Sleep deprivation (SD) especially insomnia is commonly encountered in clinic. Many factors can cause insomnia, such as anxiety, fear, pain, medication, and disruptive environment 1. Sleep disorder during the perioperative period may cause a number of complications, including delayed anesthesia emergence. However, the mechanism underlying this phenomenon remains elusive.
It has been postulated that common neural pathways between natural sleep and general anesthesia may be involved in the regulation of anesthesia emergence times 2. Orexin is a peptide that consists of orexin‐A (OXA) and orexin‐B (OXB) subtypes 3. Accumulated evidence suggests that orexinergic neurons play a critical role in the promotion and maintenance of wakefulness 4. It has been reported that orexinergic neurons participate in the control of rapid eye movement (REM) sleep and the orexin‐1 receptor is particularly necessary in the suppression of REM sleep 5. Our earlier studies demonstrated that activation of the orexinergic signals in the basal forebrain significantly increased acetylcholine efflux in the somatosensory cortical region and shortened the awakening time of rats under inhalation or intravenous anesthesia 6. These findings strongly supported the notion that orexinergic neurons might play a crucial role in the regulation of postanesthesia arousal.
It has been reported that the anesthesia behaviors of rats change after SD in terms of time of reduced loss of righting reflex (LORR) and delayed recovery of the righting reflex (RORR) 7. These alterations strongly suggest that sleep pattern changes might modulate the effectiveness of general anesthetics. Furthermore, administration of adenosine A1 and A2 receptor antagonists partially reversed the effect of SD, but the awakening time did not return to its original length 8. We hypothesize that the delayed emergence from general anesthesia after SD is associated with the orexinergic neural pathway and experimentally verified the hypothesis in the rat.
To determine whether the delayed emergence from anesthesia after SD is due to the alteration of the orexinergic neuron's activity, the animals were divided into two groups: SD group and sham‐SD group. We adopted a modified multiple‐platform method to deprive REM sleep of the rats in SD group for 24 h. The plasma OXA concentrations were measured by radioimmunoassay (RIA) after SD. Results showed that the plasmic concentration of OXA in the sham‐SD group was 25.2 ± 2.43 pg/mL, but decreased to 17.8 ± 1.29 pg/mL at 24 h after sleep deprivation.
Next, we recorded both induction and emergence time in the two groups of rats subjected to isoflurane anesthesia. We found that the SD had no effects on the induction time, but increased the duration of emergence in isoflurane anesthesia. The induction time of the SD group (1.90 ± 0.12 min) was similar to that of the sham‐SD group (1.83 ± 0.09 min, P < 0.05). However, the emergence time in the SD rats (16.9 ± 0.63 min) was significantly longer than that of the sham‐SD group (14.8 ± 0.42 min; P < 0.05).
The brain sections were double immunofluorescently stained for orexin and c‐Fos in hypothalamic neurons to determine the orexinergic neuron's activity. The orexinergic neuron activity was observed at four time points: before and after the sleep deprivation, 30 min of isoflurane anesthesia, and at emergence. Approximately 140–170 orexin‐positive neurons were detected in each section. Of them, the percentage of OXA+ and c‐Fos+ double‐labeled neurons was 45.4 ± 6.5% before SD. After 24 h SD, the total number of OXA‐positive neurons did not change significantly, whereas the number of OXA+ and c‐Fos+ double‐labeled neurons decreased significantly to 32.1 ± 3.4% (P < 0.01 vs. before SD, n = 5). The percentage of active orexinergic neurons were decreased during the isoflurane anesthesia in both groups. However, the degree of activity of orexinergic neuron in SD group was obviously lower than that in the sham‐SD group (Figure 1).
Figure 1.
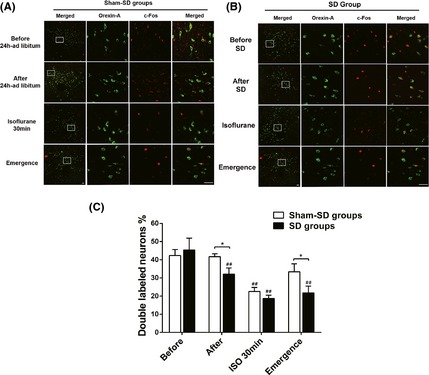
Representative microphotographs illustrating orexin (green) and c‐Fos (red) immunofluorescent staining in the rat hypothalamus at four time points in both the sham‐sleep deprivation group (A) and sleep deprivation (SD) group (B). Left column shows the merged images at low magnification, while the other three columns show higher magnification of the images in the framed areas of both the SD and sham‐SD group. Those pictures represent orexin staining, c‐Fos staining, and the merged image, respectively. (C) Histograms showing the ratio of OXA + and c‐Fos+ double‐labeled neurons compared to the total orexin‐positive neurons in the hypothalamic nuclei of the corresponding time points.
Subsequently, we administered OXA and OXB prior to anesthesia to detect the effect of exogenous administration of OXA and OXB on the emergence time in the rat with sleep deprivation. For this purpose, we divided both SD and sham‐SD rats into four groups, respectively (n = 8 each): OXA (0.5 mL of 30 μg/kg), OXA (0.5 mL of 100 μg/kg), OXB (0.5 mL of 30 μg/kg), and vehicle (0.5 mL of 0.9% NS). As orexins could cross blood–brain barrier 9, we gave the agents to the rat intraperitoneally. Results showed that injection of 30 μg/kg OXA decreased the emergence time of the rat subjected to SD to 15.9 ± 0.36 min (P < 0.01 vs. saline, 17.2 ± 0.64), and 100 μg/kg OXA decreased to 11.9 ± 0.4 min. The emergence time of the rat subjected to SD was 17.2 ± 0.47 min as intraperitoneal injection of OXB in advance, which was no different from or SD+NS group (P > 0.05). In addition, the induction time did not change significantly in any group when OXA or OXB was given (Figure 2).
Figure 2.
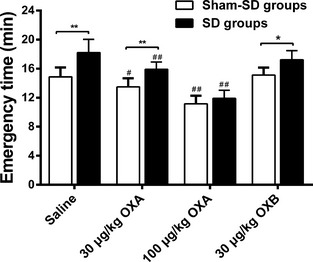
Histograms displaying the effects of intraperitoneal injection of different doses of exogenous OXA (0.5 mL of 30 μg/kg and 0.5 mL of 100 μg/kg), OXB (0.5 mL of 30 μg/kg), and vehicle (0.5 mL of 0.9% normal saline) on emergence time under isoflurane anesthesia in animals from sleep deprivation (SD) or sham‐SD group. The emergence time was significantly prolonged after SD (**P < 0.01 vs. sham‐SD group). Intraperitoneal injection of OXA (0.5 mL of 30 μg/kg) and OXA (0.5 mL of 100 μg/kg) restored the emergence time in sleep deprivation rats (##P < 0.01 vs. SD+vehicle), while intraperitoneal injection of OXB (0.5 mL of 30 μg/kg) had no effect on the prolongation of emergence time in SD rats. Values are expressed as the mean ± SEM (n = 8 each).
Our study has revealed that the orexinergic neural pathway controls, at least in part, the emergence from general anesthesia after REM sleep deprivation. The results showed that treatment of SD induced a significant reduction of orexinergic neuron activity and the decrease of plasma OXA levels and caused a prolonged emergence time in isoflurane anesthesia. Furthermore, OXA administration partially restored the emergence time that was prolonged by sleep deprivation. These findings strongly support our hypothesis that the delayed emergence from isoflurane anesthesia after SD is controlled by orexinergic neuron activity. Exogenous OXA supplement may become a new way to solve the clinical problem in the future.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Andersen JH, Boesen HC, Skovgaard OK. Sleep in the Intensive Care Unit measured by polysomnography. Minerva Anestesiol 2013;79:804–815. [PubMed] [Google Scholar]
- 2. Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 2008;9:370–386. [DOI] [PubMed] [Google Scholar]
- 3. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein‐coupled receptors that regulate feeding behavior. Cell 1998;92:1–696. [DOI] [PubMed] [Google Scholar]
- 4. Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS ONE 2011;6:e20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantor S, Mochizuki T, Janisiewicz AM, Clark E, Nishino S, Scammell TE. Orexin neurons are necessary for the circadian control of REM sleep. Sleep 2009;32:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong H, Niu J, Su B, et al. Activation of orexin signal in basal forebrain facilitates the emergence from sevoflurane anesthesia in rat. Neuropeptides 2009;43:179–185. [DOI] [PubMed] [Google Scholar]
- 7. Tung A, Szafran MJ, Bluhm B, Mendelson WB. Sleep deprivation potentiates the onset and duration of loss of righting reflex induced by propofol and isoflurane. Anesthesiology 2002;97:906–911. [DOI] [PubMed] [Google Scholar]
- 8. Tung A, Herrera S, Szafran MJ, Kasza K, Mendelson WB. Effect of sleep deprivation on righting reflex in the rat is partially reversed by administration of adenosine A1 and A2 receptor antagonists. Anesthesiology 2005;102:1158–1164. [DOI] [PubMed] [Google Scholar]
- 9. Dhuria SV, Hanson LR, Frey WN. Intranasal drug targeting of hypocretin‐1 (orexin‐A) to the central nervous system. J Pharm Sci 2009;98:2501–2515. [DOI] [PubMed] [Google Scholar]


