1. Introduction
In recent years, the prevalence of patients colonized with extended spectrum beta-lactamase (ESBL)-producing Enterobacteriacae has increased globally both in health care facilities and in the community. Similar to most gram-negative bacteria, ESBL-producing Enterobacteriacae reside in the gastrointestinal tract and decolonization is very difficult to achieve. The most common infections caused by ESBL concern the urinary tract and, to a lesser extent, bloodstream infection.
Current surgical site infection (SSI) prevention guidelines do not address the screening, decolonization and potential modification of surgical antibiotic prophylaxis in patients who are colonized with these organisms prior to surgery or the effect of these procedures as a means to prevent SSI. The objective of this systematic review was to assess the effectiveness of these measures.
2. PICO questions
Should surgical antibiotic prophylaxis be modified in areas with high (>10%) ESBL-producing Enterobacteriaceae prevalence?
Should surgical antibiotic prophylaxis be modified in patients who are known carriers of ESBL-producing Enterobacteriaceae?
Should patients be screened for ESBL-producing Enterobacteriaceae prior to surgery?
Population: inpatients and outpatients of any age undergoing any type of surgical procedure
Intervention: (1) change of surgical antibiotic prophylaxis in in areas with high ESBL-producing Enterobacteriaceae prevalence
(2) routine screening for ESBL-producing Enterobacteriaceae in both low- and high- prevalence areas prior to surgery
(3) change of antibiotic prophylaxis if the result is positive for ESBL in low- and high-prevalence areas
Comparator: (1) and (3) No change in antibiotic prophylaxis
(2) No screening
Outcomes: SSI, SSI-attributable mortality
3. Methods
The following databases were searched: Medline (PubMed); Excerpta Medica Database (EMBASE); Cumulative Index to Nursing and Allied Health Literature (CINAHL); Cochrane Central Register of Controlled Trials (CENTRAL); and WHO regional medical databases. The time limit for the review was between 1 January 1990 and 31 April 2014. There were no language restrictions. A comprehensive list of search terms was used, including Medical Subject Headings (MeSH) (Appendix 1).
Two independent reviewers screened the titles and abstracts of retrieved references for potentially relevant studies. The full text of all potentially eligible articles was obtained and two authors then independently reviewed these for eligibility based on inclusion criteria. Duplicate studies were excluded.
4. Study selection
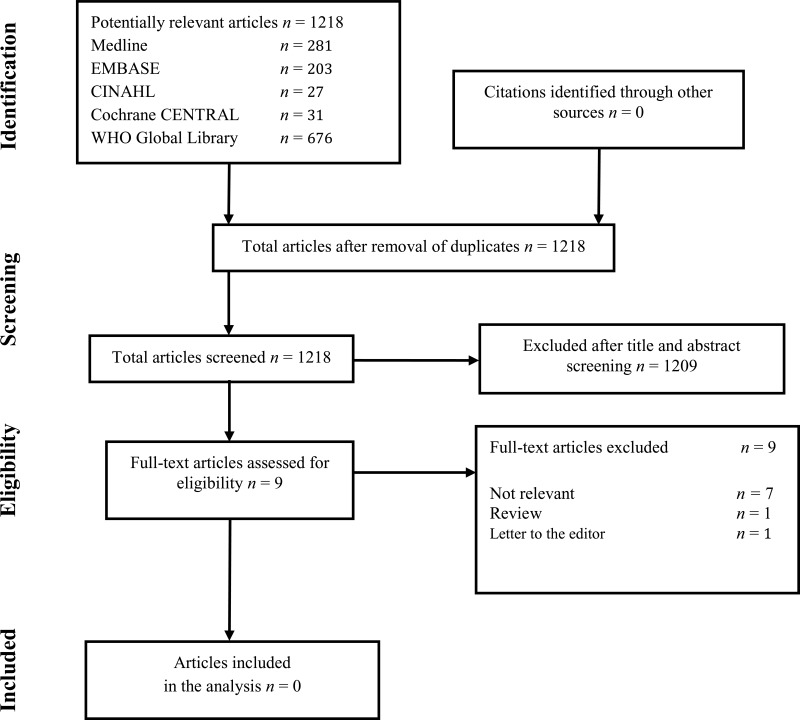
Flow chart of the study selection process
5. Summary of the findings and quality of the evidence
The literature search did not identify any studies comparing the tailored modification of surgical antibiotic prophylaxis for the prevention of SSI in areas with a high prevalence of ESBL-producing Enterobacteriacae (including patients with rectal colonization of ESBL) to no modification of standard antibiotic prophylaxis. Furthermore, no relevant studies were identified comparing routine screening for ESBL with no screening, irrespective of ESBL prevalence prior to surgery.
Two studies related to the topic were identified for consideration. In one retrospective observational study, patients received antibiotic prophylaxis at the discretion of the treating surgeon (1). A modified antibiotic regimen was given as treatment on the basis of the susceptibility of the isolated microorganisms if there was a postoperative complication. In another prospective observational study, routine screening for ESBL faecal carriage was routinely performed for all patients prior to liver transplantation (2). However, the authors failed to mention the types of infection, i.e. surgical vs. not surgical, as the objective of the study was to investigate risk factors associated with the preoperative faecal carriage of ESBL in a specific population. In both studies, the authors expressed concern that the use of carbapenems in ESBL-positive patients might increase the risk of emergence of carbapenem resistance among gram-negative bacteria, for example, carbapenem-resistant Enterobacteriaceae. However, no benefit for the prevention of SSI was shown in these very low quality studies and they were excluded after discussion.
6. Other factors considered in the review of studies
The systematic review team identified the following other factors to be considered.
Potential harms
Routine screening for ESBL prior to surgery might increase the widespread use of broad-spectrum antibiotics pre-surgery in ESBL-colonized patients, particularly carbapenems, This practice may be harmful as it is likely to further increase the emergence of resistance in gram-negative bacteria, especially carbapenem-resistant Enterobacteriaceae. The WHO global surveillance report on antimicrobial resistance has already highlighted concerns about the emergence of antibiotic-resistant bacteria due to the inappropriate use of antimicrobial agents (3). Importantly, the options for the treatment of infections are now extremely limited due to the lack of development of a new class of antimicrobial agents over the past decades
7. Key uncertainties and future research priorities
The systematic review team identified the following key uncertainties and future research priorities.
Although there is an increase in the emergence of ESBL-producing Enterobacteriaceae worldwide, no controlled trials or good quality observational studies that answer the questions of this review have been published, even in countries where ESBL-producing Enterobacteriacae are endemic. Well-designed, randomized controlled trials and good quality observational studies are urgently needed to give guidance to the surgical team and to prevent the inappropriate use of broad-spectrum antibiotics and the emergence of multidrug-resistant organisms on a global basis. As a priority, these studies should investigate whether the tailored modification of surgical antibiotic prophylaxis in areas with a high prevalence of ESBL-producing Enterobacteriaceae is more effective in reducing the risk of SSI than no modification of the standard prophylaxis, including in patients known to be colonized with ESBL.
Appendices
Appendix 1. Search terms
Medline (via PubMed)
“surgical wound infection”[Mesh] OR (surgical site infection* [TIAB] OR “SSI” OR “SSIs” OR surgical wound infection* [TIAB] OR surgical infection*[TIAB] OR post-operative wound infection* [TIAB] OR postoperative wound infection* [TIAB] OR wound infection*[TIAB]) OR “preoperative Care”[Mesh] OR “preoperative care” OR “pre-operative care” OR “perioperative Care”[Mesh] OR “perioperative care” OR “peri-operative care”
“beta-lactamases”[Mesh] OR beta-lactamase* OR “ESBL” OR “ESBLs” OR “extended spectrum beta lactamase” OR ((expanded spectrum OR extended spectrum OR extended spectra OR expanded spectra) AND (lactam* or betalactam*)) OR ((“Escherichia coli”[Mesh] OR “Escherichia coli”) AND (“Enterobacteriaceae”[Mesh] OR “Enterobacteriaceae” OR “Klebsiella”))
“epidemiological monitoring”[Mesh] OR “monitoring” OR “surveillance” [TIAB] OR “screen” or “screening” or “screened” OR “test” or “tests” or “testing” or “tested” OR “routine testing” OR “routine screening” OR “carrier state”[Mesh] OR “carrier” OR “detection” OR “detecting” OR “colonization” OR “colonized” OR “prevalence”[Mesh] OR “prevalence” OR “incidence”[Mesh] OR “incidence”
“antibiotic prophylaxis”[Mesh] OR “antibiotic prophylaxis” OR “antibacterial agents”[Mesh] OR “antibacterial” OR “anti bacterial* OR antibiotic* OR prophyla* OR “carbapenems”[Mesh] OR carbapenem OR carbapenems
Step 1 AND Step 2 AND Step 3 AND Step 4
EMBASE
‘beta lactamase’/exp OR ‘beta lactamase’ OR ‘beta-lactamase’/exp OR ‘beta-lactamase’ OR ‘beta-lactamases’/exp OR ‘beta-lactamases’ OR ‘esbl’/exp OR esbl OR ‘esbls’ OR ‘extended spectrum beta lactamase’/exp OR ‘extended spectrum beta lactamase’ OR (‘expanded spectrum’ OR ‘extended spectrum’ OR ‘extended spectra’ OR ‘expanded spectra’ AND (lactam* OR betalactam*)) OR (‘Escherichia coli’/exp OR ‘Escherichia coli’ AND (‘enterobacteriaceae infection’/exp OR ‘enterobacteriaceae infection’ OR ‘enterobacteriaceae’/exp OR ‘enterobacteriaceae’ OR ‘Klebsiella’/exp OR ‘Klebsiella’))
‘epidemiological monitoring’/exp OR ‘epidemiological monitoring’ OR ‘incidence’ OR ‘prevalence’ OR ‘heterozygote’/exp OR ‘heterozygote’ OR ‘monitoring’/exp OR monitoring OR surveillance:ab,ti OR screen OR ‘screening’/exp OR screening OR screened OR test OR tests OR testing OR tested OR ‘routine testing’ OR ‘routine screening’ OR carrier OR detection OR detecting OR colonization OR colonized OR colonised OR colonisation OR ‘prevalence’/exp OR prevalence OR ‘incidence’/exp OR incidence
‘anti-infective agent’ OR ‘anti-infective agents’/exp OR ‘anti-infective agents’ OR ‘antiinfective agent’/exp OR ‘antiinfective agent’ OR ‘antiinfective agents’ OR ‘carbapenem derivative’/exp OR ‘carbapenem derivative’ OR ‘antibiotic prophylaxis’/exp OR ‘antibiotic prophylaxis’ OR ‘antibacterial’/exp OR ‘antibacterial’ OR ‘anti bacterial’ OR antibiotic* OR prophyla* OR ‘carbapenem’/exp OR carbapenem OR ‘carbapenems’/exp OR carbapenems
#1 AND #2 AND #3
‘surgical infection’/exp OR ‘surgical site infection’:ab,ti OR ‘surgical site infections’:ab,ti OR ssis OR ‘surgical infection wound’:ab,ti OR‘surgical infection wounds’:ab,ti OR ‘surgical infection’:ab,ti OR ‘postoperative wound infection’:ab,ti OR ‘postoperative wound infections’:ab,ti OR ‘post-operative wound infection’:ab,ti OR ‘post-operative wound infections’:ab,ti OR ‘wound infection’:ab,ti OR ‘wound infections’:ab,ti OR ‘preoperative period’/exp OR ‘perioperative period’/exp OR ‘preoperative care’ OR ‘pre-operative care’ OR ‘perioperative care’ OR ‘peri-operative care’
#4 AND #5
CINAHL
(MH surgical wound infection) OR (AB surgical site infection* OR AB SSI OR AB SSIs OR AB surgical wound infection* OR AB surgical infection* OR AB post-operative wound infection* OR AB postoperative wound infection* OR AB wound infection*) OR AB postoperative wound infection* OR AB wound infection*) OR (MH “perioperative care+”) OR AB preoperative care OR AB pre-operative care OR AB perioperative care OR AB peri-operative care
AB beta-lactamase OR AB beta lactamases OR AB ESBL OR AB ESBLs OR ((AB expanded spectrum OR AB extended spectrum OR AB extended spectra OR AB expanded spectra) AND (AB lactam* OR AB betalactam*)) OR (((MH “Escherichia coli”) OR (MH “Escherichia coli infections”) OR AB Escherichia coli) AND ((MH “Enterobacteriaceae”) OR (MH “Enterobacteriaceae infections”) OR AB Enterobacteriaceae OR AB Klebsiella))
(MH “epidemiology+”) OR (MH “Incidence”) OR (MH “Prevalence”) OR (MH “Carrier State”) OR AB monitoring OR AB surveillance OR AB screen or AB screening or AB screened OR AB test or AB tests or AB testing or AB tested OR AB routine testing OR AB routine screening OR AB carrier OR AB detection OR AB detecting OR AB colonization OR AB colonized OR AB colonised OR AB colonisation OR AB prevalence OR AB incidence (MH “antibiotic prophylaxis”) OR (MH “antiinfective agents+”) OR AB anti-infective agent OR AB anti-infective agents OR AB antiinfective agent OR AB antiinfective agents OR AB antibiotic prophylaxis OR AB antibacterial OR AB anti bacterial OR AB antibiotic* OR AB prophyla* OR AB carbapenem OR AB carbapenems OR (MH “carbapenems”)
#1 AND #2 AND #3
Cochrane CENTRAL
- 1.
MeSH descriptor: [surgical wound infection] explode all trees
- 2.
MeSH descriptor: [perioperative care] explode all trees
- 3.
MeSH descriptor: [preoperative care] explode all trees
- 4.
surgical site infections or SSI or SSIs or surgical wound infection* or surgical infection* or post-operative wound infection* or postoperative wound infection* or wound infection* or “preoperative care” or “pre-operative care” or “perioperative care” or “peri-operative care”:ti,ab,kw (word variations have been searched)
- 5.
#1 or #2 or #3 or #4
- 6.
MeSH descriptor: [beta-lactamases] explode all trees
- 7.
beta-lactamase* or “ESBL” or “ESBLs” or “extended spectrum beta lactamase”:ti,ab,kw (word variations have been searched) 432
- 8.
#6 or #7
- 9.
expanded spectrum or extended spectrum or extended spectra or expanded spectra:ti,ab,kw (word variations have been searched)
- 10.
lactam* or betalactam*:ti,ab,kw (word variations have been searched)
- 11.
#9 and #10
- 12.
MeSH descriptor: [Escherichia coli] explode all trees
- 13.
“Escherichia coli”:ti,ab,kw (word variations have been searched)
- 14.
#12 or #13
- 15.
MeSH descriptor: [Enterobacteriaceae] explode all trees
- 16.
“Enterobacteriaceae” or “Klebsiella”:ti,ab,kw (word variations have been searched)
- 17.
#15 or #16
- 18.
#14 and #17
- 19.
#8 or #11 or #18
- 20.
MeSH descriptor: [epidemiological monitoring] explode all trees
- 21.
MeSH descriptor: [carrier state] explode all trees
- 22.
MeSH descriptor: [prevalence] explode all trees
- 23.
MeSH descriptor: [incidence] explode all trees
- 24.
monitoring or surveillance or screen or screening or screened or test or tests or testing or tested or “routine testing” or “routine screening” or carrier or detection or detecting or colonization or colonized or prevalence or incidence:ti,ab,kw (Word variations have been searched)
- 25.
#20 or #21 or #22 or #23 or #24
- 26.
MeSH descriptor: [antibiotic prophylaxis] explode all trees
- 27.
MeSH descriptor: [anti-bacterial agents] explode all trees
- 28.
MeSH descriptor: [carbapenems] explode all trees
- 29.
antibiotic prophylaxis or antibacterial or anti bacterial* or antibiotic* or prophyla* or carbapenem or carbapenems:ti,ab,kw (word variations have been searched)
- #30.
MeSH descriptor: [anti-infective agents] explode all trees
- #31.
#26 or #27 or #28 or #29 or #30
- #32.
#5 and #19 and #25 and #31
WHO Global Health Library
((ssi) OR (surgical site infection) OR (surgical site infections) OR (wound infection) OR (wound infections))
- ti:
title;
- ab:
abstract;
- kw:
key word
References
- 1.
Gupta
N, Sohanlal
T, Soman
R, Shetty
A, Rodrigues
C. The relevance of ESBL producing isolates in patients surgically treated for acute appendicitis. J Associ Physicians India. 2011;59:293–5. [
PubMed: 21751605]
- 2.
Bert
F, Larroque
B, Dondero
F, Durand
F, Paugam-Burtz
C, Belghiti
J, et al. Risk factors associated with preoperative fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in liver transplant recipients. Transpl Infect Dis. 2014;16:84–9. [
PubMed: 24330161]
- 3.