Abstract
Objectives: To investigate the effect of suppressor of fused (Sufu) on epidermal and dermal cellular properties and in wound healing.
Approach: Transgenic (TG) mice overexpressing human Sufu (hSufu) in the epidermis were applied to investigate the effects of Sufu on epidermal and dermal cellular properties and in wound healing.
Results: Histological staining revealed a reduction of epidermal and dermal thickness and an increase of hypodermal adipose tissue in homozygous K14-hSufu TG mice when compared with wild-type (WT) controls. TG mice exhibited significantly delayed skin wound healing. Moreover, the migratory and proliferative capabilities of cultured keratinocytes were decreased in K14-hSufuTG mice. Transforming growth factor-β treatment increased the expression of α-smooth muscle actin more in WT than in TG fibroblasts. Sufu overexpression significantly decreased the expression of β-catenin, glioma transcription factor 1 (Gli1), and matrix metalloproteinase-3 in wounds of K14-hSufu TG mice when compared with controls, probably indicating a delaying effect of Sufu on wound healing via blocking the hedgehog (Hh)/Gli and Wnt/β-catenin pathway.
Innovation: Our results indicate a new property of Sufu in the process of skin wound healing. It provides an important basis for Sufu as a potential target for skin wound healing.
Conclusion: Our findings suggest that Sufu overexpression in the epidermis impairs wound healing via dampening the Hh/Gli and Wnt/β-catenin signaling pathway. These data provide an important basis for further analyses of Sufu in skin wound healing.
Keywords: suppressor of fused, wound healing, β-catenin, transgenic mouse, skin

Xiao-Yong Man, MD, PhD

Min Zheng, MD, PhD
Introduction
Cutaneous wound healing is an interactive physiological process. It rapidly and efficiently repairs the integrity of the skin. Wound healing involves three stages: (1) inflammation; (2) migration and proliferation of keratinocytes, fibroblasts, and endothelial cells, resulting in reepithelialization and angiogenesis; and (3) remodeling.1–3 The process of wound healing is complex and requires dynamic interactions among several cell types, including endothelial cells, blood cells, epidermal keratinocytes, and dermal fibroblasts.4
Suppressor of fused (Sufu) is a negative regulator of the hedgehog (Hh) signaling pathway that interacts with the glioma transcription factors (Gli).5 The Hh signaling pathway is involved in cell proliferation, differentiation, and stem cell maintenance.6 Moreover there is evidence that the Hh signaling pathway is pivotal in adult wound healing,7 inflammation,8 and vascularization.9
Sufu is also known to display an important role in vertebrates. In mice, Sufu deletion led to embryo lethality.10 At present, Sufu was implicated in the regulation of the Wnt pathway,11 probably coordinating the Wnt and Hh pathways. Moreover, Sufu prevented the development of malignant tumors.12–15 In glioma, Sufu inhibited cell proliferation, invasion, and vasculogenic mimicry.16 Furthermore, Sufu was associated with human immunity by its single-nucleotide polymorphism involvement in graft-versus-host disease.6
Although Sufu has been studied in various immune-related diseases, the role of Sufu in cutaneous wound healing is yet unknown.
To determine whether Sufu plays a role in cutaneous wound healing, we studied the impact of Sufu in a Sufu transgenic (TG) mouse, in a full-thickness excisional cutaneous wound healing model.
Clinical Problem Addressed
While recently some studies have highlighted the role of Sufu in some cell types related to cutaneous repair—including Sufu's contribution to myofibroblast development17 and Sufu's prevention in human dermal fibroblast activation and migration18—it is necessary to study its function in cutaneous repair in depth. We used TG mice overexpressing human Sufu (hSufu) in the epidermis to investigate the effects of Sufu on epidermal and dermal cellular properties and wound healing. Moreover, we assessed its potential as a possible strategy for the development of therapeutics for cutaneous wounds.
Materials and Methods
Ethical approval
All animal experiments were performed in accordance with protocols approved by the Second Affiliated Hospital, Zhejiang University School of Medicine Animal Care Committee.
Generation of transgenic mice overexpressing Sufu in the epidermis
TG mice overexpressing hSufu in the epidermis were successfully constructed by including a K14 promoter. K14-hSufu TG mice were obtained from Cyagen Laboratories (Suzhou, China) and housed in a specific pathogen-free animal barrier facility in the Laboratory Animal Research Center of Zhejiang Chinese Medical University. The presence of the transgene was verified by polymerase chain reaction (PCR) (forward primer: GTTCGACAGTCCGCTACACTAGACC; reverse primer: AGACCCCTAGGAATGCTCGTCA). To obtain homozygous Sufu mice, Sufu heterozygous mice were intercrossed for at least seven generations. Homozygous Sufu male mice on a C57BL/6 background were used in our experiments. Age- and sex-matched wild-type (WT) C57BL/6 male mice were purchased from Shanghai SLAC Laboratory Animal Center (Shanghai, China) and used as controls.
In vivo wound healing assay
Male K14-hSufu TG mice and WT littermates (6–8 weeks old) were anesthetized by intraperitoneal injection of 50 mg/kg sodium pentobarbital and the dorsal skin was shaved and cleaned with 70% ethanol before wounding. Full-thickness incision wounds (the diameter: 1 cm) were generated using a sterile biopsy punch (Acuderm, Ft. Lauderdale, FL) on the dorsal skin of hSufu TG (n = 6, the number of animals taken for this experiment) and WT (n = 6) mice. Wounds were left uncovered and animals were housed individually. We measured the wounds using a caliper ruler. Length and width of the wounds were used to calculate the wound area. Healing was defined as the decrease in wound diameter over time and was expressed as the percentage compared with the day-0 wound diameter.
Skin histopathology
For histopathological analysis, mouse skin tissue specimen was fixed in 4% (w/v) buffered formalin for 24 h. Fixed skin samples embedded in paraffin and tissue sections were deparaffinized and stained with hematoxylin and eosin (H&E). Histological wound measurements were made on Masson trichrome (Solarbio, Beijing, China)-stained sections. Dermal thickness was measured at 40 × magnification. Five areas of the epidermis and adjacent dermis were measured separately. All measurements were conducted by a single blinded observer. The width of each wound and the distance traversed by the epithelium were measured in H&E-stained sections at the specified time points. The percentage of reepithelialization was calculated as follows: (Length of the minor axis covered by epithelium)/(Length of the minor axis between the original wound edges) × 100.
Immunofluorescence
Immunofluorescence was performed according to a previously published protocol.19 Briefly, skin biopsy specimens (n = 6) were processed, fixed, preheated, and blocked using 10% normal fetal bovine serum for 1 h. The following antibodies were used for immunofluorescence assays: anti-Sufu (1:200, sc10933; Santa Cruz Biotech, Santa Cruz, CA), anti-α-SMA (1:200, ab125044; Abcam, Cambridge, MA), anti-β-catenin (1:200, sc7199; Santa Cruz Biotech), anti-k10 (1:200, sc51581; Santa Cruz Biotech), anti-k14 (1:200, sc53253; Santa Cruz Biotech), and secondary antibodies labeled with Alexa 488 (green) and Cy3 (red) (1:5000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Cell nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; Roche Molecular Biochemicals, Mannheim, Germany) for 5 min at a concentration of 5 μg/mL (applied after incubation with a secondary antibody). For each case, a negative control incubated with nonimmune mouse immunoglobulin G (Sigma-Aldrich, St. Louis, MO) was included. Sufu, β-catenin, k10, and k16 were detected using a positive fluorescence microscope (Leica, Wetzlar, Germany). α-Smooth muscle actin (α-SMA) expression in fibroblasts was detected using an inverted fluorescence microscope (Leica).
Immunohistochemical analyses
The back skin of the mice was fixed in 10% formalin and embedded in paraffin. An immunohistochemical analysis was performed using the standard ABC-peroxidase Kit (Vector Laboratories, Burlingame, Ontario, Canada and CA), as suggested by the manufacturer. Affinity-purified biotinylated anti-rabbit IgG was purchased from Vector Laboratories. Other antibodies used in this study included Ki67 (12202; Cell Signaling Technology). A representative picture showing similar results for each group was chosen for this publication. Negative controls without primary antibodies showed no immunolabeling.
Keratinocyte and dermal fibroblast isolation and culture
K14-hSufu TG and WT neonatal pups were sacrificed by excessive anesthesia and the dorsal skin was dissected and treated as described previously18: briefly, samples were placed in 0.5% dispase (Invitrogen, Carlsbad, CA) overnight at 4°C. Detached keratinocytes were cultured in keratinocyte serum-free medium with keratinocyte growth supplements (Thermo Fisher Scientific, Rockford, IL) and refreshed every 48 h. Then, the dermis was separated from the epidermis and incubated with 0.1% collagenase (Invitrogen) overnight and the released fibroblasts were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum.
In vitro wound healing and proliferative assay
Keratinocytes and fibroblasts were cultured separately to confluence in six-well plates. Scratch wounds were created using a sterile P200 pipette tip. After washing cells three times, they were refed with medium. Cell migration into the wound space was estimated at 0 and 24 h after wounding, using image analysis. Three representative images from each well of the scratched area were taken using a microscope to estimate the migration ability of keratinocytes and fibroblasts, and the cell migration rate was analyzed using ImageJ software (National Institutes of Health Bethesda, MD). All scratch assays were performed in quadruplicate.
Keratinocyte and fibroblast proliferation was determined separately using a cholecystokinin octapeptide (CCK8) assay. Briefly, cells were seeded (1 × 104 cells/well) in a 96-well plate and incubated for 5 h to permit cell adhesion. Then, proliferation was studied every 24 h up to 48 h. At this time point, the Cell Counting Kit-8 (Dojindo Molecular Technologies, Kumamoto, Japan) was added to measure cell proliferation. Absorbance was read at 450 nm using a plate reader, and results were presented as the average of three independent experiments. All cell proliferation assays were performed in quadruplicate.
Fibroblast differentiation assay
The cells were serum starved for 48 h and then treated with 10 ng/mL transforming growth factor-β1 (TGF-β1; Peprotech, Rocky Hill, NJ) or medium vehicle for 48 h.
Western blot analysis
Protein extraction and Western blot analysis of tissue and primary cell lysates were performed as previously described.19 Briefly, an equal amount of protein (20 μg/well) was loaded and run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Then, the gels were transferred onto nitrocellulose filter membranes (Millipore, Billerica, MA) and probed with the primary antibody for the first time. The primary antibodies, anti-Sufu (sc10933), anti-MMP-3 (sc21732), anti-β-catenin (sc7199), anti-Gli1 (sc20687), anti-β-actin (sc47778), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, sc47724), were all obtained from Santa Cruz Biotech. The secondary antibodies used in immunoblotting studies were HRP-conjugated sheep anti-rabbit or sheep anti-mouse IgG antibodies (1:5000; Jackson ImmunoResearch Laboratories, Inc.). Signals were detected using an enhanced chemiluminescence kit (Millipore).
Quantitative real-time polymerase chain reaction analysis
Quantitative real-time PCR was performed according to our previously published protocol.18 Primers for Sufu (5′-ACAGAGTCCATGAGTTTAC-3′, 5′-CCCAGTTTCTCTCTTCAG-3′) and GAPDH (5′-AGCTTCGGCACATATTTCATCTG-3′, 5′-CGTTCACTCCCATGACAAACA-3′) were designed using Beacon designer software (version 8.20; Premier Biosoft International, Palo Alto, CA).
Statistical analysis
Data are expressed as mean ± standard error of the mean. All statistical tests were performed using Prism GraphPad 6.00 (GraphPad Software, La Jolla, CA). Student's two-tailed t-tests were used to compare means between groups. p-Values <0.05 indicated statistical significance.
Results
Generation and verification of TG mice that overexpress hSufu in the epidermis
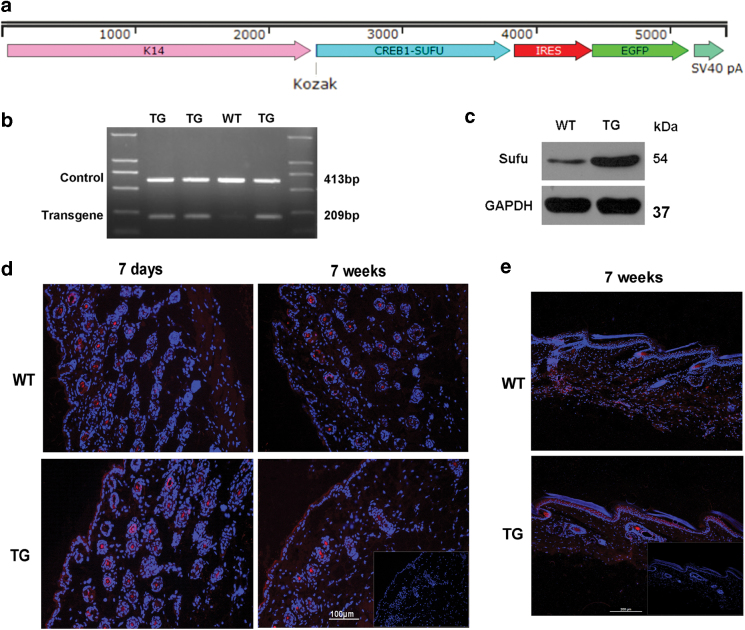
A TG mouse overexpressing hSufu in the epidermis was generated. hSufu gene was cloned into the downstream of the K14 promoter (Fig. 1a) to spatially restrict overexpression of Sufu to the epidermis. Homozygous TG mice were used in our study. The K14-hSufu TG mice were identified by PCR (Fig. 1b). The overexpression of hSufu was confirmed by Western blot analysis (Fig. 1c). Elevated expression of Sufu in the epidermis and hair follicles in 7-day-old and 7-week-old TG animals was compared with WT littermates, and was also demonstrated in dorsal skin and tail skin through immunofluorescence staining (Fig. 1d, e).
Figure 1.
Verification of Sufu overexpression in the epidermis of TG mice. (a) Sufu expression vector. The Sufu cDNA was inserted into the K14 expression plasmid comprising the human K14 promoter and the K14 polyA sequence. Sufu overexpression in the epidermis of hSufu TG mice was confirmed by (b) polymerase chain reaction and (c) Western blot analyses. (d) Immunofluorescence staining revealed elevated Sufu levels in the skin of neonatal and 7-week-old mice localized in epidermal keratinocytes and hair follicle cells (Sufu—red, DAPI—blue). (e) Immunofluorescence staining revealed elevated Sufu levels in the tail skin of neonatal and 7-week-old mice localized in epidermal keratinocytes (Sufu—red, DAPI—blue). Scale bar = 0.1 mm. DAPI, 4′,6-diamidino-2-phenylindole; hSufu, human suppressor of fused; TG, transgenic. Color images are available online.
Characterization of the K14-hSufu TG mouse model
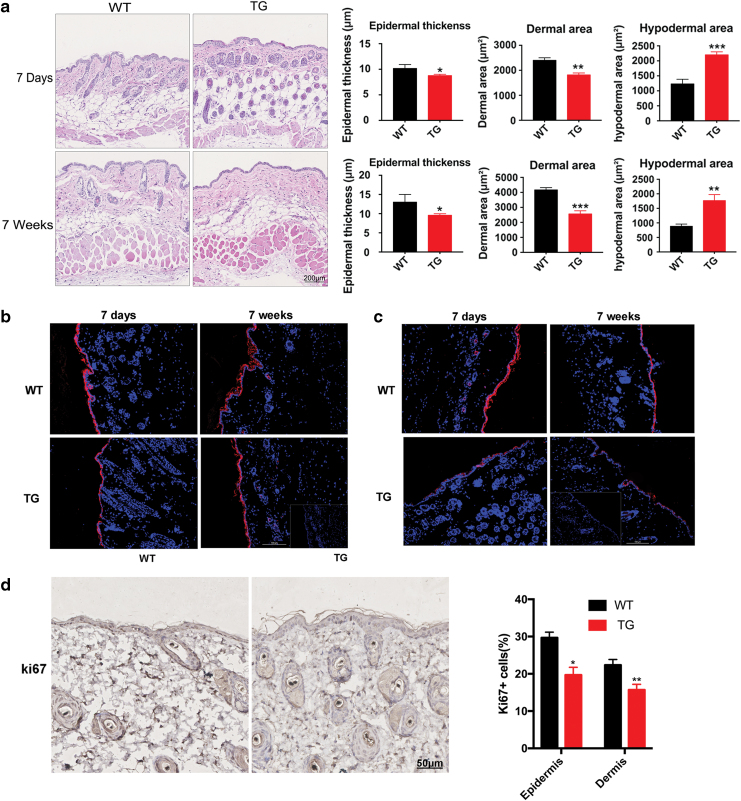
We examined Sufu morphology in the skin of newborn and adult mice. The homozygous TG mice are viable, have a normal life span, and do not show an overt cutaneous phenotype. We observed the following microscopic phenotypic changes: the epidermis and dermis of the dorsal skin were significantly reduced in thickness in the 7-day-old and 7-week-old TG mice compared with that of littermates. In contrast, the hypodermal adipose tissue was significantly increased in Sufu TG mice at both ages (Fig. 2a, Supplementary Fig. S1).
Figure 2.
Dermal skin thickness was reduced and hypodermal adipose tissue was increased in TG mice compared with WT controls. (a) Histological analysis of dorsal skin sections revealed that the dermal area of TG mice was reduced compared with WT controls 7 days after birth and persisted into adulthood. n = 6, *p < 0.05; **p < 0.01; ***p < 0.001 by two-tailed Student's t-test. (b) Immunofluorescence labeling of K10 in the skin of WT and TG mice (K10—red, DAPI—blue). Scale bar = 0.1 mm. (c) Immunofluorescence labeling of K14 in the skin of WT and TG mice (K14—red, DAPI—blue). Scale bar = 0.1 mm. (d) Representative immunohistochemical analyses of Ki67 (brown-stained nuclei in the epidermis and dermis) in the skin of WT and TG mice. Scale bar = 50 μm. *p < 0.05; **p < 0.01. DAPI, 4′,6-diamidino-2-phenylindole; TG, transgenic; WT, wild type. Color images are available online.
We hypothesized that the epidermal and dermal thickness reduction observed in the Sufu TG mice could be due to alternations in the epidermal proliferation and/or differentiation. Therefore, we examined the expression of proliferation and differentiation markers, including K10, K14, and Ki67. In the skin of Sufu TG mice, the suprabasal layer expressing K10 was slightly reduced, in addition, the expression of K14 was modestly reduced in Sufu TG skin (Fig. 2b, c). These results indicated that keratinocytes overexpressing Sufu might have impaired cell differentiation, reducing the thickness of the epidermis and dermis. Ki-67 immunostaining showed that the number of Ki-67-positive keratinocytes and fibroblasts was decreased in TG mice compared with WT mice (p < 0.05, p < 0.01, respectively) (Fig. 2d), indicating the down acceleration of keratinocyte and fibroblast proliferation by Sufu overexpression. In all, TG mice showed a significantly thinner epidermis and dermis and degraded expression of differentiation and proliferation markers (K10, K14, and Ki-67).
Cutaneous wound healing was delayed in hSufu TG mice
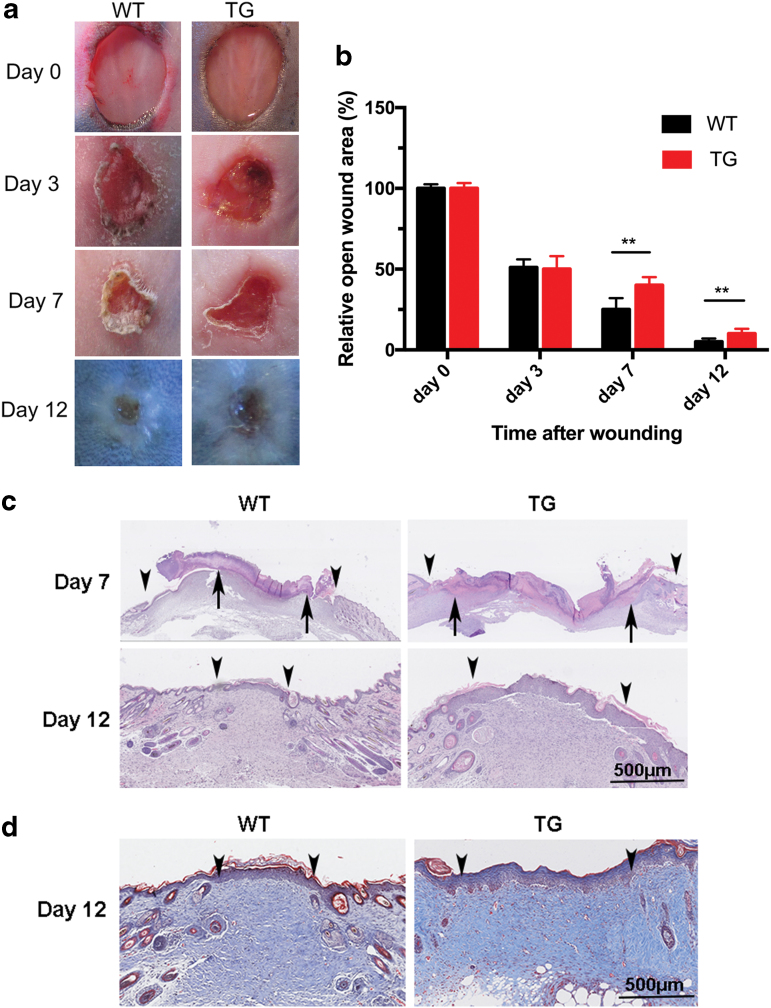
To investigate the role of Sufu in skin homeostasis, the effect of Sufu overexpression in skin repair was examined. Full-thickness incision wounds were created. The percentage of original wound area was larger in TG mice than in the WT controls from day 7. This finding suggested that wound healing was significantly delayed in TG mice (p < 0.01), as shown in Fig. 3a and b. Significantly extended epithelial rete was also observed in TG mice at days 7 and 12 after injury (p < 0.05, Fig. 3c). Masson's trichrome staining of excisional wound sections made on day 12 postinjury revealed that the area of granulation tissue in the midwound region of the TG mice was significantly larger than that of the WT controls (p < 0.05, Fig. 3d).
Figure 3.
Cutaneous wound healing was delayed in TG mice. (a) Representative images of wounds generated with dermal punch biopsies on the dorsal skin of 7-week-old male mice show significant delays in wound closure in TG mice compared with WT controls. Duplicate wounds, n = 6, (b) the proportion of the wound remaining open relative to the initial wound area at each time point. Data are shown as mean ± standard error of the mean from six to eight wounds and are representative of three independent experiments with similar results. **p < 0.01, by two-tailed Student's t-test. (c) Hematoxylin and eosin staining of reepithelialization (wound margin [arrowheads] and the leading edge of epithelial wound [arrows]). Scale bar = 500 μm. (d) Masson's trichrome-stained wound sections at 12 days postinjury show the extent of granulation tissue at the midpoint of the wound (wound margin [arrowheads]). Scar bar = 500 μm. TG, transgenic; WT, wild type. Color images are available online.
Overexpression of Sufu restrained keratinocyte migration and proliferation
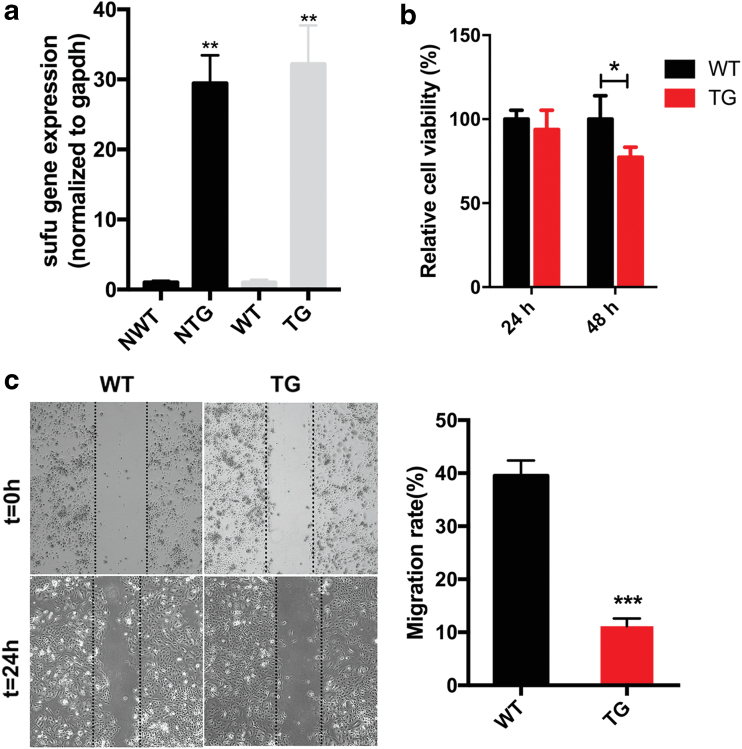
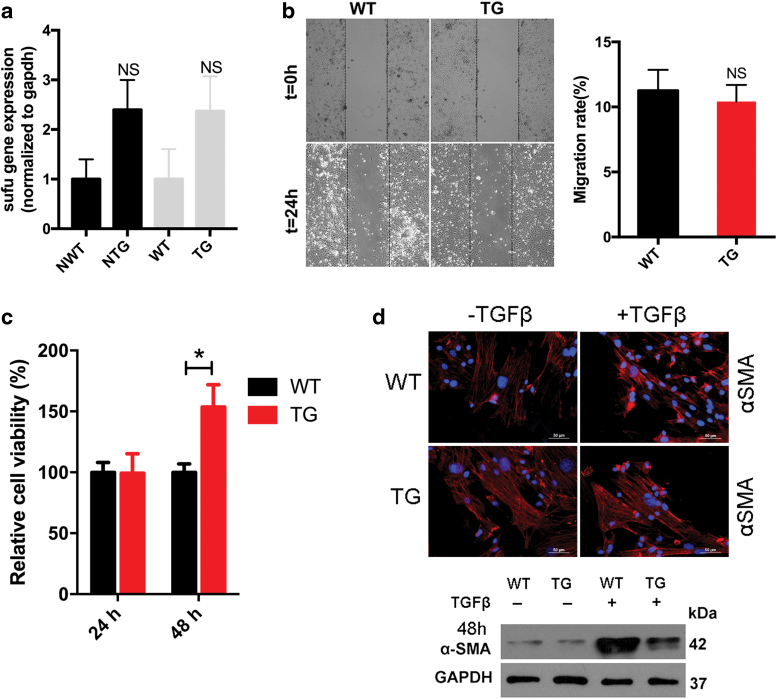
Keratinocyte migration is one of the essential biological processes for epidermal repair.20 Therefore, the role of Sufu in keratinocyte migration was investigated. The transcription of Sufu was confirmed in neonatal and 7-week-old animals by quantitative polymerase chain reaction (Fig. 4a). We first performed the assay with primary keratinocytes isolated from hSufu TG mice. Scratch wound assays (Fig. 4c) indicated that Sufu overexpression significantly reduced the migration rate of keratinocytes after 24 h of scratching by 23.5% ± 3.1% (p < 0.001) compared with our controls. The proliferation capability of keratinocytes was greater in the WT mice than in the Sufu TG mice at 48 h (p < 0.05) (Fig. 4b).
Figure 4.
Primary keratinocytes derived from TG neonatal skin are less migratory and proliferative than those from WT controls. (a) qPCR analysis on Sufu in primary epidermal keratinocytes of NTG and NWT control mice and 7-week-old TG and WT control mice. (b) Proliferation analysis of keratinocytes in TG and WT fibroblasts at 24 and 48 h. (c) Scratch wound experiments of primary keratinocytes of TG mice and controls. All values are expressed as mean ± standard deviation from three different independent experimental sets. *p < 0.05; **p < 0.01; ***p < 0.001. NTG, neonatal TG; NWT, neonatal WT; qPCR; Sufu, suppressor of fused; TG, transgenic; WT, wild type. Color images are available online.
Overexpression of Sufu in the epidermis affects the dermis
Sufu expression in the primary dermal fibroblasts was similar in TG and WT neonatal and 7-week-old mice (Fig. 5a). No significant differences between WT and TG fibroblasts in the total migration distance after the scratch wound assay in vitro were observed (Fig. 5b). However, proliferation capability increased in TG fibroblasts compared with WT fibroblasts at 48 h (Fig. 5c). Morphologically, TG fibroblasts did not respond to TGF-β1 stimulation, whereas WT fibroblasts acquired a contractile myofibroblast morphology after stimulation. Moreover, TGF-β1 treatment increased the expression of α-SMA in WT, compared with TG fibroblasts (Fig. 5d).
Figure 5.
Sufu overexpression in the epidermis affects the dermis. (a) Sufu expression in the primary dermal fibroblasts was similar in NTG and NWT control mice and 7-week-old TG and WT mice. (b) Scratch wound experiments revealed no significant differences in migration distance between Sufu TG primary and control fibroblasts. (c) CCK8 analysis revealed increased proliferation capability in TG fibroblasts compared with control fibroblasts at 24 and 48 h. (d) Immunofluorescence revealed that the morphology of WT fibroblasts differentiated into contractile myofibroblasts in the presence of TGF-β1, whereas the morphology of TG fibroblasts did not change (SMA—red; DAPI—blue). TGF-β treatment increased the expression of α-SMA in WT fibroblasts compared with TG fibroblasts. GAPDH was used as a loading control. *p < 0.05. α-SMA, α-smooth muscle actin; CCK8, cholecystokinin octapeptide; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NTG, neonatal TG; NWT, neonatal WT; Sufu, suppressor of fused; TG, transgenic; TGF-β1, transforming growth factor-β1; WT, wild type. Color images are available online.
Overexpression of Sufu retards wound healing via blocking Hh/Gli and Wnt/β-catenin pathway
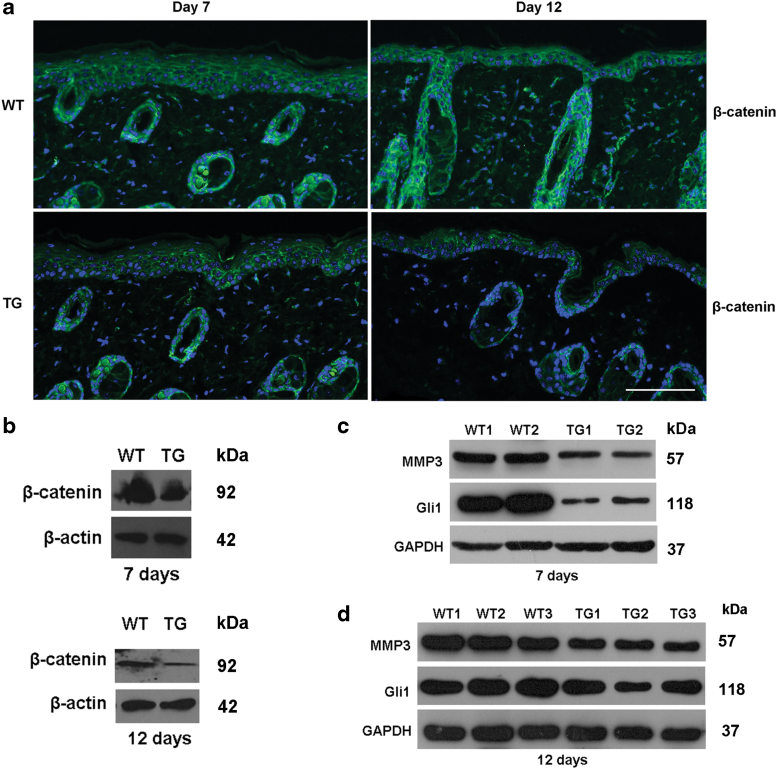
We then sought to identify the mechanism by which Sufu affects the process of cutaneous wound healing. β-Catenin was less expressed in the epidermis of the TG mice on days 7 and 12 after wound compared with the control (Fig. 6a). Western blot analysis further verified results, as shown in Fig. 6b. Sufu overexpression was associated with a reduction in the levels of matrix metalloproteinase-3 (MMP-3) (Fig. 6c) in the wounds of TG mice compared with our controls. There was also a marked decrease in Gli1 expression on days 7 and 12 of wound healing in TG mice compared with WT controls. There was no change in the expression of GAPDH (Fig. 6d).
Figure 6.
Overexpression of Sufu retards wound healing via blocking the Hh/Gli and Wnt/β-catenin pathway. (a) Representative immunofluorescence staining images (n = 5 independent experiments) for β-catenin in control and TG wounds at day 7 and 12 postwounding. (b–d) Western blot analyses of β-catenin, MMP-3, and Gli1 at day 7 and 12 postinjury in the control and TG wounds. Gli, glioma transcription factor; Hh, hedgehog; MMP-3, matrix metalloproteinase-3; Sufu, suppressor of fused; TG, transgenic. Color images are available online.
Our data suggest that the overexpression of Sufu retards cutaneous wound healing by inhibition of the Hh/Gli and Wnt/β-catenin signaling.
Discussion
Our findings indicate a possible link between Sufu overexpression within the epidermis and the regulation of cellular properties of keratinocytes and dermal fibroblasts. Moreover, it might delay cutaneous wound healing.
Our results demonstrated that epidermal overexpression of Sufu results in decreased epidermal thickness. Epidermal differentiation and proliferation were downregulated in Sufu TG mice, leading to reduced expression of k14, k16, and Ki67. Several studies have already implicated K14 and other type 1 keratins, such as K10, as significant players in epidermal homeostasis.21,22
In addition, the dermal thickness was reduced; this may suggest an additional mechanism of cross talk from the epidermis to fibroblasts that modulate production in the dermis. Such a finding would be consistent with previous publications describing a cross talk between keratinocytes and endothelial cells through induction of secreted proangiogenic factors.23
However, the question of whether the effects observed are a primary consequence of Sufu overexpression and what further factors do play a role may have to be investigated in additional studies.
Sufu overexpression in the epidermis significantly delayed the cutaneous wound healing after injury to the dorsal skin of 7-week-old mice. Thus, it might be possible that Sufu orchestrates the signaling cascade necessary for the healing response.
Our findings indicate that Sufu overexpression in the epidermis inhibits both, the proliferation and migration of keratinocytes, which are crucial cellular events during the proliferative phase of wound healing and essential for reepithelialization.24 Furthermore, our findings are consistent with the role of Sufu as an inhibitor of glioma cell migration and proliferation.16 Whether the antiproliferative role of Sufu is of functional importance in hyperproliferative skin diseases (e.g., psoriasis) is under investigation at present. Moreover, Sufu overexpression in the epidermis had an impact on the dermis, such that Ki67 expression was downregulated in TG dermis and that TG fibroblasts did not differentiate into α-SMA-enriched myofibroblasts, which serve to contract the wound, in response to TGF-β1. Previous studies have reported similar findings.25–27 This differentiation and proliferation defect may be resulted from the absence of factors necessary for the normal differentiation and contraction of fibroblasts or due to other molecules in this signaling pathway.
To understand and enhance the wound repair process, it is essential to understand the molecular mechanisms underlying the function of Sufu in wound repair. It is interesting to find the ability of Sufu to interact with the canonical Wnt effector β-catenin. Sufu overexpression was associated with a significant decrease in β-catenin of the TG mice compared with the control.
The Wnt/β-catenin pathway, which is also called the canonical Wnt pathway,28 plays pivotal roles in cutaneous wound healing.29,30 The Wnt/β-catenin pathway is important for the regulation of cell proliferation and the motility in wounds.31,32 A previous in vitro study showed that overexpressed murine Sufu is able to bind to β-catenin and thereby exporting it from the nucleus. This again results in a negative regulation of β-catenin-dependent transcription.14 Moreover, β-catenin was shown to inhibit keratinocyte migration and activated fibroblast proliferation, causing aggressive fibromatosis and hyperplasia in cutaneous wounds33 and inhibiting epithelial cell migration in wounds in a human skin organ culture model. β-Catenin is also a component of the adherent junction,34 which may be important during reepithelialization, because epidermal cells migrate and proliferate to cover the surface of the tissue defect and reestablish cadherin–catenin-mediated adherens junction with adjacent cells.2 In all, we believed that the moderate regulation of Wnt/β-catenin signaling pathway in cutaneous wound is important for enhancing wound healing and Sufu plays an important role in modulating its function.
In addition, we have shown that epidermal Sufu overexpression delayed wound healing as indicated by a reduction in the levels of MMP-3. Matrix metallopeptidase 3 is associated with keratinocyte migration and wound repair in mouse skin. A decrease in MMP-3 expression impairs wound healing and reduces keratinocyte migration.35–37 We found that an increase in epidermal MMP-3 expression stimulated by wound healing was significantly inhibited in TG mice compared with WT mice. Our study suggested that overexpression of Sufu suppresses cell migration likely through inhibiting expressions and activations of MMP-3. β-Catenin may act as a mediator, linking the expression of factors important in the later remodeling phase (MMP-3).
At the mechanistic level, Sufu overexpression significantly reduced Gli1 expression, which is a transcription factor in the Hh signaling pathway and a direct target of Sufu.5 In vertebrates, Sufu represses Gli1 function, in part, by tethering it in the cytoplasm.38,39 Previous studies have found that Gli1 may regulate cell proliferation in HaCaT cells40 and knockdown of Gli1 restrained proliferation, invasion, and migration of cutaneous squamous cell carcinoma cells.41 However, further investigation is needed to identify the mechanism underlying Sufu-Gli1-mediated modulation of cutaneous wound healing. Of particular interest is that Sufu may act as a dual inhibitor of Hh/Gli1 and Wnt/β-catenin signaling pathways and may have an integrating role in cutaneous wound healing.
We found that overexpression of epidermal Sufu regulated the cellular properties of keratinocytes and dermal fibroblasts and delayed wound healing via inhibition of Hh/Gli1 and Wnt/β-catenin signaling pathways.
Taken together, our results suggest a novel aspect of Sufu in regulating the cellular properties of keratinocytes and dermal fibroblasts and in cutaneous wound healing, in which Sufu, β-catenin, and Gli1 interplay and provide a potential target for the development of new treatments for cutaneous wound healing.
Innovation
Our results suggest a new aspect of Sufu in the skin wound healing process and provide potential targets for the development of new therapies for skin wound healing.
Key Findings
Overexpression of Sufu may delay cutaneous wound healing.
Sufu restrains keratinocyte migration and proliferation.
Sufu retards wound healing through blocking the Hh/Gli and Wnt/β-catenin pathway.
Supplementary Material
Abbreviations and Acronyms
- α-SMA
α-smooth muscle actin
- CCK8
cholecystokinin octapeptide
- DAPI
4′,6-diamidino-2-phenylindole
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- Gli
glioma transcription factor
- H&E
hematoxylin and eosin
- Hh
hedgehog
- hSufu
human Sufu
- MMP-3
matrix metalloproteinase-3
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- Sufu
suppressor of fused
- TG
transgenic
- TGF-β1
transforming growth factor-β1
- WT
wild type
Acknowledgment and Funding Sources
This research was supported by grants from the National Natural Science Foundation of China (NFSC) (81573039, 81630082, 81602739, and 91542124).
About the Authors
Xiao-Yong Man, MD, PhD, Min Zheng, MD, PhD, and Sui-Qing Cai, MD, are Professors in the Department of Dermatology, Second Affiliated Hospital, Zhejiang University School of Medicine, where their laboratories study the mechanisms underlying psoriasis and impaired wound healing. Bei-Bei Yang, Bing-Xi Yan, and Ping Wang are PhD candidates and conducted research in wound healing. Yu-Xin Zheng is MSc in the Second Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou, China. Lilla Landeck, MD, is associate professor in the Ernst von Bergmann General Hospital in Potsdam, Teaching Hospital of the Charité–Humboldt University, Berlin, Germany, where she studies mechanism of atopic dermatitis. Hua-Li Cao, MD, PhD, Jia-Qi Chen, MD, PhD, Wei Li, MD, PhD, and Min Min, MD, PhD are in the Department of Dermatology, Second Affiliated Hospital, Zhejiang University School of Medicine in Hangzhou, China.
Author Disclosure and Ghostwriting
The article was written by the authors and ghostwriting services were not used.
Supplementary Material
References
- 1. Martin P. Wound healing—aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 2. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 3. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi Y, Yoshikawa K. Cutaneous wound healing: an update. J Dermatol 2001;28:521–534 [DOI] [PubMed] [Google Scholar]
- 5. Cherry AL, Finta C, Karlstrom M, et al. Structural basis of SUFU-GLI interaction in human Hedgehog signalling regulation. Acta Crystallogr D Biol Crystallogr 2013;69:2563–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katz MC, Michaelis S, Siegmund DM, et al. Association analysis between SUFU polymorphism rs17114808 and acute graft versus host disease after hematopoietic stem cell transplantation. Bone Marrow Transplant 2018;53:377–382 [DOI] [PubMed] [Google Scholar]
- 7. Le H, Kleinerman R, Lerman OZ, et al. Hedgehog signaling is essential for normal wound healing. Wound Repair Regen 2008;16:768–773 [DOI] [PubMed] [Google Scholar]
- 8. Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci 2009;29:10299–10308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kazmers NH, McKenzie JA, Shen TS, Long F, Silva MJ. Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing. Bone 2015;81:524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Svard J, Heby-Henricson K, Persson-Lek M, et al. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell 2006;10:187–197 [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Zapico ME. Primers on molecular pathways GLI: more than just Hedgehog? Pancreatology 2008;8:227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev 2010;24:670–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee Y, Kawagoe R, Sasai K, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene 2007;26:6442–6447 [DOI] [PubMed] [Google Scholar]
- 14. Meng X, Poon R, Zhang X, et al. Suppressor of fused negatively regulates beta-catenin signaling. J Biol Chem 2001;276:40113–40119 [DOI] [PubMed] [Google Scholar]
- 15. Merchant M, Vajdos FF, Ultsch M, et al. Suppressor of fused regulates Gli activity through a dual binding mechanism. Mol Cell Biol 2004;24:8627–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu X, Wang X, Du W, et al. Suppressor of fused (Sufu) represses Gli1 transcription and nuclear accumulation, inhibits glioma cell proliferation, invasion and vasculogenic mimicry, improving glioma chemo-sensitivity and prognosis. Oncotarget 2014;5:11681–11694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin C, Chen MH, Yao E, et al. Differential regulation of Gli proteins by Sufu in the lung affects PDGF signaling and myofibroblast development. Dev Biol 2014;392:324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao HL, Zhou J, Chen XB, et al. Inhibition of the hedgehog pathway leads to antifibrotic effects in dermal fibrosis. Discov Med 2016;22:311–318 [PubMed] [Google Scholar]
- 19. Man XY, Li W, Chen JQ, et al. Impaired nuclear translocation of glucocorticoid receptors: novel findings from psoriatic epidermal keratinocytes. Cell Mol Life Sci 2013;70:2205–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci 2003;116:3227–3238 [DOI] [PubMed] [Google Scholar]
- 21. Reichelt J, Magin TM. Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J Cell Sci 2002;115:2639–2650 [DOI] [PubMed] [Google Scholar]
- 22. Wei X, Fricker K, Enk AH, Hadaschik EN. Altered expression of keratin 14 in lesional epidermis of autoimmune skin diseases. Int J Dermatol 2016;55:620–628 [DOI] [PubMed] [Google Scholar]
- 23. Mitchell K, Szekeres C, Milano V, et al. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci 2009;122:1778–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res 2005;304:274–286 [DOI] [PubMed] [Google Scholar]
- 25. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363 [DOI] [PubMed] [Google Scholar]
- 27. Penuela S, Kelly JJ, Churko JM, Barr KJ, Berger AC, Laird DW. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J Invest Dermatol 2014;134:2026–2035 [DOI] [PubMed] [Google Scholar]
- 28. Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol 2008;468:5–15 [DOI] [PubMed] [Google Scholar]
- 29. Zhang DL, Gu LJ, Liu L, et al. Effect of Wnt signaling pathway on wound healing. Biochem Biophys Res Commun 2009;378:149–151 [DOI] [PubMed] [Google Scholar]
- 30. Wei J, Melichian D, Komura K, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum 2011;63:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheon S, Poon R, Yu C, et al. Prolonged beta-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab Invest 2005;85:416–425 [DOI] [PubMed] [Google Scholar]
- 32. Cheon SS, Cheah AY, Turley S, et al. Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A 2002;99:6973–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol 2005;167:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko K, Arora P, Lee W, McCulloch C. Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am J Physiol Cell Physiol 2000;279:C147–C157 [DOI] [PubMed] [Google Scholar]
- 35. Bullard KM, Lund L, Mudgett JS, et al. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg 1999;230:260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lund LR, Romer J, Bugge TH, et al. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J 1999;18:4645–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gawronska-Kozak B. Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biol 2011;30:290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stone DM, Murone M, Luoh S, et al. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci 1999;112(Pt 23):4437–4448 [DOI] [PubMed] [Google Scholar]
- 39. Kogerman P, Grimm T, Kogerman L, et al. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol 1999;1:312–319 [DOI] [PubMed] [Google Scholar]
- 40. Eichberger T, Sander V, Schnidar H, et al. Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics 2006;87:616–632 [DOI] [PubMed] [Google Scholar]
- 41. Sun Q, Bai J, Lv R.. Hedgehog/Gli1 signal pathway facilitates proliferation, invasion, and migration of cutaneous SCC through regulating VEGF. Tumour Biol 2016. DOI: 10.1007/s13277-016-5435-x [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.