Abstract
Airway remodeling in chronic obstructive pulmonary disease (COPD) originates, in part, from smoking-induced changes in airway basal stem/progenitor cells (BC). Based on the knowledge that the bone morphogenetic protein 4 (BMP4) influences epithelial progenitor function in the developing and adult mouse lung, we hypothesized that BMP4 signaling may regulate the biology of adult human airway BC relevant to COPD. BMP4 signaling components in human airway epithelium were analyzed at the mRNA and protein levels, and the differentiation of BC was assessed using the BC expansion and air-liquid interface models in the absence/presence of BMP4, BMP receptor inhibitor and/or siRNAs against BMP receptors and downstream signaling. The data demonstrates that in cigarette smokers, BMP4 is up-regulated in ciliated and intermediate undifferentiated cells and expression of the BMP4 receptor BMPR1A is enriched in BC. BMP4-induced BC to acquire a smoking-related abnormal phenotype in vitro mediated by BMPR1A/Smad signaling, characterized by decreased capacity to differentiate into the normal mucociliary epithelium, while generating squamous metaplasia. In summary, exaggerated BMP4 signaling promotes cigarette smoking-relevant airway epithelial remodeling by inducing abnormal phenotypes in human airway BC progenitor cells. Targeting of BMP4 signaling in airway BC may represent a novel target to prevent/treat COPD-associated airway disease.
Introduction
The human airway epithelium is a pseudostratified columnar epithelium, composed of basal cells (BC), intermediate undifferentiated, ciliated, and secretory cells [1–3]. BC, small cuboidal cells residing on the airway basement membrane, serve as the stem/progenitor cells of the human airway epithelium, capable of differentiating into the ciliated and secretory cells that are central to the physical barrier, host defense and mucociliary clearance [1, 2]. The intermediate cells are BC-derived precursors committed to differentiate into ciliated and secretory cells [4, 5].
Cigarette smoking, the major cause of chronic obstructive pulmonary disease (COPD), is associated with marked changes to the morphology of airway epithelium, including BC and intermediate cell hyperplasia, mucous cell hyperplasia, squamous cell metaplasia, loss of ciliated cells and shortened cilia [5–13]. Increasing evidence supports the concept that reprogramming of airway basal stem/progenitor cells is central to the early pathogenesis of cigarette smoking-induced derangement of the airway epithelium [14, 15]. BC isolated from smokers have a significant transcriptomic difference compared to the BC from nonsmokers, including reprogramming of genes associated with the genetic risk for COPD [16]. Smoking skews the normal function of BC by suppression of airway epithelial differentiation and tight junction formation [12, 17], in part through the crosstalk between BC and other cell types [5, 18]. For example, activation of EGFR in BC alters the differentiation program of BC toward the squamous and epithelial-mesenchymal transition (EMT)-like phenotypes characteristic of smoking-induced changes to the airway epithelium in vivo [5].
In the assessment of human airway epithelium in healthy nonsmokers, asymptomatic smokers and COPD smokers, we observed that bone morphogenetic protein 4 (BMP4) expression is up-regulated in the airway epithelium of asymptomatic and COPD smokers. Based on the knowledge that BMP4, a secreted transforming growth factor-β family member, controls epithelial progenitor function in the developing and adult mouse lung by regulating early mesoderm formation [19], airway branching morphogenesis [20], promoting proximal airway differentiation [21], and induces EMT-like changes in a human airway epithelial cell line [22], we hypothesized that excessive BMP4 signaling may play a functional role in dysregulating the biology of adult human airway BC relevant to smoking and COPD. The data demonstrates that in cigarette smokers, BMP4 expression is up-regulated in ciliated and intermediate undifferentiated cells, and BMP4 receptor BMPR1A expression is enriched in BC. BMP4-treated normal BC acquires a COPD-like “fatigue” phenotype, characterized by decreased capacity to differentiate into the normal mucociliary epithelium, while generating squamous metaplasia. These effects are mediated by BMPR1A/Smad signaling and inhibition of BMPR1A restores the stem/progenitor cell potential of airway BC, providing a possible target for therapeutic intervention in the smoking-induced lung disorders.
Methods
Human airway epithelium and BC were obtained from nonsmokers, asymptomatic and COPD smokers by bronchoscopy and in vitro primary BC culture. In vitro primary BC and air-liquid interface (ALI) cultures were carried out to study the effects of BMP4 on BC differentiation. Gene expression of the airway epithelium and BC in vivo and in vitro were assessed by RNA-sequencing/microarray and TaqMan real-time PCR. Airway epithelial morphology and localization of proteins were accessed by immunohistochemistry/immunofluorescence staining. Phosphorylation of BMP4 downstream signaling was assessed by Western blot. Details are in Supplemental Methods.
Results
BMP4 is Up-regulated in the Airway Epithelium of Asymptomatic and COPD Smokers
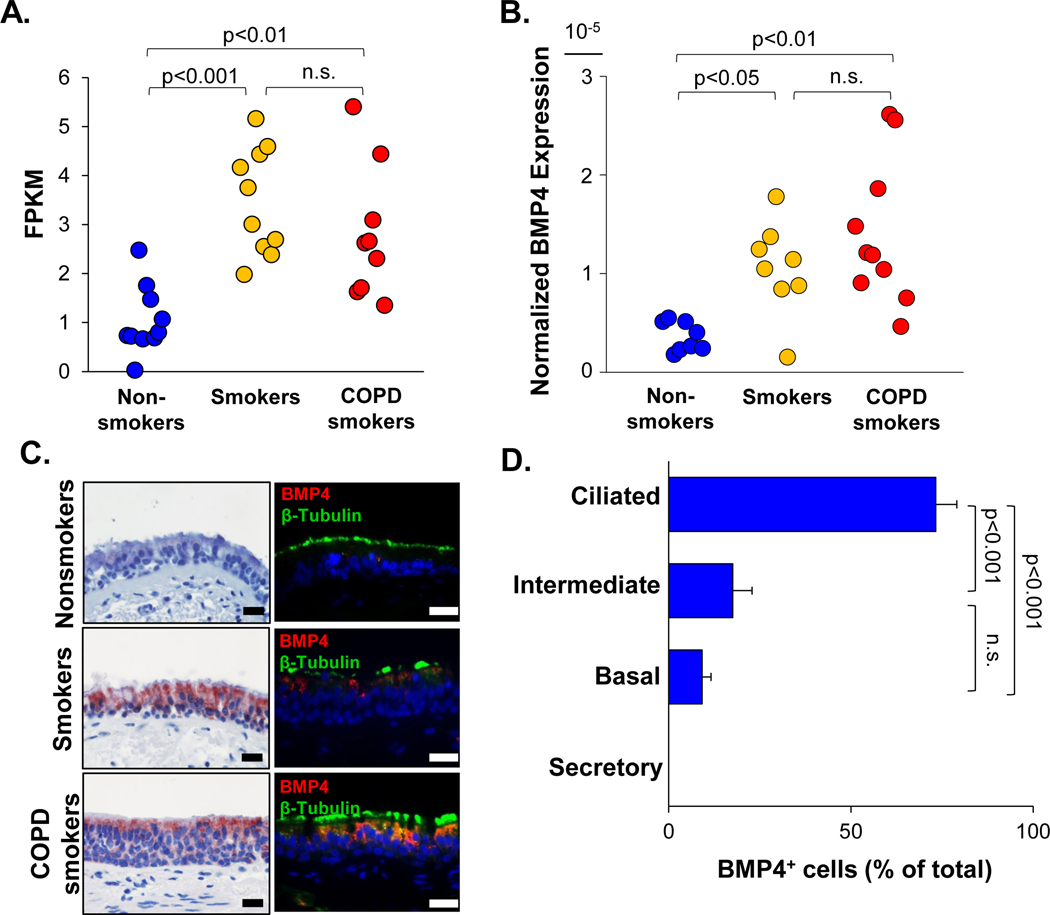
To determine whether cigarette smoking was associated with abnormal BMP signaling in the airway epithelium, gene expression of BMPs and BMP antagonists in the large airway epithelium and BC from nonsmokers, asymptomatic and COPD smokers were quantified by RNA-sequencing. Expression of BMP5, BMP6 and BMP antagonists NOG, CHRD and CHRDL2 were very low in both airway epithelium and airway BC (average FPKM<0.2). BMP2 and BMP antagonists FST, FSTL3, and TWSG1 were highly expressed in the airway BC compared to the expression in the complete airway epithelium, and the expression did not change in the asymptomatic and COPD smokers (Supplemental Figure 1A–B, Supplemental Table I). BMP7 was expressed in nonsmokers and mildly increased in the asymptomatic and COPD smokers (Supplemental Figure 1A, Supplemental Table I). BMP4 gene expression was relatively low in nonsmokers, but significantly up-regulated in asymptomatic and COPD smokers in the airway epithelium. There was no significant difference between asymptomatic smokers and COPD smokers (Figure 1A, Supplemental Figure 2A, Supplemental Table I). The BMP4 up-regulation in the airway epithelium of asymptomatic and COPD smokers was further confirmed by TaqMan quantitative PCR (Figure 1B). Although there was no significant difference in the BMP4 expression of the airway BC from nonsmokers, asymptomatic and COPD smokers, a sub-group of the asymptomatic and COPD smokers had higher BMP4 expression than any of the nonsmokers (Supplemental Figure 2A). Comparing the airway epithelium and airway BC from asymptomatic smokers, the BMP4 expression was highly expressed in the total airway epithelium (Supplemental Figure 2A), suggesting that the up-regulated BMP4 is mainly expressed in the differentiated airway epithelial cells. The data was in agreement with the in vitro TaqMan data that BMP4 expression went up during BC differentiation in the ALI model (Supplemental Figure 2B).
Figure 1.
Up-regulation of BMP4 in the human airway epithelium of asymptomatic smokers and COPD smokers. A. RNA-seq (fragments per kilobase of transcript per million mapped reads, FPKM) assessment of BMP4 expression in human airway epithelium of nonsmokers (blue, n=10), asymptomatic smokers (yellow, n=10) and COPD smokers (red, n=9). B. TaqMan quantitative PCR normalized BMP4 gene expression in human airway epithelium of nonsmokers (blue, n=8), asymptomatic smokers (yellow, n=8) and COPD smokers (red, n=10). C. BMP4 protein expression in the human airway epithelium. Shown is BMP4 immunohistochemistry staining (left panel) and immunofluorescence co-localization (right panel) of BMP4 (red) and ciliated cell marker β-tubulin (green) in the human airway epithelium of nonsmokers (top), asymptomatic smokers (middle) and COPD smokers (bottom). Scale bar - 20 μm. Nuclei are stained with DAPI (blue). D. Distribution (% of total airway epithelial cells) of BMP4+ cells in ciliated, intermediate, basal and secretory cells based on immunohistochemistry staining on the asymptomatic smokers (n=6) and COPD (n=6) smokers (based on assessment of a total of 444 cells from 12 different samples). No secretory cells expressed BMP4. p values are indicated in the figure, n.s. - not significant.
Using a unique microarray dataset from human small airway epithelium (SAE) at multi-time points (month 0, 3, 6 and 12), we observed that BMP4 was up-regulated at all the time points in the COPD smokers when compared to the nonsmokers (Supplemental Figure 3). When the COPD smokers quit smoking after the initial bronchoscopy at month 0, their BMP4 expression in SAE dramatically decreased at month 3, and approached the normal level in nonsmokers at month 6 and 12. However, when the COPD smokers stopped smoking and re-started smoking after month 3, their BMP4 expression in SAE went up again (Supplemental Figure 3). In summary, in addition to RNA-seq data of large airway epithelium, the microarray data confirm that BMP4 expression in human small airway epithelium is also up-regulated in COPD smokers, and cigarette smoking is a “trigger” for the BMP4 expression in the airway epithelium.
To determine the expression and localization of the BMP4 protein in human airway epithelium, immunohistochemistry and immunofluorescence staining was carried out on the human airway epithelial biopsies. BMP4 protein was barely detected in the normal airway epithelium of nonsmokers, but markedly increased in the β-tubulin positive ciliated cells of the normal airway epithelium from asymptomatic and COPD smokers (Figure 1C, Supplemental Figure 4). In the abnormal airway epithelium with BC hyperplasia, mucous cell hyperplasia and squamous metaplasia from asymptomatic and COPD smokers, the BMP4 protein was up-regulated in the ciliated cells as well (Figure 3A, Supplemental Figure 4). Besides expression in the ciliated cells, BMP4 was also up-regulated in a subset of BC and intermediate cells from asymptomatic and COPD smokers, but not expressed in the secretory cells (Supplemental Figure 4), which was consistent with the RNA-seq data in the airway BC. The cellular distribution of BMP4+ cells from asymptomatic and COPD smokers, which included both the normal and abnormal morphologies of the airway epithelium, showed that BMP4 was predominately induced in the ciliated cells, as well a few intermediate and basal cells (Figure 1D). However, the BMP4 protein was not detected in the mesenchyme underneath the epithelial cells (Figure 1C, 3A, Supplemental Figure 4)
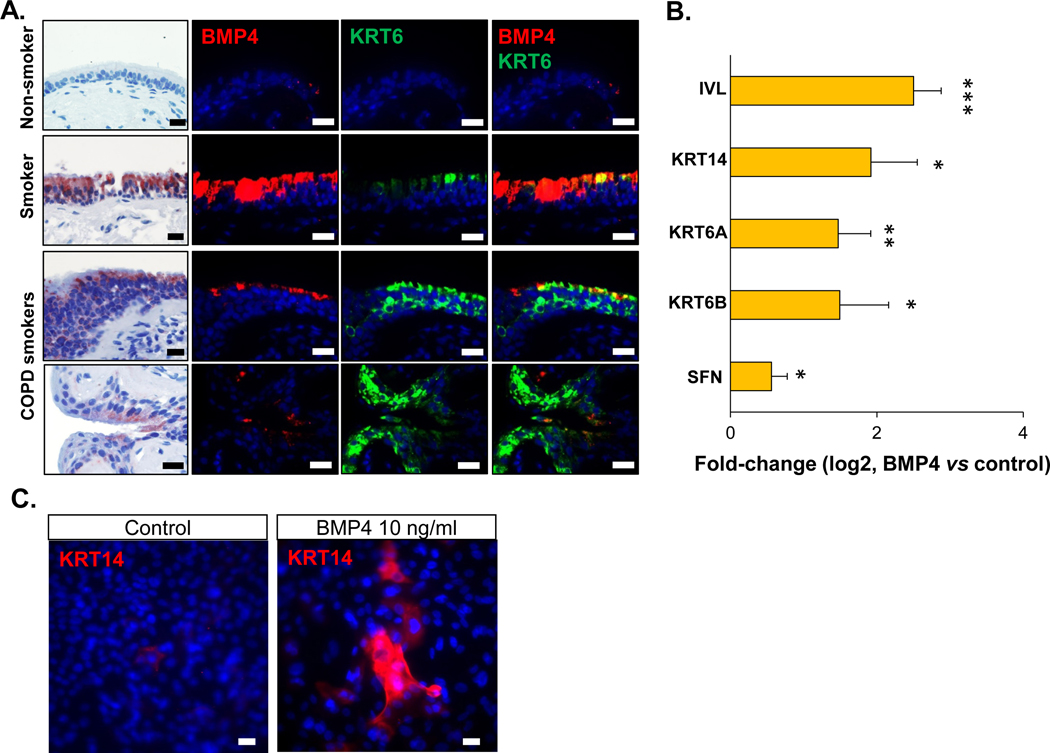
Figure 3.
BMP4 induction of differentiation toward squamous cells. A. From left to right: 1st column - representative BMP4 immunohistochemistry staining (positive cells are shown in red), 2nd column - immunofluorescence staining of BMP4 (red), 3rd column - immunofluorescence staining of squamous cell marker keratin 6 (KRT6, green), 4th column – overlap of 2nd and 3rd column. These staining were performed on the human airway epithelium of nonsmoker with normal morphology (1st row), asymptomatic smoker (2nd row) and COPD smokers (3rd and 4th rows) with abnormal morphology. Nuclei are stained with DAPI (blue). Scale bar - 20 μm. The biopsy images with immunohistochemistry and immunofluorescence staining in each row were from the same sample. B. TaqMan assessment of fold-change (log2) in the expression of squamous cell-related genes (IVL, KRT14, KRT6A, KRT6B and SFN) from the airway epithelium after 14 days ALI culture with BMP4 (10 ng/ml) stimulation from the basolateral side vs untreated control. * p<0.05, ** p<0.01, *** p<0.001, n=3 or 4. C. Immunofluorescence top staining of the squamous cell marker KRT14 (red) on the airway epithelium after 14 days ALI culture with BMP4 (10 ng/ml) stimulation from the basolateral side (right) vs untreated control (left). Nuclei are stained with DAPI (blue). Scale bar – 20 μm.
BMP4 Suppresses Normal BC Proliferation and Differentiation
BC are the stem/progenitor cells in human airways and are able to self-renew and differentiate into normal ciliated and secretory cells. To determine the effect of BMP4 on the BC self-renewal and proliferation, we applied BMP4 to the cultured normal nonsmoker BC and identified that BMP4 significantly decreased the BC number and suppressed the BC proliferation (Supplemental Figure 5).
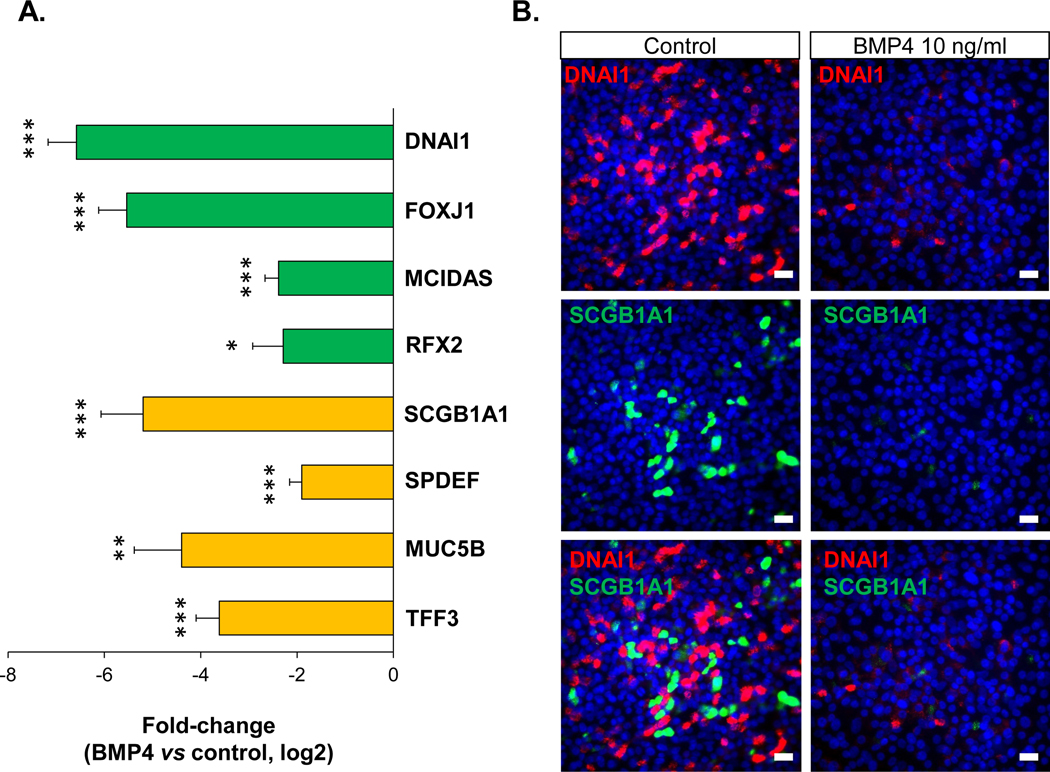
To study the effects of up-regulated BMP4 on the normal BC differentiation, we used ALI culture to assess human airway BC differentiation. The data demonstrated that BMP4 has a broad inhibitory effect on the normal BC differentiation into a mucociliary epithelium. Compared to the untreated control, the airway epithelium generated from the BMP4-treated BC had a significantly lower expression of ciliated cell-related genes, including dynein DNAI1, transcription factor FOXJ1, and the transcription factors MCIDAS and RFX2 that control early ciliogenesis (Figure 2A, Supplemental Figure 6A). Consistent with the gene expression data, the immunofluorescence staining of DNAI1 showed that BMP4 decreased the proportion of DNAI1+ ciliated cells that were derived from the BC in ALI culture (Figure 2B, Supplemental Figure 6B–C). Moreover, BMP4 significantly suppressed the cilia beating of the ciliated cells derived from healthy nonsmoker BC (Supplemental Video 1A, D).
Figure 2.
BMP4 suppression of normal airway BC differentiation. To mimic the effects of the up-regulated BMP4 observed in the airway epithelium in asymptomatic smokers and COPD smokers, BMP4 (10 ng/ml) was added to the basolateral side when established the ALI cultures, and expression of airway epithelial differentiation-related genes and protein were assessed. A. TaqMan assessment of fold-change (log2) in the mRNA expression of ciliated cell differentiation-related genes (FOXJ1, DNAI1, MCIDAS and RFX2) and secretory cell differentiation-related genes (SCGB1A1, SPDEF, MUC5B and TFF3) on the airway epithelium after 14 days ALI culture with BMP4 treatment vs untreated control. * p<0.05, ** p<0.01, *** p<0.001, n=3 or 4. B. Immunofluorescence top staining of ciliated cell marker DNAI1 (red) and secretory cell marker SCGB1A1 (green) on the airway epithelium after 28 days ALI culture with BMP4 (10 ng/ml) stimulation from the basolateral side (right) vs untreated control (left). Top – DNAI1 (red), middle – SCGB1A1 (green), bottom – overlapping of DNAI1 and SCGB1A1. Nuclei are stained with DAPI (blue). Scale bar – 20 μm.
Similarly, BMP4 had an inhibitory effect on the secretory cell differentiation. The secretory cell related genes SCGB1A1, SPDEF, MUC5B and TFF3 were significantly down-regulated by BMP4 stimulation (Figure 2A–B, Supplemental Figure 6A). Stimulation of the BC with different levels of BMP4 at various time points in ALI showed that the suppression effects of ciliated and secretory cell differentiation by BMP4 was sustained during differentiation in ALI culture in a concentration-dependent manner (Supplemental Figure 6A). TaqMan analysis also showed that the expression of the early differentiation markers for ciliated cells (MCIDAS, DNAH5, IFT172) and secretory cells (SPDEF, TFF3) were all suppressed (Supplemental Figure 6D) in the cultured BC by BMP4 stimulation. These data suggested that BMP4 begins to modulate the ciliated and secretory cell differentiation program in the BC at a very early stage.
BMP4 Promotes Squamous Cell Differentiation
As described above, the BMP4 up-regulation in asymptomatic smokers and COPD smokers was associated with abnormal morphology of airway epithelium, including squamous metaplasia (Figure 3A, Supplemental Figure 4). To further confirm this observation, immunofluorescence co-staining of BMP4 with intermediate/squamous cell marker KRT6 was assessed in the airway epithelium from nonsmokers, asymptomatic and COPD smokers. In the nonsmoker samples with normal morphology, BMP4 and KRT6 expression were very low. However, in the asymptomatic and COPD smokers’ samples with normal and squamous metaplasia morphology, BMP4 and KRT6 expression were both markedly increased, and a small subset of BMP4+ cells expressed KRT6 (Figure 3A). Based on the staining data that BMP4 up-regulation appeared to be related to squamous metaplasia, we hypothesized that BMP4 shifted the normal BC differentiation to the abnormal squamous cells. To assess this hypothesis, we stimulated the human airway BC with BMP4 in both ALI differentiation culture and BC culture models. The TaqMan data showed that BMP4 could induce up-regulation of a series of squamous cell markers, including IVL, KRT14, KRT6A and KRT6B, in both models (Figure 3B, Supplemental Figure7A, E). BMP4 induced up-regulation of SFN in the ALI, but not in BC culture (Figure 3B, Supplemental Figure 7E). The TaqMan data were strengthened by the immunofluorescence staining of squamous cell marker on the airway epithelium derived from BC with or without BMP4 stimulation. In the airway epithelium derived from BMP4 treated BC, more cells were positive for squamous cell markers KRT14 and IVL, while in the control groups, the % of IVL+ and KRT14+ cells were significantly lower (Figure 3C, Supplemental Figure 7B–D). BMP4 not only suppressed ciliated and secretory cell differentiation, but also induced squamous cell differentiation in a concentration-dependent manner (Supplemental Figure 7A).
Effects of BMP4 on the Fully Differentiated Airway Epithelium
In addition, stimulation the fully differentiated airway epithelium with BMP4 from the basolateral side also suppressed the expression of ciliated cell (FOXJ1 and MCIDAS) and secretory cell (SCGB1A1, SPDEF, MUC5B and TFF3) markers, and induced the up-regulation of squamous cell markers (IVL, KRT14 and KRT6A; Supplemental Figure 8). However, apical application of BMP4 had no effects on the expression of ciliated cell (FOXJ1), secretory cell (SCGB1A1, MUC5B and TFF3), squamous cell (IVL and KRT14) markers, and mildly suppressed the expression of ciliated cell marker DNAI1 (Supplemental Figure 9).
BMP Type I Receptor Enriched in Airway BC Mediates BMP4 Induced Epithelial Remodeling
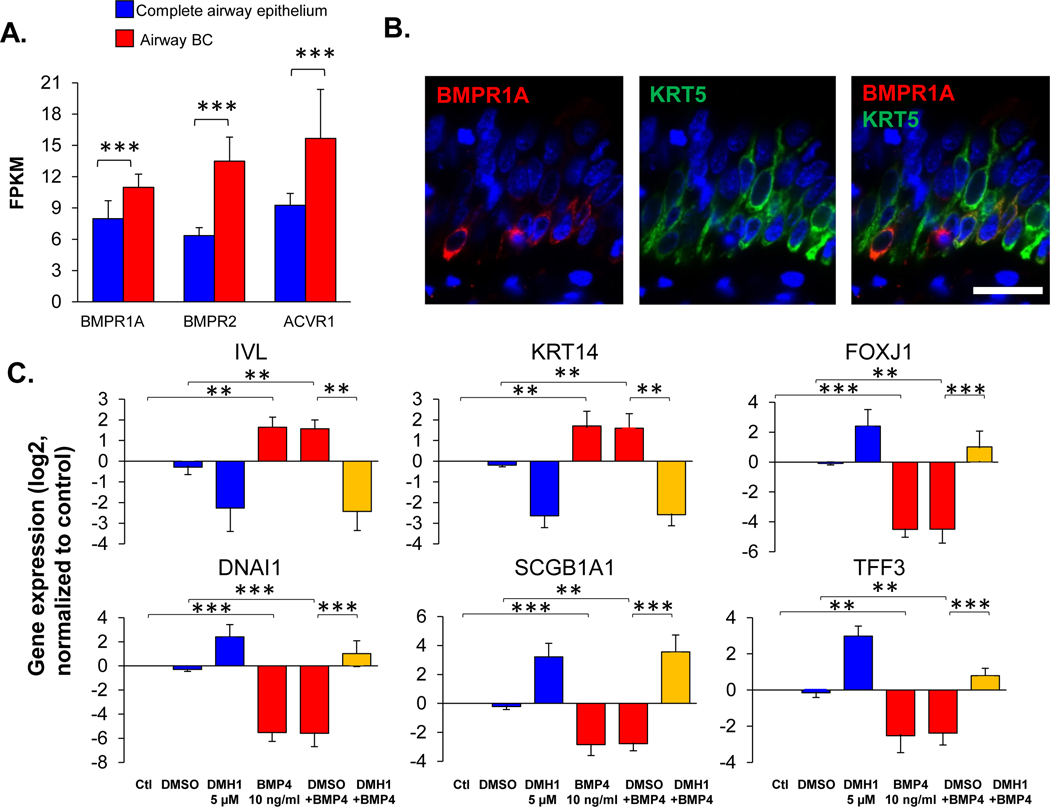
BMP4 signals through two receptor families: BMP receptors (BMPR1A, BMPR1B, and BMPR2) and activin receptors (ACVR1, ACVR2A, and ACVR2B) [23]. Using the RNA-sequencing data, we examined the expression of BMP4 receptors in total human airway epithelium and BC samples. The data demonstrated that the BMPR1A expression slightly decreased, and BMPR1B expression increased in the airway epithelium from asymptomatic and COPD smokers, but not in the airway BC. Expression of BMPR1B, ACVR2A and ACVR2B were higher in the airway epithelium compared to the expression in BC (Supplemental Figure 1C, Supplemental Table II). In contrast, BMPR1A, BMPR2 and ACVR1 gene expression was higher in the BC than in the complete airway epithelium, suggesting that BMP4 may regulate BC function through these receptors enriched in BC. The same pattern of BMP4 receptor enrichment in BC was observed in nonsmokers, asymptomatic and COPD smokers, respectively (Figure 4A, Supplemental Figure 1C, and Supplemental Table II). Immunofluorescence staining further confirmed that BMPR1A protein was mainly expressed in a subset of KRT5 positive BC (Figure 4B).
Figure 4.
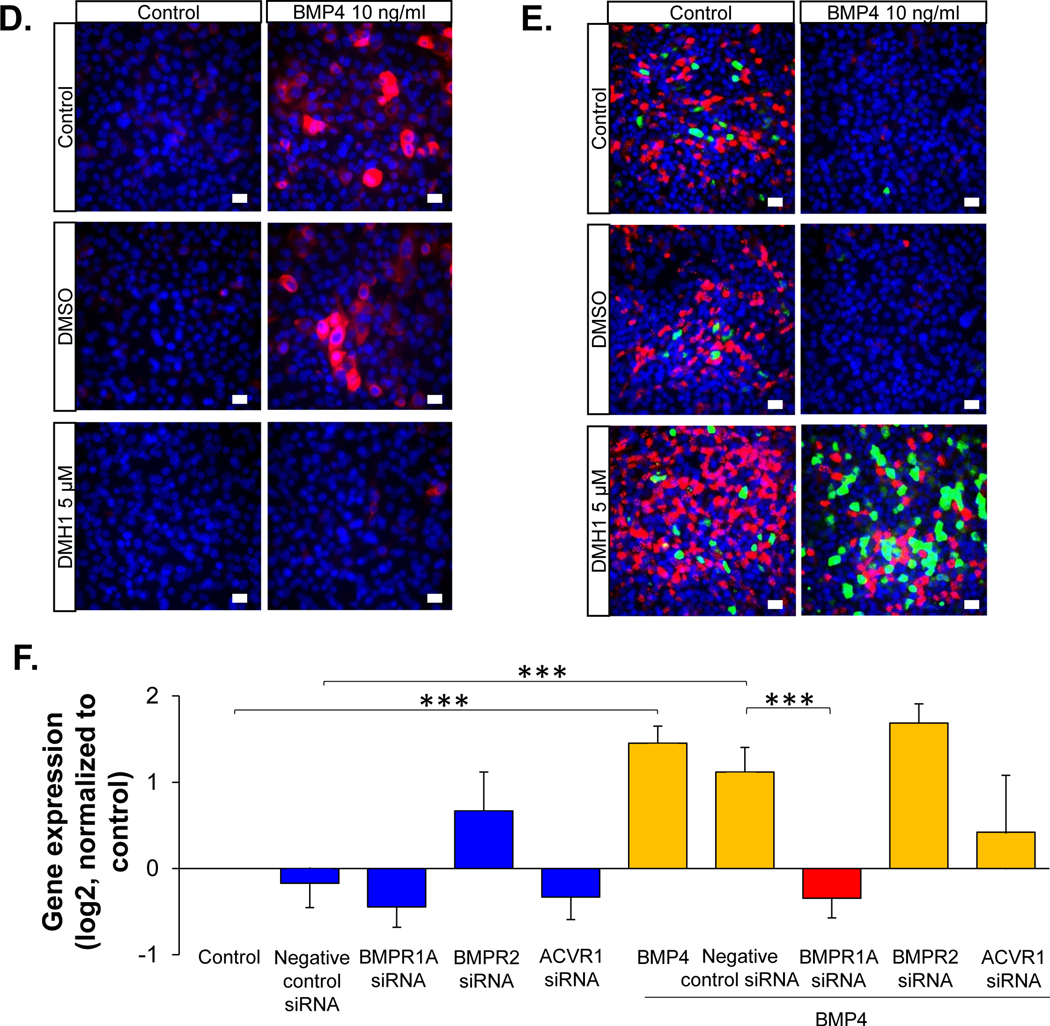
BMP receptor-mediated BMP4 induced airway epithelial remodeling. A. RNA-seq assessment of the BMP4 receptors (BMPR1A, BMPR2 and ACVR1) in the human airway epithelium (n=29, blue) and airway BC (n=42, red). Detailed expression the BMP4 receptors for each individuals with different phenotypes are shown in Supplemental Figure 1C and Supplemental Table II. B. Representative immunofluorescence co-localization of BMPR1A (red) and the basal cell marker keratin 5 (KRT5, green) in the normal human airway epithelium. Scale bar - 20 μm. Nuclei are stained with DAPI (blue). C. TaqMan analysis of the fold-change (log2) of the expression of various genes related to airway BC differentiation (IVL, KRT14 – squamous cells; FOXJ1, DNAI1 – ciliated cells; SCGB1A1, TFF3 – secretory cells). The gene symbols are shown on top of the bar plots. Data were generated from the airway epithelium in ALI culture (day 14) with BMP4 (10 ng/ml) stimulation from the basolateral side vs untreated control in the absence or presence of BMP type I receptor inhibitor DMH1 (5 μM). DMSO was used as the negative control, n=3. D. Representative immunofluorescence top staining of squamous cell marker KRT14 (red) on the airway epithelium after 14 days ALI culture. E. Representative immunofluorescence top staining of ciliated cell marker DNAI1 (red) and secretory cell marker SCGB1A1 (green) on the airway epithelium after 28 days ALI culture. In D.-E., left column – untreated control, right column – BMP4 stimulation. Top row - untreated control, middle row – negative control DMSO, bottom row – DMH1 (5 μM). Nuclei are stained with DAPI (blue). Scale bar – 20 μm. F. TaqMan assessment of the squamous cell marker IVL in BC with BMP4 (10 ng/ml) stimulation vs untreated control for 48 hr in the presence of siRNA silencing of BMPR1A, BMPR2 or ACVR1. The data were normalized to the untreated control (log2), n=3 or 4. ** p<0.01, *** p<0.001.
To assess whether BMP receptors mediate BMP4 induced airway epithelial remodeling, DMH1, a selective inhibitor of BMP type I receptors, was applied to the ALI culture while stimulating the BC with BMP4. The data showed that DMH1 reversed the BMP4 induced up-regulation of squamous cell markers (IVL, KRT14), down-regulation of ciliated (FOXJ1, DNAI1) and secretory cell (SCGB1A1, TFF3) markers (Figure 4C). Immunofluorescence staining of the airway epithelium with squamous cell markers KRT14, ciliated cell marker DNAI1 and secretory cell marker SCGB1A1 validated that DMH1 could block BMP4 induced airway epithelial remodeling (Figure 4D–E). Furthermore, DMH1 reversed the BMP4 suppressed cilia cell beating as well (Supplemental Video 1).
To further evaluate the specific receptor that mediates the BMP4-induced abnormal BC differentiation, BMPR1A, BMPR2 and ACVR1 siRNAs were applied to the cultured BC with BMP4 treatment vs control. BMPR2 siRNA had no effect on the cultured BC, but BMPR1A siRNA significantly decreased the BMP4 induced up-regulation of the squamous cell marker IVL (Figure 4F). Although not significant, ACVR1 siRNA tended to suppress the BMP4 induced up-regulation of IVL. Additional evidence in the ALI differentiation culture confirmed that silencing of BMPR1A could reverse BMP4 induced squamous metaplasia (Supplemental Figure 10A), and the suppression of ciliated cell differentiation (Supplemental Figure 10B). Efficiency of all the siRNAs were validated by the TaqMan analysis of the receptors expression in the cultured BC and airway epithelium in ALI (Supplemental Figure 11A–D).
Smad Signaling Mediates BMP4 Induced Squamous Metaplasia
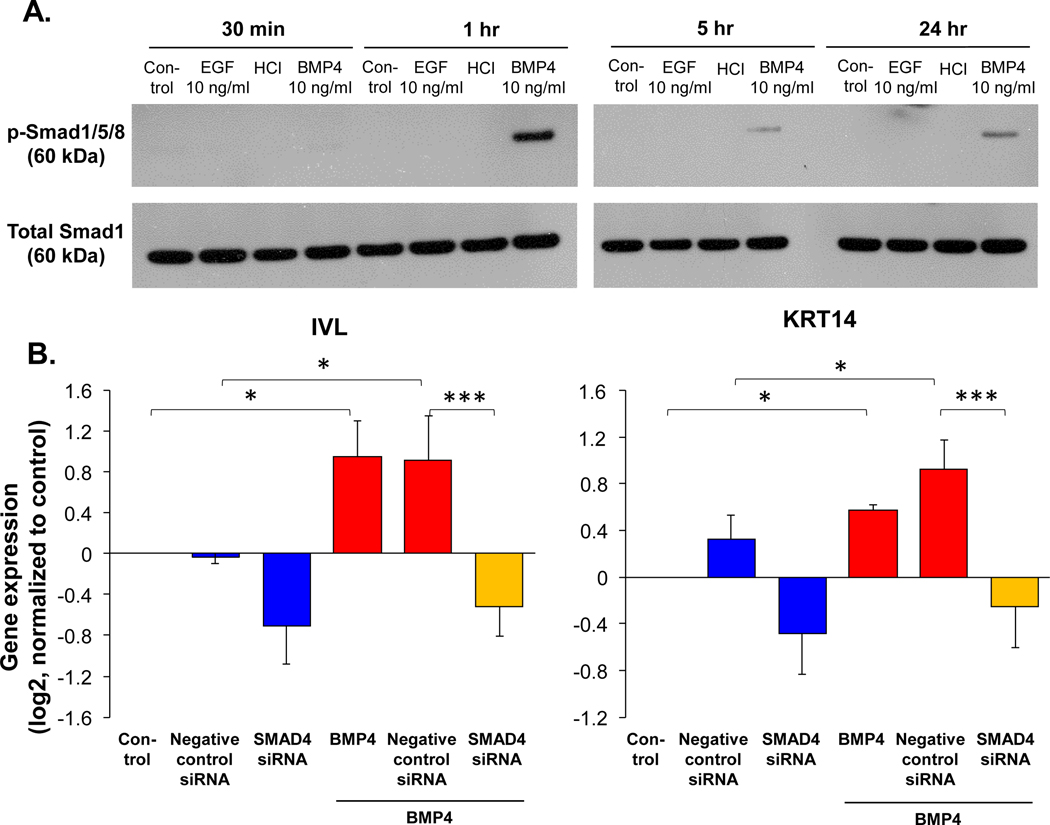
To assess whether the BMP4 downstream Smad signaling was activated by BMP4 on human airway BC, Western blot analysis of p-Smad1/5/8 was performed on the BC in ALI culture. BMP4 activated Smad1/5/8 phosphorylation at 1 hr and the activation continued but with weaker signaling at 5 and 24 hour (Figure 5A). To evaluate whether Smad signaling mediated BMP4 induced squamous metaplasia, Smad4, which translocates the p-Smad1/5/8 to the nucleus, was silenced by siRNA in cultured BC (Supplemental Figure 12). TaqMan analysis showed that BMP4 induced expression of squamous cell markers (IVL and KRT14) were both decreased (Figure 5B), suggesting that Smad signaling mediated BMP4 induced human airway squamous metaplasia.
Figure 5.
Smad signaling mediated BMP4-induced squamous cell differentiation. A. Western blot analysis shows the phosphorylated Smad 1/5/8 (upper) and total Smad 1 (lower) protein expression of the airway BC in ALI culture (day 0) with or without BMP4 (10 ng/ml) stimulation for 30 min, 1, 5 and 24 hours. BMP4 solvent HCl and EGF (10 ng/ml; EGF does not activate BMP4 downstream Smad signaling [23] were used as negative controls. B. TaqMan assessment of the squamous cell markers IVL (right) and KRT14 (left) in BC with BMP4 (10 ng/ml) stimulation vs untreated control for 48 hr while silencing Smad4 by siRNA. Negative control siRNA was used as negative control, and the data were normalized to the untreated control (log2), n=4. * p<0.05, *** p<0.001.
Inhibition of BMP4 Signaling Reverses Cigarette Smoke Extract (CSE) and EGF-induced Abnormal Phenotypes
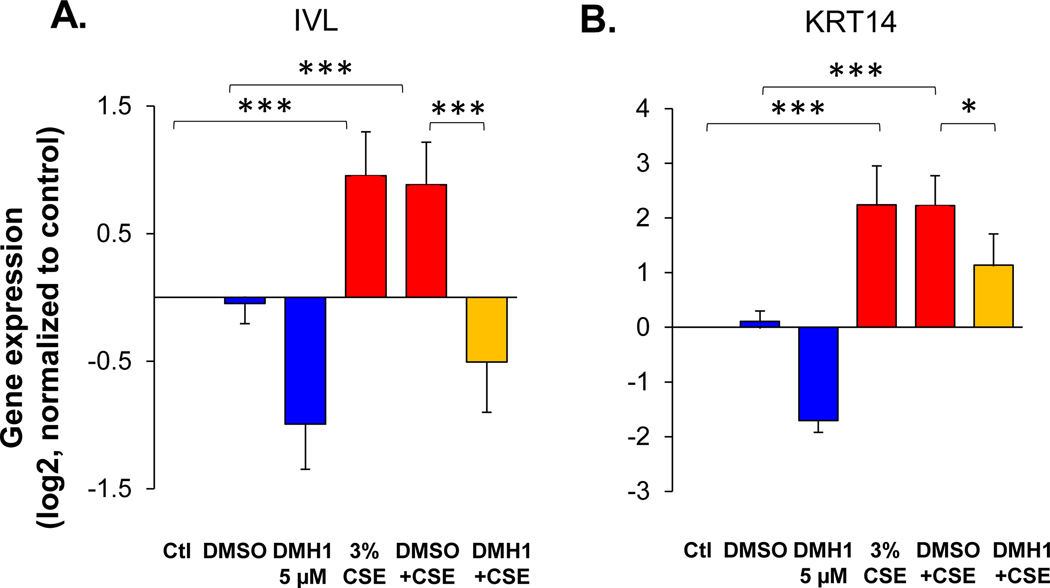
CSE is known to alter BC differentiation to suppress the generation of mucociliary epithelium and induce squamous cell differentiation [12] (Figure 6, Supplemental Figure 13). To assess whether BMP4 signaling was involved in the CSE induced airway epithelial remodeling, BMP type I receptor inhibitor DMH1 was applied to the BC differentiation culture in ALI when treated by CSE. The gene expression data showed that the DMH1 significantly reversed the CSE-induced up-regulation of the squamous cell markers IVL (Figure 6A) and KRT14 (Figure 6B), and down-regulation of ciliated cell markers (FOXJ1 and DNAI1; Supplemental Figure 13a) and secretory cell markers (SCGB1A1 and TFF3; Supplemental Figure 13B).
Figure 6.
DMH1 suppresses cigarette smoke extract (CSE)-induced squamous cell differentiation. TaqMan assessment of the fold-change (log2) in the gene expression of the squamous cell markers (A.) IVL and (B.) KRT14 in the airway epithelium derived from BC after 14 days culture in ALI culture. In the CSE-treated groups, the BC were exposed to 3% CSE stimulation from the basolateral side vs untreated control in the absent or present of BMP4 type I receptor inhibitor DMH1 (5 μM). DMSO was used as negative control, n=4. * p<0.05, *** p<0.001.
Like CSE, we previously identified that EGF signaling plays a role in reprogramming the BC differentiation to the smoking-relevant phenotypes [5, 18]. Here, we found that inhibition of BMP4 signaling by DMH1 could restore the EGF-induced abnormal phenotypes in the BC differentiation process, including down-regulation of ciliated (FOXJ1 and DNAI1) and secretory cell (SCGB1A1) markers, and up-regulation of squamous cell markers (IVL and KRT14; Supplemental Figure 14A). However, BMP4 had no effect on the expression of amphiregulin (AREG; an EGFR ligand), the up-regulation of which is associated with BC and mucous cell hyperplasia (Supplemental Figure 14B).
Discussion
COPD is a cigarette smoking-related chronic lung disease characterized by long-term reduced airflow with a chronic cough, increased sputum production and shortness of breath [24]. The early pathogenesis of COPD is associated with dramatic architectural changes of the airway epithelium, including basal and intermediate cell hyperplasia, decreased number of ciliated cells, shortening cilia length, mucus over-production, goblet cell hyperplasia, and squamous metaplasia [5–13]. The present study identifies a novel mechanism that regulates the airway epithelial remodeling in asymptomatic smokers and COPD smokers based on the observation that expression of BMP4 is up-regulated in the differentiated airway epithelium of asymptomatic and COPD smokers. The up-regulated BMP4 interacts with BC, the stem/progenitor cells in human airway, to suppress the normal mucociliary differentiation and induce the squamous metaplasia, processes directly relevant to the pathogenesis of COPD (Supplemental Figure 17).
BMP4 Expression in the Airway Epithelium
BMP4, a ligand classified with the TGF-beta superfamily, is expressed in many tissues and plays a critical role in embryogenesis, embryonic development, and homeostasis of adult organs [23]. In the developing embryonic mouse lung, BMP4 is expressed in the distal endoderm [25] and dynamics of BMP4 expression in endoderm during the lung development determines lung bud morphogenesis [20] and epithelial proximal-distal patterning [21]. In the adult mouse airway, BMP4 is highly expressed in fibroblast-like cells in the mesenchyme and weakly expressed in some luminal epithelial cells in the steady state. During the injury, BMP4 expression decreases in the mesenchyme and disappears in the epithelial cells [26]. In addition, BMP4 has been reported to be expressed in mouse lung endothelial cells, suppressed by naphthalene injury and induced by bleomycin injury [27].
There is limited information available on the BMP4 expression in the human airway epithelium. Consistent with our data, the BMP4 mRNA is detected in the primary cultured human bronchial epithelial cells, and the expression of BMP4 is increased in the ALI culture [28], suggesting BMP4 may be involved in the mucociliary differentiation. In the present study, taking advantage of the RNA-sequencing of bronchoscopy brushing epithelial cells and immunostainings of the human airway epithelial biopsies from nonsmokers, asymptomatic and COPD smokers, we found that the BMP4 expression is very low in the normal airway epithelium. In contrast, in the asymptomatic and COPD smokers, BMP4 expression was significantly up-regulated, mostly in the ciliated and intermediate cells, as well as in a subset of BC. Unlike the BMP4 expression in the mouse airway, BMP4 expression in asymptomatic healthy smokers and COPD smokers was not detected in mesenchyme and epithelial secretory cells in the human airway. Importantly, BMP4+ cells were not only observed in smokers’ normal airway epithelium, but were markedly increased is the abnormal airway epithelium in asymptomatic and COPD smokers that also had abundant KRT6 expression. The expression correlation between BMP4 and KRT6, which has been related to squamous differentiation [5, 18, 29], suggests that BMP4 is relevant to the generation of the remodeled airway epithelium. In contrast to the up-regulation of BMP4, the expression of other BMPs (BMP2, BMP5 and BMP6), BMP antagonists (NOG, FST, FSTL3, CHRD, CHRDL2 and TWIG2) and the BMP4 receptors (BMPR2, ACVR1 and ACVR2A) did not change in the airway epithelium obtained from asymptomatic and COPD smokers. BMP7, BMPR1B expression were increased and BMPR1A expression decreased in asymptomatic and COPD smokers, respectively, but with less dramatic change. Together, these data suggest that BMP4 is the main component in the BMP pathway that induces abnormal airway epithelium in asymptomatic smokers and COPD smokers. However, the mechanism of how cigarette smoking enhances the BMP4 expression is unknown. It is reported that the hedgehog signaling is evoked by chronic cigarette smoke exposure in human airway epithelial cells [30] and hedgehog signaling induces the expression of BMP4 [31]. If so, the exaggerated BMP4 in smokers may be the result of the activation of hedgehog signaling in the epithelial cells. BC are stem/progenitor cells capable of differentiating into mucociliary epithelium, and the BC from COPD smokers are quite different with the BC from nonsmokers [32]. These observations suggest another hypothesis that the enhanced BMP4 in the differentiated airway epithelium are due to the altered differentiation of BC in COPD smokers.
Exaggerated BMP4 in Airway Epithelium Induces Cigarette Smoking-relevant Phenotypes
In the mouse, conditional knockout BMP4 in the anterior foregut causes the loss of the trachea [33]. During morphogenesis of the mouse lung, BMP4 in endoderm cooperates with FGF10 in the distal mesenchyme to regulate the branching morphogenesis [20]. BMP4 also controls the endodermal proximal-distal patterning during the lung development [21]. In the resting adult mouse airway epithelium, BMP4 inhibits BC proliferation, while following an injury, BMP4 signaling is suppressed, allowing airway epithelial proliferation and repair [26].
In humans, BMP4 has been shown to activate EMT response in the airway epithelial cells [22, 34]. BMP4 is also crucial for human pluripotent stem cell differentiation to lung cells [35, 36]. However the effects of BMP4 to human BC are still unknown. BC are the stem/progenitor cells in human airway epithelium, and BC from COPD smokers fail to generate the normal airway epithelium [37] suggesting human airway BC play a central role in the pathogenesis of smoking-related lung diseases. Based on (1) cigarette smoking reduces the junctional barrier integrity of the airway epithelium, partially mediated by the EGF-EGFR signaling [5, 17], to allow the particles/proteins to go across the barrier junctions of the airway epithelium, (2) our observation that BMP4 is expressed abundantly in the airway epithelium of smokers, we hypothesized that up-regulated BMP4 passes through the damaged barrier junctions of the airway epithelium, and interacts with BC to switch the normal BC self-renewal and differentiation toward smoking relevant abnormal airway epithelium. From our data, the BC exposed to BMP4 have decreased proliferation rate and were not able to differentiate into the normal mucociliary epithelium and reprogrammed to generate squamous cells. Interestingly, the expression of SPDEF, a transcription factor that regulates goblet and club cell differentiation [38, 39], was also suppressed by BMP4. These data are in agreement with the reduction of club cells in COPD smokers [40], while opposite to the expected phenotypes of goblet cell hyperplasia in smokers [7, 10], suggesting a complicated mechanism that regulate the smoking-related phenotypes in human airway epithelium.
BMP Signaling Pathway Reprogramming of Human Airway Epithelial Differentiation
BMP4 signals through canonical Smad dependent and other non-canonical signaling pathways [23]. In the canonical signaling pathway, BMP4 binds to the receptors, including type I and II receptors to initiate the transduction cascade. Activation of the receptor by BMP4 phosphorylates the intracellular Smad1/5/8 [23]. With the help of Smad4, the phosphorylated SMAD1/5/8 trans-locates to the nucleus to regulate the gene expression. Non-canonical pathways, like MAPK, PI3K and p38, can also be activated by BMP4 [41–43]. BMPR1A, one of the BMP type I receptors, is critical for the mouse lung development [44]. Deletion of BMPR1A decreases epithelial proliferation in the distal embryonic lung and leads to apoptosis and abnormal morphogenesis in mouse lung [44]. We identified that BMPR1A serves as the major receptor to mediate BMP4-induced smoking-relevant abnormal phenotypes, and its downstream Smad signaling mediates squamous cell differentiation as well. As BMPR1A is only expressed in a subset of BC in vivo, we consider the BMP4-induced smoking-relevant phenotypes (decreased mucociliary epithelium and increased squamous metaplasia) would reside in a “local” area of the airway epithelium. The “local changes” of the airway epithelium mediated by BMP4 may represent an early pathogenesis of smoking-related diseases, including COPD.
We previously reported that CSE and EGF/amphiregulin-EGFR signaling altered the basal cell differentiation program toward the smoking-related lesions [5, 12, 18], which is similar to the phenotype induced by BMP4. Interesting, our data showed that blocking the BMP receptor reversed the CSE/EGF-induced smoking-related lesions, suggesting that CSE/EGF and BMP4 might share some common signaling pathways in the BC. Map kinase (MAPK) signaling might be a candidate, as it is the downstream signaling pathway for EGF and non-canonical downstream signaling for BMP4 [45]. However, BMP4 did not change the expression of amphiregulin, suggesting that BMP4 signaling is not entirely overlapping with EGF-amphiregulin signaling.
In summary, our study identified a novel BMP4 dependent mechanism related to abnormal airway epithelial reprogramming associated with smoking and COPD. Modulation of BMP4 or the downstream receptors and signaling decades in human airway BC may be a potential therapeutic approach to cure or prevent the pathogenesis of smoking induced airway disease.
Supplementary Material
Acknowledgements:
We thank R. Shaykhiev and I. Chao for the help in experimental design and technical assistance; and Nahla Mohamed for editorial help. These studies were supported, in part, by HL107882, HL107882-S, HL1189541, and UL1 TR000457. The biologic samples in the study were obtained from a total of 100 subjects; 2 of these subjects were also enrolled in the bronchoscopy subset of SPIROMICS.
The authors thank the SPIROMICS participants and participating physicians, investigators and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E Alexis, PhD; Wayne H Anderson, PhD; Igor Barjaktarevic, MD, PhD; R Graham Barr, MD, DrPH; Eugene R Bleecker, MD; Richard C Boucher, MD; Russell P Bowler, MD, PhD; Elizabeth E Carretta, MPH; Stephanie A Christenson, MD; Alejandro P Comellas, MD; Christopher B Cooper, MD, PhD; David J Couper, PhD; Gerard J Criner, MD; Ronald G Crystal, MD; Jeffrey L Curtis, MD; Claire M Doerschuk, MD; Mark T Dransfield, MD; Christine M Freeman, PhD; MeiLan K Han, MD, MS; Nadia N Hansel, MD, MPH; Annette T Hastie, PhD; Eric A Hoffman, PhD; Robert J Kaner, MD; Richard E Kanner, MD; Eric C Kleerup, MD; Jerry A Krishnan, MD, PhD; Lisa M LaVange, PhD; Stephen C Lazarus, MD; Fernando J Martinez, MD, MS; Deborah A Meyers, PhD; Wendy C Moore, MD; John D Newell Jr, MD; Laura Paulin, MD, MHS; Stephen Peters, MD, PhD; Cheryl Pirozzi, MD; Elizabeth C Oelsner, MD, MPH; Wanda K O’Neal, PhD; Victor E Ortega, MD, PhD; Robert Paine, III, MD; Nirupama Putcha, MD, MHS; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P Tashkin, MD;; J Michael Wells, MD; Robert A Wise, MD; and Prescott G Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Thomas Croxton, PhD, MD. SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc..; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; and Sunovion.
References
- 1.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc 2008: 5(7): 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 2010: 3(9–10): 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol 1994: 10(6): 613–624. [DOI] [PubMed] [Google Scholar]
- 4.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011: 8(6): 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, Brekman A, Crystal RG. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci U S A 2013: 110(29): 12102–12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auerbach O, Forman JB, Gere JB, Kassouny DY, Muehsam GE, Petrick TG, Smolin HJ, Stout AP. Changes in the bronchial epithelium in relation to smoking and cancer of the lung; a report of progress. NEnglJMed 1957: 256(3): 97–104. [DOI] [PubMed] [Google Scholar]
- 7.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978: 298(23): 1277–1281. [DOI] [PubMed] [Google Scholar]
- 8.Hessel J, Heldrich J, Fuller J, Staudt MR, Radisch S, Hollmann C, Harvey BG, Kaner RJ, Salit J, Yee-Levin J, Sridhar S, Pillai S, Hilton H, Wolff G, Bitter H, Visvanathan S, Fine J, Stevenson CS, Crystal RG, Tilley AE. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS One 2014: 9(1): e85453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004: 350(26): 2645–2653. [DOI] [PubMed] [Google Scholar]
- 10.Hogg JC, Pare PD, Hackett TL. The Contribution of Small Airway Obstruction to the Pathogenesis of Chronic Obstructive Pulmonary Disease. Physiol Rev 2017: 97(2): 529–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS One 2009: 4(12): e8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brekman A, Walters MS, Tilley AE, Crystal RG. FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro. Am J Respir Cell Mol Biol 2014: 51(5): 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol 2015: 77: 379–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crystal RG. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014: 190(12): 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc 2014: 11 Suppl 5: S252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan DM, Vincent TL, Salit J, Walters MS, Agosto-Perez F, Shaykhiev R, Strulovici-Barel Y, Downey RJ, Buro-Auriemma LJ, Staudt MR, Hackett NR, Mezey JG, Crystal RG. Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PLoS One 2014: 9(2): e88051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, Strulovici-Barel Y, Salit J, Harvey BG, Crystal RG. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci 2011: 68(5): 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo WL, Yang J, Gomi K, Chao I, Crystal RG, Shaykhiev R. EGF-Amphiregulin Interplay in Airway Stem/Progenitor Cells Links the Pathogenesis of Smoking-Induced Lesions in the Human Airway Epithelium. Stem Cells 2017: 35(3): 824–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 1995: 9(17): 2105–2116. [DOI] [PubMed] [Google Scholar]
- 20.Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 2000: 127(12): 2695–2704. [DOI] [PubMed] [Google Scholar]
- 21.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 1999: 126(18): 4005–4015. [DOI] [PubMed] [Google Scholar]
- 22.Molloy EL, Adams A, Moore JB, Masterson JC, Madrigal-Estebas L, Mahon BP, O’Dea S. BMP4 induces an epithelial-mesenchymal transition-like response in adult airway epithelial cells. Growth Factors 2008: 26(1): 12–22. [DOI] [PubMed] [Google Scholar]
- 23.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal 2011: 23(4): 609–620. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004: 364(9434): 613–620. [DOI] [PubMed] [Google Scholar]
- 25.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 1996: 122(6): 1693–1702. [DOI] [PubMed] [Google Scholar]
- 26.Tadokoro T, Gao X, Hong CC, Hotten D, Hogan BL. BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development 2016: 143(5): 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 2014: 156(3): 440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross AJ, Dailey LA, Brighton LE, Devlin RB. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol 2007: 37(2): 169–185. [DOI] [PubMed] [Google Scholar]
- 29.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, Barzcak A, Xiao Y, Erle DJ, Nishimura SL. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest 2007: 117(11): 3551–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemjabbar-Alaoui H, Dasari V, Sidhu SS, Mengistab A, Finkbeiner W, Gallup M, Basbaum C. Wnt and Hedgehog are critical mediators of cigarette smoke-induced lung cancer. PLoS One 2006: 1: e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang DH, Tiwari A, Kim ME, Clemons NJ, Regmi NL, Hodges WA, Berman DM, Montgomery EA, Watkins DN, Zhang X, Zhang Q, Jie C, Spechler SJ, Souza RF. Hedgehog signaling regulates FOXA2 in esophageal embryogenesis and Barrett’s metaplasia. J Clin Invest 2014: 124(9): 3767–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh M, Miller YE, Nakachi I, Kwon JB, Baron AE, Brantley AE, Merrick DT, Franklin WA, Keith RL, Vandivier RW. Exhaustion of Airway Basal Progenitor Cells in Early and Established Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2018: 197(7): 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol 2008: 322(1): 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormack N, O’Dea S. Regulation of epithelial to mesenchymal transition by bone morphogenetic proteins. Cell Signal 2013: 25(12): 2856–2862. [DOI] [PubMed] [Google Scholar]
- 35.Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 2014: 32(1): 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 2012: 30(9): 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staudt MR, Buro-Auriemma LJ, Walters MS, Salit J, Vincent T, Shaykhiev R, Mezey JG, Tilley AE, Kaner RJ, Ho MW, Crystal RG. Airway Basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014: 190(8): 955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009: 119(10): 2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest 2015: 125(5): 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lumsden AB, McLean A, Lamb D. Goblet and Clara cells of human distal airways: evidence for smoking induced changes in their numbers. Thorax 1984: 39(11): 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003: 425(6958): 577–584. [DOI] [PubMed] [Google Scholar]
- 42.Lee MY, Lim HW, Lee SH, Han HJ. Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells 2009: 27(8): 1858–1868. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 1995: 270(5244): 2008–2011. [DOI] [PubMed] [Google Scholar]
- 44.Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol 2006: 291(1): 67–82. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q, Heinke J, Vargas A, Winnik S, Krauss T, Bode C, Patterson C, Moser M. ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res 2007: 76(3): 390–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.