SUMMARY
Objective
The goal of the study was to determine whether the level of OTOLIN-1, a protein whose expression is highly restricted to the inner ear,is increased in the body fluids of patients with inner ear disorders in comparison to healthy subjects.
Methods
In the preliminary part of the study, OTOLIN-1 levels were measured in the serum, urine, and saliva of patients with an acute onset of Ménière´s disease and in healthy individuals. Subsequently, only serum OTOLIN-1 levels were taken into account and were compared between patients with acute onset of Ménière´s disease, sudden hearing loss, vestibular neuritis and healthy subjects.
Results
The most reliable diagnostic parameter was OTOLIN-1 levels in serum. Serum samples of patients with Ménière’s disease and sudden hearing loss showed significantly higher OTOLIN-1 levels than those from healthy individuals. In addition, there was no significant difference between the serum concentration of OTOLIN-1 in patients with vestibular neuritis and the control group.
Conclusions
Serum levels of OTOLIN-1 can potentially be used as a biomarker for acute onset of inner ear disorders due to its significant increase in patients with acute Meniere´s disease and sudden hearing loss in comparison to healthy individuals.
KEY WORDS: Ménière disease, OTOLIN-1, sudden hearing loss, biomarker, inner ear
RIASSUNTO
Obiettivo
Scopo principale dello studio è stato determinare se il livello di OTOLIN-1, una proteina la cui espressione è altamente ristretta all’orecchio interno, aumenti nei fluidi corporei di pazienti con diversi disturbi dell’orecchio interno, rispetto a soggetti sani.
Metodi
Nella parte preliminare dello studio, la concentrazione di OTOLIN-1 è stata misurata nel siero, urina e saliva di pazienti con esordio acuto della malattia di Ménière e di soggetti sani. In seguito, solo i livelli di OTOLIN-1 nel siero sono stati considerati e paragonati in pazienti con malattia di Ménière, ipoacusia improvvisa, neurite vestibolare e soggetti sani.
Risultati
Il parametro diagnostico più affidabile è stato il livello sierico di OTOLIN-1. La concentrazione sierica di OTOLIN-1 nei pazienti affetti da malattia di Ménière e ipoacusia improvvisa era significativamente più alta rispetto agli individui sani. I livelli della proteina nel siero dei pazienti con neurite vestibolare e del gruppo di controllo non differivano significativamente.
Conclusioni
La concentrazione sierica di OTOLIN-1 può essere potenzialmente utilizzata come un biomarcatore per i disturbi dell’orecchio interno, dato l’incremento significativo in pazienti con malattia di Ménière e ipoacusia improvvisa rispetto a individui sani.
PAROLE CHIAVE: malattia di Ménière, OTOLIN-1, ipoacusia improvvisa, biomarker, orecchio interno
Introduction
The cellular and molecular structures of the inner ear are difficult to assess due to its anatomy and delicacy. Currently, diagnoses of inner ear pathologies such as sudden hearing loss (SHL), Ménière’s disease (MD), or benign paroxysmal positional vertigo (BPPV) is based on a classic diagnostic battery that requires clinical assessment combined with audiological examinations and imaging studies. In recent years, there is a growing interest in identifying inner ear biomarkers that can potentially simplify the diagnosis of inner ear diseases 1. Advances in the understanding of the inner ear biology have led to the identification of several proteins that are specifically expressed in the cells of the cochlea and vestibule, and thereby represent promising inner ear biomarkers 1. Among these, OTOLIN-1 is a collagen-like protein that serves as an organic scaffold for the deposition of calcium carbonate into otoconia 2-4. It is expressed in the supporting cells of the vestibular maculae and cristae, in the tectorial membrane, in marginal cells of the stria vascularis, and in Claudius cells 2. Recent studies have shown that OTOLIN-1 is detectable in serum and that its level significantly increases in patients affected by BPPV in comparison to healthy subjects 5,6, during aging 7, and after ear surgery 8. Therefore, it has the characteristics of a biomarker, since it is specific for an organ, is easily accessible and measurable and can differentiate between healthy subjects and patients.
The first aim of the study was to expand the range of body fluids where OTOLIN-1 can reliably be detected by analyzing its presence in saliva, urine and serum and to choose the most appropriate fluid for the further analysis. The second aim was to determine whether additional pathologies, such as SHL, MD and vestibular neuritis (VN), cause an increase in OTOLIN-1 level in the fluid of choice in comparison to healthy controls and thus assess its specificity for inner ear disorders.
Materials and methods
Patients
The study consisted of four patient groups divided as follows: 20 with acute onset of MD (mean age 51.2 years), 20 with SHL (mean age 48.8 years), 18 with VN (mean age 58.1 years) and 17 healthy controls (mean age 45.8 years). The healthy control group consisted of individuals with no previous history of MD, SHL, VN, BPPV, or ear operations.
Inclusion criteria for SHL patients were pure-tone audiometry with an increase in the threshold of at least 30 dB in three consecutive frequencies and with the onset of symptoms within 72 hours 9,10. Exclusion criteria were the absence of an acute vertigo attack, previous history of MD and ear operations.
The MD group included patients with probable or definite MD in its acute onset. According to the AAO-HNS criteria 11, probable MD is diagnosed in presence of one definitive episode of vertigo lasting 20 minutes or longer, plus hearing loss, tinnitus, or aural fullness in the affected ear. Definite MD is diagnosed in presence of two or more spontaneous episodes of vertigo lasting 20 minutes or longer, documented hearing loss and tinnitus or aural fullness. Additionally, these patients underwent electrocochleography test and/or glycerol dehydration test. Exclusion criteria were a history of vestibular neuritis, ear operations and decompensated hypertension.
For VN patients, the following inclusion criteria were applied: sudden onset of severe dizziness, spontaneous horizontal-torsional nystagmus at the vestibular examination, abnormal head impulse test, nausea, vomiting, head shaking nystagmus, positive Romberg test, positive Unterberger test and positive caloric test. Exclusion criteria were a history of MD, ear operations, presence of neurological symptoms and absence of acute hearing loss.
Diagnostic procedures
All audiometry procedures were performed in soundproof rooms. Pure-tone audiometry was performed with a computer audiometry system (Audio-DATA, CAS AD2117) at frequencies from 0.125 to 8 kHz for air conduction hearing thresholds and from 0.250 to 6 kHz for bone conduction hearing thresholds.
Electrocochleography (ECochG) was performed using a needle electrode positioned at the promontory. The outer hair cells were stimulated with the use of clicks. The procedure was performed using a Natus CareFusion Nicolet EDX device. The summating potential (SP) and the action potential of the auditory nerve (AP) were detected. A SP/AP limit was set to 0.30-0.40. Values exceeding this limit suggest endolymphatic hydrops 12-14.
The glycerol dehydration test was performed by administering 1.5 mg/kg glycerol for body weight orally early in the morning and after fasting. Three consecutive pure-tone audiometry tests were performed after the administration. The glycerol test was considered positive if the hearing threshold rose by at least 10 dB in three consecutive frequencies.
Head shaking nystagmus test was performed using a chair with the patient sitting upright in front of the examiner. The clinician tilted the patient’s head down 30° and rotated on the horizontal plane in both directions 30 times. The patient was asked to keep his eyes closed during the procedure and was asked to open them at the end of the rotations. Eye movements were observed with Frenzel’s glasses. The presence of three or more nystagmus beats indicated vestibular impairment.
Romberg test was performed with the patient standing up, with the feet together in parallel position, the arms along the body and the eyes closed for one minute. If unsteadiness occurs, the test was positive.
For the Unterberger test, the patients marched in place with closed eyes, raising their knees up to approximately 45°, performing 50 steps (one per second) with their arms extended in front of them at 90°. Patients who showed a right or left rotation equal or superior to 45° were classified as having a change in dynamic balance 15.
Caloric nystagmus test was performed using a Hortmann Video-CNG-Analyser videonystagmography system (Otometrics Natus Medical Denmark ApS, Taastrup, Denmark). Both ears were irrigated with 50 ml of water at 30 °C or 44 °C for 30 seconds. During the procedure, patients remained supine with a 30° elevation of the upper body. Nystagmus was evaluated by the mean maximal slow phase velocity after warm and cool irrigation over 30 seconds. In our laboratory, caloric asymmetry higher than 20% after warm and cool irrigation is defined as canal paresis.
Biochemical analysis
Blood, urine and saliva were collected from patients at admission to the hospital. The blood was centrifuged at 1500 xg for 20 minutes and the supernatant (serum) was stored at -20 °C, together with urine and saliva, until further analysis. Human OTOLIN-1 was quantified by a highly sensitive enzyme-linked immunosorbent assay (ELISA) kit (MyBiosource.com, San Diego, CA), following the manufacturer’s instructions. Serum samples were assayed in duplicate.
Statistical analysis
Data storage and statistical analysis was performed with Microsoft® Office Excel 2010 (Microsoft Corp., Redmond, Wash.), GraphPad Prism 9.0.0(121) (GraphPad Software, San Diego, CA) and SigmaPlot 14.5 (Systat Software, San Jose, CA) using a 5% criterion for statistical significance. The Mann Whitney test was used for statistical comparison between two groups. A Spearman’s correlation test was applied to measure the correlation between OTOLIN-1 serum levels and degree of hearing loss and SP/AP values. To quantify the sensitivity and specificity of serum OTOLIN-1 in distinguishing patients with MD or SHL from healthy subjects, a Receiver-operating Characteristic (ROC) was performed. The level of significance was set at p < 0.05.
Results
Quantification of OTOLIN-1 in body fluids
In the preliminary part of the study, the concentration of OTOLIN-1 was measured in the serum, urine and saliva of 10 patients with acute MD, as an example of inner ear disease, and in 10 healthy individuals. In serum (Fig. 1A), the concentration of OTOLIN-1 ranged from 60.5 to 893.3 pg/ml with a mean concentration of 502.2 ± 240.2 pg/ml in patients with MD (n = 9, one patient refused blood sampling), and from 26.7 to 367.9 pg/ml with a mean value of 145.5 ± 109.2 pg/ml in control subjects (n = 10). Patients with MD had a higher level of OTOLIN-1 in serum in comparison to healthy subjects and the difference was statistically significant as shown by Mann Whitney test (p = 0.0007; p < 0.05). In saliva (Fig. 1B), the concentration of OTOLIN-1 ranged from 17.2 to 1844.2 pg/ml with a mean value of 809.5 ± 547.2 pg/ml in patients with MD (n = 10), and from 611.9 to 1470.5 pg/ml with a mean value of 1046.0 ± 277.2 pg/ml in healthy individuals (n = 10). Overall, in saliva OTOLIN-1 was more concentrated than in serum. In contrast, OTOLIN-1 was less concentrated in urine (Fig. 1C). In patients with MD, it ranged from 0 to 66.9 pg/ml, with the exception of one patient (1750 pg/ml), and in healthy subjects it ranged from 0 to 91.4 pg/ml, with the exception of one individual (947.1 pg/ml). The average OTOLIN-1 level in urine of MD patients (n = 10) and control subjects (n = 10) were 183.1 ± 550.9 pg/ml and 100.7 ± 299.5 pg/ml, respectively. In the saliva and urine, there was no significant difference between patients with MD and control subjects (p = 0.07; p > 0.05, p = 0.12; p > 0.05 respectively). Due to the wide scatter of OTOLIN-1 levels in saliva and the low concentration of the protein in urine in both MD patients and control subjects, it was decided to monitor OTOLIN-1 only in serum samples in further experiments.
Figure 1.
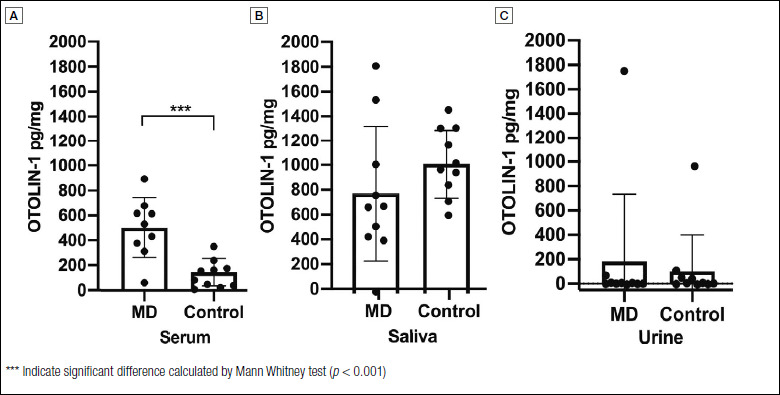
OTOLIN-1 levels in serum (A), saliva (B) and urine (C) of Ménière’s disease patients (MD) and healthy controls. Columns and error bars represent mean values ± SD. Individual data are shown with dots.
Quantification of OTOLIN-1 in serum of patients with MD, SHL, VN and healthy controls
In the second part of the study, OTOLIN-1 serum levels in patients with two different inner ear disorders (MD and SHL) and with retro-cochlear injury (VN) were compared with healthy subjects (Fig. 2). The average concentration of OTOLIN-1 was 439.1 ± 318.5 pg/ml in patients with MD (n = 20), 299.5 ± 224.1 pg/ml in patients with SHL (n = 20), 206.1 ± 136.3 pg/ml in patients with VN (n = 18) and 171.6 ± 107.0 pg/ml in healthy individuals (n = 17). OTOLIN-1 was significantly higher in patients with MD in comparison to control subjects as shown by Mann Whitney test (p = 0.0003; p < 0.05), and to patients with VN (p = 0.001; p < 0.05). OTOLIN-1 was also significantly higher in patients with SHL than in the healthy individuals (p = 0.032; p < 0.05). There was no difference between patients with VN and the control subjects (p = 0.27; p > 0.05). The data showed a significantly higher level of OTOLIN-1 in MD and SHL patients in comparison to control individuals. Additionally, to have a preliminary estimate of the potential of serum OTOLIN-1 in distinguishing patients with MD or SHL from healthy individuals, ROC analysis was performed. The optimal cut-off value to discriminate MD patients and control subjects was 243 pg/ml, which had a sensitivity of 80.0%, a specificity of 76.5% and an area under the curve of 0.82 (95% CI: 0.675-0.966). For the prediction of SHL, the optimal cut-off value was 196 pg/ml, which had a sensitivity of 60.0%, a specificity of 64.7%, and an area under the curve of 0.68 (95% CI: 0.505-0.853). To evaluate the potential of serum OTOLIN-1 as a common biomarker for inner ear diseases, ROC analysis was performed comparing the serum OTOLIN-1 values of both the MD and SHL patients with healthy controls. The optimal cut-off value to discriminate between patients with inner ear disorders and control subjects was 222 pg/ml, which had a sensitivity of 62.5%, a specificity of 70.6%, and an area under the curve of 0.75 (95% CI: 0.625-0.875).
Figure 2.
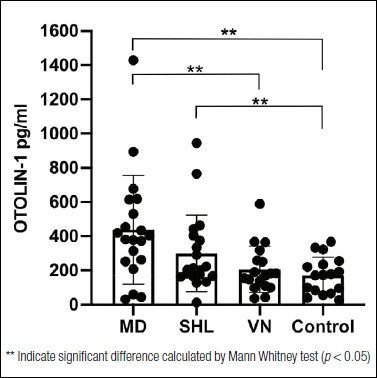
OTOLIN-1 levels in the serum of Ménière’s disease patients (MD), patients with sudden hearing loss (SHL), patients with vestibular neuritis (VN), and healthy controls. Columns and error bars represent mean values ± SD. Individual data are shown with dots.
Relationship between OTOLIN-1 and audiological parameters in MD and SHL patients
It was investigated whether there was a correlation between the severity of hearing loss and OTOLIN-1 levels in MD and SHL patients. Hearing loss was evaluated based on pure tone audiometry. For MD patients, the pure tone average was evaluated for lower frequencies, due to low-tone hearing loss caused by the disease (Tab. I). The frequencies considered were between 125 Hz and 1000 Hz, and the pure tone average for MD patients, referred to as low tone PTA (ltPTA) in this work, had a mean value of 44.29 dB. For SHL patients, the pure tone average (PTA) was measured for frequencies between 500 Hz and 2000 Hz (Tab. I) and had a mean value of 52.25 dB. The potential correlation between ltPTA and OTOLIN-1 in MD patients and PTA and OTOLIN-1 in SHL subjects was tested with a Spearman’s correlation test that gave, respectively, rs = -0.016 (p = 0.94; p > 0.05) and rs = 0.27 (p = 0.24; p > 0.05), indicating no association between the variables. Additionally, it was tested whether there was a relationship between the hydrops index, SP/AP and OTOLIN-1 levels in MD patients. No correlation could be found between protein levels and the SP/AP coefficient, rs = -0.15 (p = 0.56; p > 0.05).
Table I.
Audiologic diagnostic values in Ménière’s disease patients (MD) and patients with sudden hearing loss (SHL).
| MD patients | Age | Sex | ltPTA 1 | ECohG 2 | Glycerol Test | SHL patients | Age | Sex | PTA 3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 41 | 42 | / | + | 1 | 55 | M | 90 |
| 2 | F | 70 | 55 | 0.56 | / | 2 | 19 | M | 6.67 |
| 3 | F | 68 | 70 | / | + | 3 | 62 | M | 116.67 |
| 4 | F | 32 | 50 | 0.32 | - | 4 | 50 | M | 8.33 |
| 5 | M | 23 | 42 | 0.55 | + | 5 | 41 | F | 115 |
| 6 | M | 55 | 47 | 0.71 | + | 6 | 76 | F | 113.33 |
| 7 | F | 65 | 8 | 0.26 | + | 7 | 57 | M | 23.33 |
| 8 | F | 49 | 63 | 0.23 | + | 8 | 81 | F | 30 |
| 9 | F | 78 | 36.25 | 0.42 | - | 9 | 22 | M | 50 |
| 10 | F | 84 | 42.50 | 0.59 | / | 10 | 62 | M | 120 |
| 11 | F | 33 | 67.50 | 0.63 | - | 11 | 38 | M | 11.67 |
| 12 | F | 27 | 22.50 | 0.53 | + | 12 | 41 | F | 11.68 |
| 13 | F | 71 | 52.50 | 0.39 | + | 13 | 36 | M | 28.33 |
| 14 | M | 33 | 42.50 | 0.55 | + | 14 | 67 | F | 21.67 |
| 15 | F | 33 | 38.75 | / | + | 15 | 56 | F | 45 |
| 16 | F | 55 | 35 | 0.62 | / | 16 | 56 | F | 41.67 |
| 17 | M | 20 | 22.50 | 0.57 | - | 17 | 19 | M | 30 |
| 18 | M | 49 | 38.75 | 0.51 | + | 18 | 19 | F | 63.33 |
| 19 | F | 58 | 28.75 | 0.34 | - | 19 | 51 | F | 63.33 |
| 20 | M | 66 | 81.25 | 0.68 | + | 20 | 68 | M | 30 |
1 Low tone pure tone average;
2 Electrocochleography;
3 Pure tone average.
Relationship between OTOLIN-1 and age of subjects
OTOLIN-1 serum concentration has previously been described to increase with aging 7. To verify this information in our cohort of subjects, OTOLIN-1 serum levels of the control group were matched with age. The control individuals were subdivided into three groups: 20-40 years old, 41-60 years old, > 60 years old (Fig. 3A). Mean OTOLIN-1 values were 137.1 ± 286.4 pg/ml for the 20-40 year group (n = 6), 202.9 ± 161.2 pg/ml in those 41-60 year (n = 7), and 168.7 ± 312.1 pg/ml for controls > 60 years (n = 4). The youngest group showed a tendency towards lower OTOLIN-1 levels in serum in comparison to the older groups. However, these differences were not statistically significant as determined by Mann Whitney test between the groups 20-40 and 41-60 (p = 0.18; p > 0.05) and between the group 20-40 and > 60 (p = 0.39; p > 0.05). To investigate the relationship between OTOLIN-1 and age in patients with MD and SHL, that were shown (Fig. 2) to significantly differentiate from controls as far as OTOLIN-1 levels is concerned, patients were divided into the three age groups described above. In MD subjects (Fig. 3B), the protein mean values were 484.6 ± 266.9 pg/ml for the 20-40 year group (n = 7), 298.6 ± 129.2 pg/ml for those 41-61 years (n = 6), and 514.1 ± 455.4 pg/ml for > 60 years (n = 7). No significant differences were found between the younger and older patients as determined by Mann Whitney test between the groups 20-40 and > 60 (p = 0.40; p > 0.05) and between the groups 41-60 and > 60 (p = 0.22; p > 0.05). In SHL patients (Fig. 3C), mean OTOLIN-1 values were 458.5 ± 330.2 pg/ml for the 20-40 year group (n = 4), 278.5 ± 212.7 pg/ml for those 41-61 years (n = 10), and 368.5 ± 129.5 pg/ml for > 60 years (n = 6). Although younger SHL patients had, in tendency, higher values of OTOLIN-1, no significant differences were found, as determines by Mann Whitney test between the groups 20-40 and > 60 (p = 0.08; p > 0.05) and between the groups 41-60 and > 60 (p = 0.18; p > 0.05).
Figure 3.
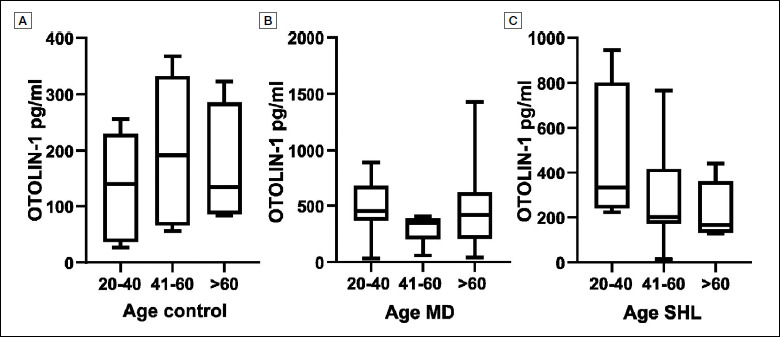
OTOLIN-1 levels in healthy subjects (A), MD (B) and SHL (C) divided in different age groups.
Discussion
One of the characteristics of an effective biomarker is easy sampling. As shown previously 5-8, OTOLIN-1 is detectable in serum and requires a blood withdrawal. In contrast, the collections of saliva and urine are easier to obtain and are not invasive. In our study, we tested the possibility to detect OTOLIN-1 in these two additional body fluids. OTOLIN-1 levels in saliva of healthy subjects and MD patients showed high heterogeneity. This observation can be explained by the different volume of saliva produced by individuals, for example, dehydrated subjects have less saliva leading to a more concentrated protein in the sample. On the other hand, the concentration of OTOLIN-1 in urine was very low in all groups, with the exception of two individuals. The protein has a molecular weight of 70 kDa that exceeds the renal glomerular filtration capacity, and for this reason it is retained in blood and is barely detectable in urine. The two individuals with high OTOLIN-1 urine levels may have a damaged glomerular membrane as a consequence of diabetic nephropathy 16. Even though we showed that OTOLIN-1 is detectable in saliva and urine, the sampling drawbacks of these two body fluids lead us to conclude that OTOLIN-1 in serum is the most reliable diagnostic parameter.
We demonstrated that the serum concentration of OTOLIN-1 in MD and SHL patients was significantly higher than in the control group. In particular, OTOLIN-1 serum levels were highest in MD patients. On the other hand, OTOLIN-1 levels in VN patients was not significantly different from controls. Taking into account that OTOLIN-1 has been shown to increase as a consequence of BPPV 5,6 and after mastoidectomy 8, we suggest that OTOLIN-1 levels correlate positively with isolated damage to the inner ear.
Pathologies involving mainly retro-cochlear structures, such as the VN, may not further alter the serum level of the protein. From our results, we propose a new hypothesis on the pathophysiological construct of the inner ear, in which a pathological event resulting in inner ear damage releases a large amount of OTOLIN-1 into the bloodstream. The exact mechanism of OTOLIN-1 release in the bloodstream is yet to be elucidated. A possible explanation could be the increase of the physiological filtration of OTOLIN-1 through the blood-labyrinthine barrier that also occurs in healthy subjects. Although serum OTOLIN-1 levels increased in MD and SHL patients, we did not find a positive correlation between this level and severity of hearing loss. We also did not find a correlation between the extent of endolymphatic hydrops, measured by the SP/AP ratio, and serum level of the protein in MD subjects. We conclude that OTOLIN-1 serum concentrations can be used in diagnosis of inner ear diseases, but that it does not provide information about the severity of disease.
Additionally, the relationship between OTOLIN-1 levels and age was investigated. OTOLIN-1 serum levels have been reported to increase with increasing age of healthy subjects 7. Our data showed that in tendency, OTOLIN-1 levels were higher in the serum of older healthy subjects in comparison to younger ones, but statistical significance was not reached. We have also investigated the relationship between OTOLIN-1 levels and age in our cohort of patients. In contrast to healthy subjects, in SHL younger patients OTOLIN-1 levels tended to be higher than in older subjects, whereas no tendency and no significant differences could be found in individuals with MD. While aging might increase OTOLIN-1 levels in control subjects, it does not seem to play the same role in patients with inner ear disorders or, alternatively, the aging effect is masked by the pathology.
To properly define the potential clinical applications of OTOLIN-1, it is necessary to determine age-specific thresholds of protein serum levels. Although our study is a small-scale preliminary investigation, we sketched potential serum OTOLIN-1 cut-off values that can be used to differentiate MD or SHL affected subjects from healthy individuals. The cut-off value for diagnosing MD appears to be particularly reliable, having high sensitivity and specificity. The threshold to differentiate SHL patients from healthy subjects has a good compromise of sensitivity and specificity. As the serum OTOLIN-1 levels of MD patients are much higher than for SHL patients, it is clear that a higher cut-off value is needed to discriminate MD patients.
In our work, we evaluated OTOLIN-1 as a common marker for inner ear diseases. We estimated a cut-off value that provides a preliminary indication of the potential of OTOLIN-1 in distinguishing healthy and inner ear disorders affected subjects. Further studies with a higher number of controls and patients will be necessary to calculate more reliable cut-off ranges of the protein in healthy subjects and the thresholds to be applied for different inner ear pathologies, especially in relation to the age of the patient.
Our results showed that OTOLIN-1 appears to be a promising biomarker for the diagnosis of MD. This opens up a number of opportunities for differential diagnosis, for example in patients with acute vertigo, allowing subjects with MD to be differentiated from individuals with VN or neurological conditions (e.g. multiple sclerosis, vestibular migraine or stroke).
The potential use of OTOLIN-1 as a biomarker for inner ear disorders led us to envisage several applications, such as monitoring inner ear damage in patients undergoing chemotherapy or ototoxic antibiotic therapy. Additionally, it could be used as a supplementary diagnostic aid in the evaluation of patients with specific audio-vestibular symptoms following head trauma to assess damage to the labyrinth. Likewise, it could be a marker of specific injury after otologic surgery. It would be interesting to investigate its possible prognostic value by examining the serum levels of the protein during follow-up of patients with inner ear diseases and correlate these with the clinical picture; in particular, this would allow to evaluate the effects of therapies.
A limitation of the study is the small number of individuals enrolled, which allowed only preliminary evaluation of the potential of OTOLIN-1 as a biomarker for inner ear disorders. Furthermore, it was not possible to know whether some of our patients had an acoustic neuroma instead of only an inner ear disorder, because it was not always possible to obtain the results of the suggested MRI from patients and we had no information from the local ENT physicians. However, our study is the first in which OTOLIN-1 has been detected in other body fluids other than serum. In addition, we showed that the serum level increases in MD and SHL in comparison to healthy subjects and VN patients. Preliminary cut-off values were sketched out. A further aspect of our study that has never been previously considered is the investigation of the correlations between OTOLIN-1 and the entity of hearing loss, as well as between the protein and hydrops. We hope that the growing interest in this area of otology will lead to further studies in order to increase the sample size, thus enabling more accurate cut-off values to be calculated.
Conclusions
Our study provides evidence that OTOLIN-1 can be used as a serum marker for inner ear damage. In the foreseeable future, OTOLIN-1 can potentially be applied as an additional examination tool to improve the specificity of the diagnostic process in inner ear diseases, for example in MD. Furthermore, it could be implemented to assess damage to the inner ear following procedures such as ear surgeries, use of ototoxic drugs, or a traumatic event resulting in a labyrinthine concussion.
Acknowledgements
We thank Chiara Baccolini for valuable advice in analysing the data and writing the manuscript.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research was funded by the Cluster of Excellence of the German Research Foundation (DFG; “Deutsche Forschungsgemeinschaft”) EXC 2177/1 “Hearing4all”, and the Hannover Medical School, Hannover, Germany.
Author contributions
EA conceived and designed the study, carried out the sampling, the data analysis and wrote the manuscript. HS performed the biochemical analysis and contributed with the data analysis. GL helped with sample collection and the design of the study. AW and AL-S gave a valuable contribution in reviewing the manuscript. TL and KW designed the study and reviewed the manuscript.
Ethical consideration
The ethics approval was given by the Hannover Medical School Ethics Committee (approval no. 3231-2016 and no. 8199-2018).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from each participant/patient for study participation and data publication.
Figures and tables
References
- 1.Gomaa NA, Jimoh Z, Campbell S, et al. Biomarkers for inner ear disorders: scoping review on the role of biomarkers in hearing and balance disorders. Diagnostics (Basel) 2020;11. https://doi.org/10.3390/diagnostics11010042 10.3390/diagnostics11010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deans MR, Peterson JM, Wong GW. Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PLoS One 2010;5:e12765. https://doi.org/10.1371/journal.pone.0012765 10.1371/journal.pone.0012765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murayama E, Herbomel P, Kawakami A, et al. Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae. Mech Dev 2005;122:791-803. https://doi.org/10.1016/j.mod.2005.03.002 10.1016/j.mod.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Murayama E, Takagi Y, Nagasawa H. Immunohistochemical localization of two otolith matrix proteins in the otolith and inner ear of the rainbow trout, Oncorhynchus mykiss: comparative aspects between the adult inner ear and embryonic otocysts. Histochem Cell Biol 2004;121:155-166. https://doi.org/10.1007/s00418-003-0605-5 10.1007/s00418-003-0605-5 [DOI] [PubMed] [Google Scholar]
- 5.Parham K, Sacks D, Bixby C, et al. Inner ear protein as a biomarker in circulation? Otolaryngol Head Neck Surg 2014;151:1038-1040. https://doi.org/10.1177/0194599814551127 10.1177/0194599814551127 [DOI] [PubMed] [Google Scholar]
- 6.Sacks D, Parham K. Preliminary report on the investigation of the association between BPPV and osteoporosis using biomarkers. Otol Neurotol 2015;36:1532-1536. https://doi.org/10.1097/MAO.0000000000000853 10.1097/MAO.0000000000000853 [DOI] [PubMed] [Google Scholar]
- 7.Tabtabai R, Haynes L, Kuchel GA, et al. Age-related increase in blood levels of Otolin-1 in humans. Otol Neurotol 2017;38:865-869. https://doi.org/10.1097/MAO.0000000000001426 10.1097/MAO.0000000000001426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dogan M, Sahin M, Kurtulmus Y. Otolin-1, as a potential marker for inner ear trauma after mastoidectomy. J Int Adv Otol 2019;15:200-203. https://doi.org/10.5152/iao.2019.5155 10.5152/iao.2019.5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurgel RK, Jackler RK, Dobie RA, et al. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 2012;147:803-807. https://doi.org/10.1177/0194599812458401 10.1177/0194599812458401 [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg 2019;161:S1-S45. https://doi.org/10.1177/0194599812436449 10.1177/0194599812436449 [DOI] [PubMed] [Google Scholar]
- 11.Committee on Hearing and Equilibrium Guidelines for the Diagnosis and Evaluation of Therapy in Meniere’s Disease. Otolaryngol Head Neck Surg 1995;113:181-185. https://doi.org/10.1016/S0194-5998(95)70102-8 10.1016/S0194-5998(95)70102-8 [DOI] [PubMed] [Google Scholar]
- 12.Moon IJ, Park GY, Choi J, et al. Predictive value of electrocochleography for determining hearing outcomes in Meniere’s disease. Otol Neurotol 2012;33:204-210. https://doi.org/10.1097/MAO.0b013e318241b88c 10.1097/MAO.0b013e318241b88c [DOI] [PubMed] [Google Scholar]
- 13.Gibson WP, Prasher DK, Kilkenny GP. Diagnostic significance of transtympanic electrocochleography in Meniere’s disease. Ann Otol Rhinol Laryngol 1983;92:155-159. https://doi.org/10.1177/000348948309200212 10.1177/000348948309200212 [DOI] [PubMed] [Google Scholar]
- 14.Chung WH, Cho DY, Choi JY, et al. Clinical usefulness of extratympanic electrocochleography in the diagnosis of Meniere’s disease. Otol Neurotol 2004;25:144-149. https://doi.org/10.1097/00129492-200403000-00011 10.1097/00129492-200403000-00011 [DOI] [PubMed] [Google Scholar]
- 15.Melo RS, Marinho S, Freire MEA, et al. Static and dynamic balance of children and adolescents with sensorineural hearing loss. Einstein (Sao Paulo) 2017;15:262-268. https://doi.org/10.1590/S1679-45082017AO3976 10.1590/S1679-45082017AO3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 2015;95:405-511. https://doi.org/10.1152/physrev.00042.2012 10.1152/physrev.00042.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


