998;63(2):541–46. doi: 10.1086/301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartek J. Microcephalin guards against small brains, genetic instability, and cancer. Cancer Cell. 2006;10(2):91–93. doi: 10.1016/j.ccr.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesh T, Suresh PS. Emerging roles of MCPH1: Expedition from primary microcephaly to cancer. Eur J Cell Biol. 2014;93(3):98–105. doi: 10.1016/j.ejcb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhou ZW, Tapias A, Bruhn C, et al. DNA damage response in microcephaly development of MCPH1 mouse model. DNA Repair. 2013;12(8):645–55. doi: 10.1016/j.dnarep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Gruber R, Zhou Z, Sukchev M, et al. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat Cell Biol. 2011;13(11):1325–34. doi: 10.1038/ncb2342. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Gao H, Lin SY, et al. Mcph1/Brit1 deficiency promotes genomic instability and tumor formation in a mouse model. Oncogene. 2015;34(33):4368–78. doi: 10.1038/onc.2014.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partipilo G, Simone G, Scattone A, et al. Expression of proteins involved in DNA damage response in familial and sporadic breast cancer patients. Int J Cancer. 2016;138(1):110–20. doi: 10.1002/ijc.29699. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Bai Y, Li Y, et al. Overexpression of MCPH1 inhibits uncontrolled cell growth by promoting cell apoptosis and arresting the cell cycle in S and G2/M phase in lung cancer cells. Oncol Lett. 2016;11(1):365–72. doi: 10.3892/ol.2015.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilbao C, Ramirez R, Rodriguez G, et al. Double strand break repair components are frequent targets of microsatellite instability in endometrial cancer. Eur J Cancer. 2010;46(15):2821–27. doi: 10.1016/j.ejca.2010.06.116. [DOI] [PubMed] [Google Scholar]
- 24.Wang N, Lu H, Chen W, et al. Primary microcephaly gene MCPH1 shows a novel molecular biomarker of human renal carcinoma and is regulated by miR-27a. Int J Clin Exp Pathol. 2014;7(8):4895–903. [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Han Z, Zhao N, et al. Inhibition of DNMT suppresses the stemness of colorectal cancer cells through down-regulating Wnt signaling pathway. Cell Signal. 2018;47:79–87. doi: 10.1016/j.cellsig.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Yang YZ, Zhang XY, Li J. [MSC Senescence-related signaling pathway – review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26(1):307–10. doi: 10.7534/j.issn.1009-2137.2018.01.055. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 27.Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: Modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101. doi: 10.1186/s13045-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y, Yu K, Wang G, et al. Lanatoside C inhibits cell proliferation and induces apoptosis through attenuating Wnt/beta-catenin/c-Myc signaling pathway in human gastric cancer cell. Biochem Pharmacol. 2018;150:280–92. doi: 10.1016/j.bcp.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Liang H, Zhang Q, Lu J, et al. MSX2 induces trophoblast invasion in human placenta. PLoS One. 2016;11(4):e0153656. doi: 10.1371/journal.pone.0153656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao MR, Qiu W, Li YX, et al. Dual effect of transforming growth factor beta1 on cell adhesion and invasion in human placenta trophoblast cells. Reproduction. 2006;132(2):333–41. doi: 10.1530/rep.1.01112. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang B, Luo X, Rao H, et al. [Expression and significance of SATB1 and wnt/beta-catenin signaling molecule in the placenta of preeclampsia]. Zhonghua Fu Chan Ke Za Zhi. 2015;50(4):283–90. [in Chinese] [PubMed] [Google Scholar]
- 32.Cathcart J. Assessment of matrix metalloproteinases by gelatin zymography. Methods Mol Biol. 2016;1406:151–59. doi: 10.1007/978-1-4939-3444-7_12. [DOI] [PubMed] [Google Scholar]
- 33.Chang S, Chen W, Yang J. Another formula for calculating the gene change rate in real-time RT-PCR. Mol Biol Rep. 2009;36(8):2165–68. doi: 10.1007/s11033-008-9430-1. [DOI] [PubMed] [Google Scholar]
- 34.Mai L, Yi F, Gou X, et al. The overexpression of MCPH1 inhibits cell growth through regulating cell cycle-related proteins and activating cytochrome c-caspase 3 signaling in cervical cancer. Mol Cell Biochem. 2014;392(1–2):95–107. doi: 10.1007/s11010-014-2022-6. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wu XB, Fan JJ, et al. MCPH1 protein expression in normal and neoplastic lung tissues. Asian Pac J Cancer Prev. 2013;14(12):7295–300. doi: 10.7314/apjcp.2013.14.12.7295. [DOI] [PubMed] [Google Scholar]
- 36.Venkatesh T, Nagashri MN, Swamy SS, et al. Primary microcephaly gene MCPH1 shows signatures of tumor suppressors and is regulated by miR-27a in oral squamous cell carcinoma. PLoS One. 2013;8(3):e54643. doi: 10.1371/journal.pone.0054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bass KE, Morrish D, Roth I, et al. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: Evidence that paracrine factors modify this process. Dev Biol. 1994;164(2):550–61. doi: 10.1006/dbio.1994.1223. [DOI] [PubMed] [Google Scholar]
- 38.Kilani RT, Mackova M, Davidge ST, et al. Endogenous tumor necrosis factor alpha mediates enhanced apoptosis of cultured villous trophoblasts from intrauterine growth-restricted placentae. Reproduction. 2007;133(1):257–64. doi: 10.1530/REP-06-0080. [DOI] [PubMed] [Google Scholar]
- 39.Duval F, Dos Santos E, Moindjie H, et al. Adiponectin limits differentiation and trophoblast invasion in human endometrial cells. J Mol Endocrinol. 2017;59(3):285–97. doi: 10.1530/JME-17-0046. [DOI] [PubMed] [Google Scholar]
- 40.Sugiura T, Berditchevski F. Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2) J Cell Biol. 1999;146(6):1375–89. doi: 10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong T, Chen J, Ling Y, et al. Down-regulation of neuropathy target esterase in preeclampsia placenta inhibits human trophoblast cell invasion via modulating MMP-9 levels. Cell Physiol Biochem. 2018;45(3):1013–22. doi: 10.1159/000487296. [DOI] [PubMed] [Google Scholar]
- 42.Dai X, Cai Y. Down-regulation of microRNA let-7d inhibits the proliferation and invasion of trophoblast cells in preeclampsia. J Cell Biochem. 2018;119(1):1141–51. doi: 10.1002/jcb.26282. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Cao Q, Li X, Wang Z. Knockdown of TACC3 inhibits trophoblast cell migration and invasion through the PI3K/Akt signaling pathway. Mol Med Rep. 2016;14(4):3437–42. doi: 10.3892/mmr.2016.5659. [DOI] [PubMed] [Google Scholar]
- 44.Rao H, Bai Y, Zhang F, et al. The role of SATB1 in HTR8/SVneo cells and pathological mechanism of preeclampsia. J Matern Fetal Neonatal Med. 2018 doi: 10.1080/14767058.2018.1425387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Zuo Q, Huang S, Zou Y, et al. The Lnc RNA SPRY4-IT1 modulates trophoblast cell invasion and migration by affecting the epithelial-mesenchymal transition. Sci Rep. 2016;6:37183. doi: 10.1038/srep37183. [DOI] [PMC free article] [PubMed] [Google Scholar]